Insights into Sinus-Lift Bone Grafting Materials: What’s Changed?
Abstract
1. Introduction
- SL bone augmentation techniques
- SL postsurgical complications
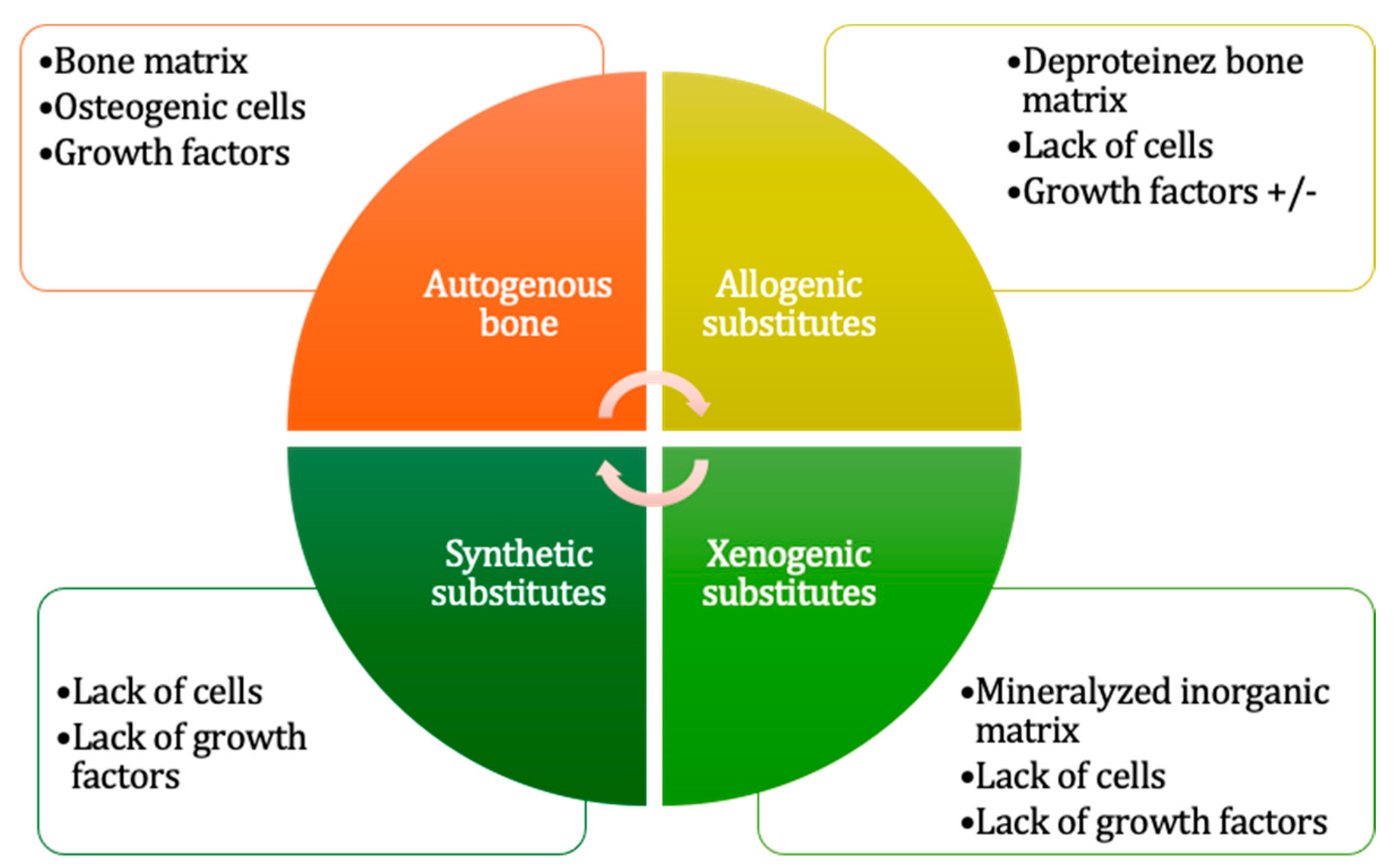
- Grafting materials
- Autogenous grafts
- 2.
- Allografts
- 3.
- Bone substitutes
- 4.
- Growth adjuvants
- 5.
- Synthetic membranes
- Grafting procedure evaluation
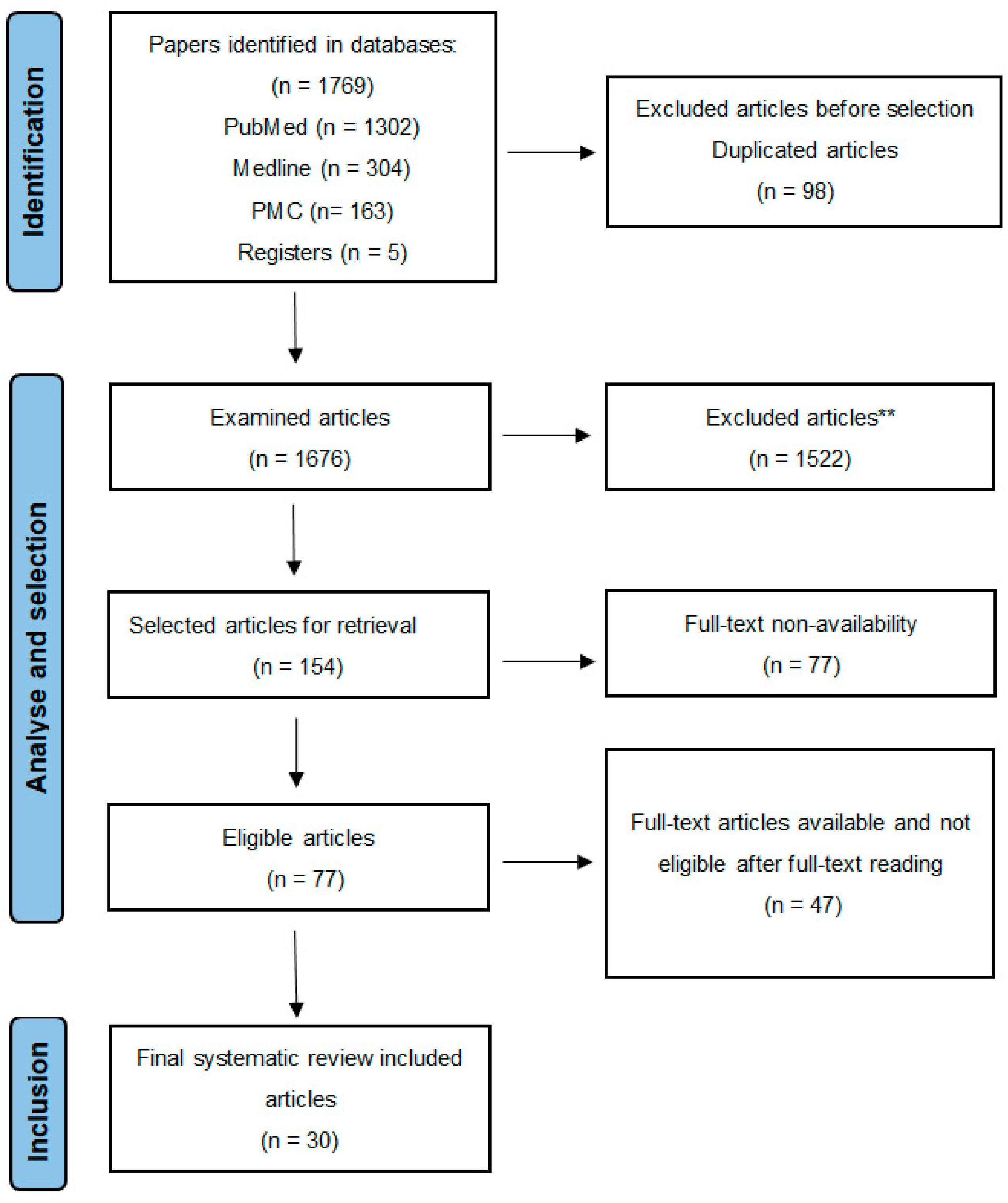
2. Materials and Methods
- Statistical analyses
3. Results
3.1. SL New Bone Regeneration Ratio
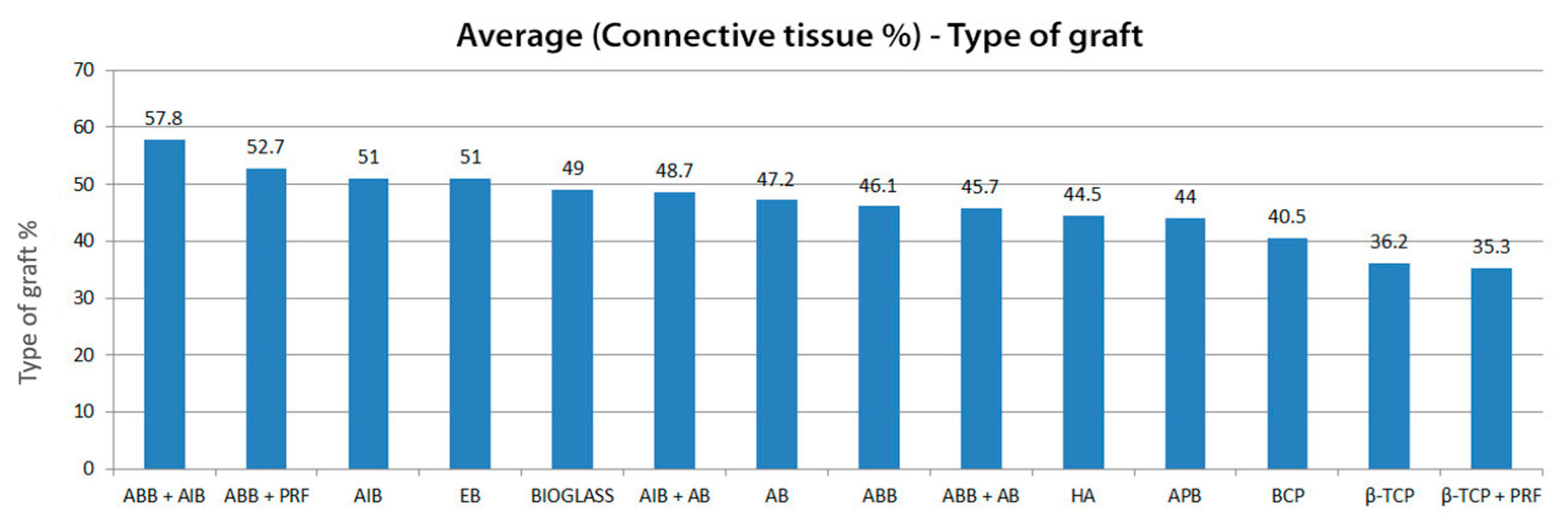
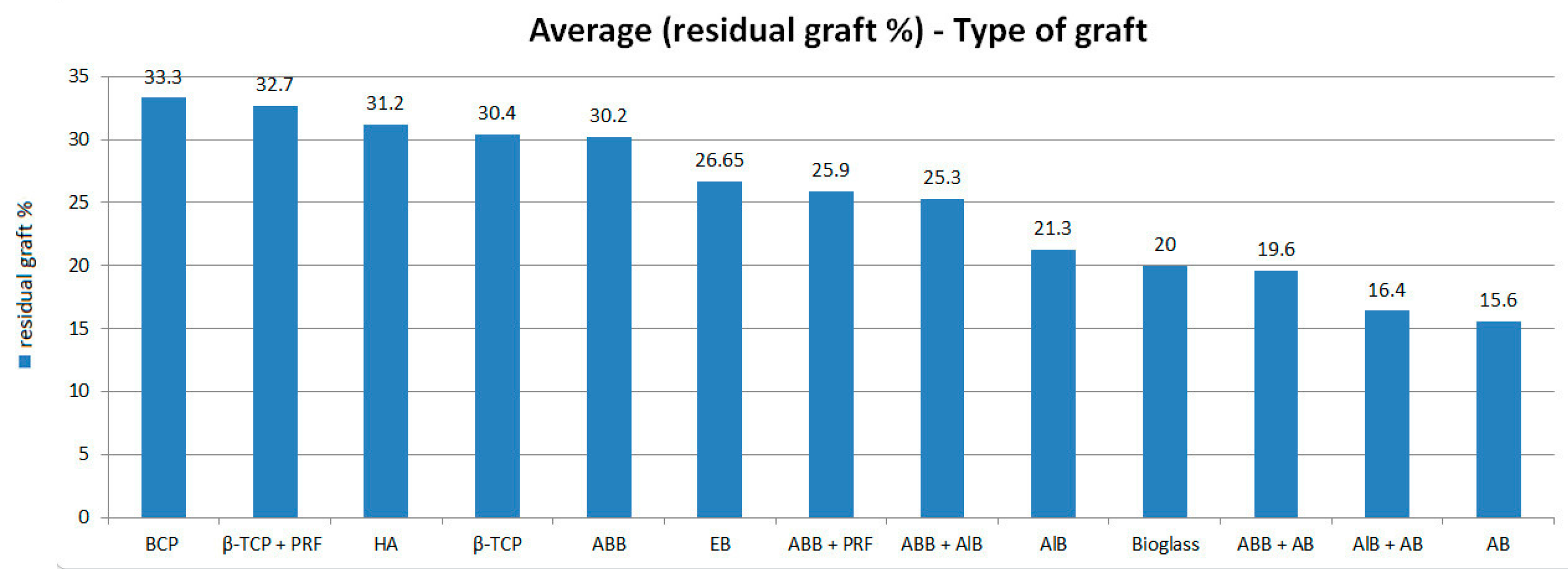
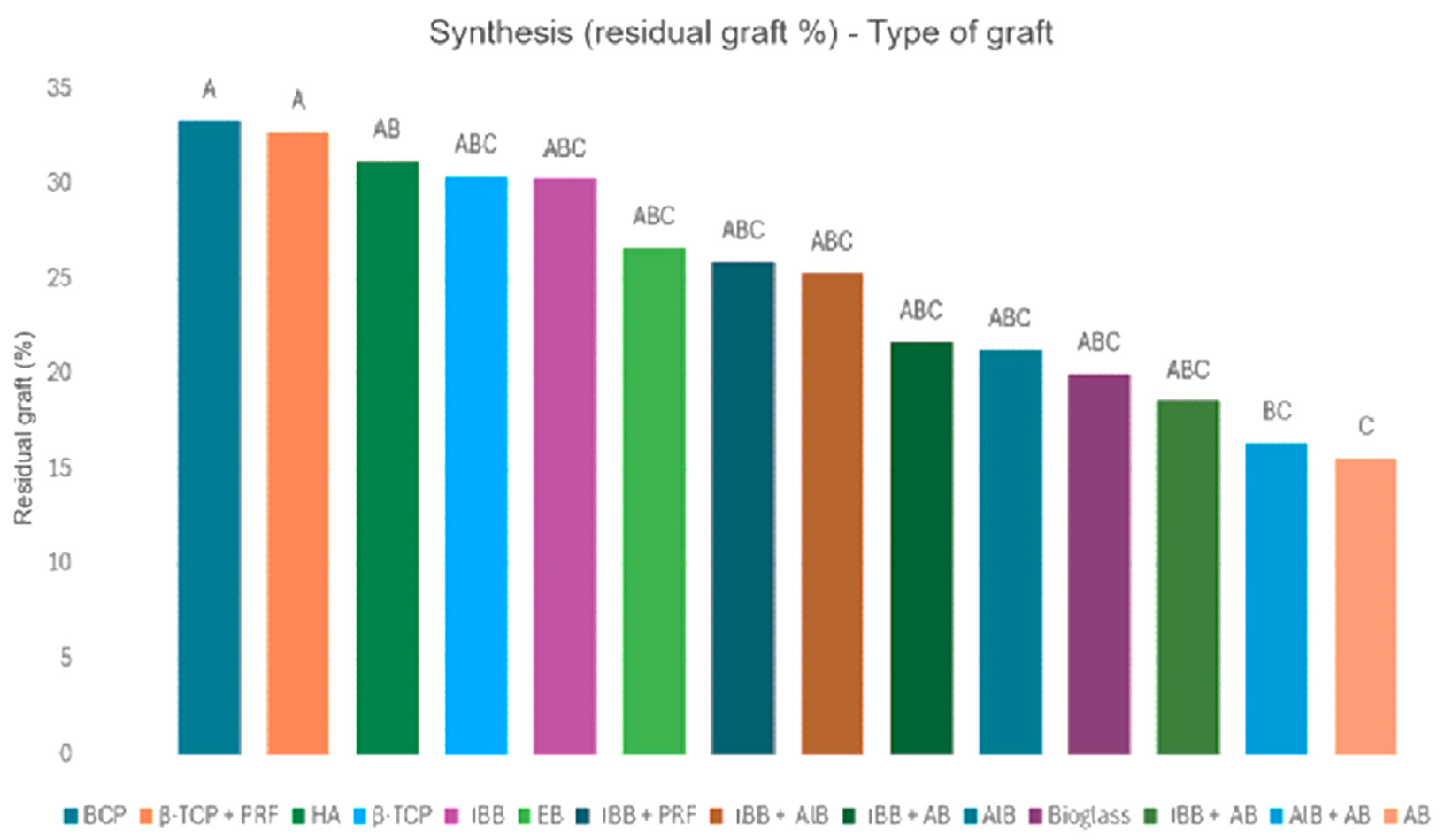
3.2. Connective Tissue Quantity and Residual Graft
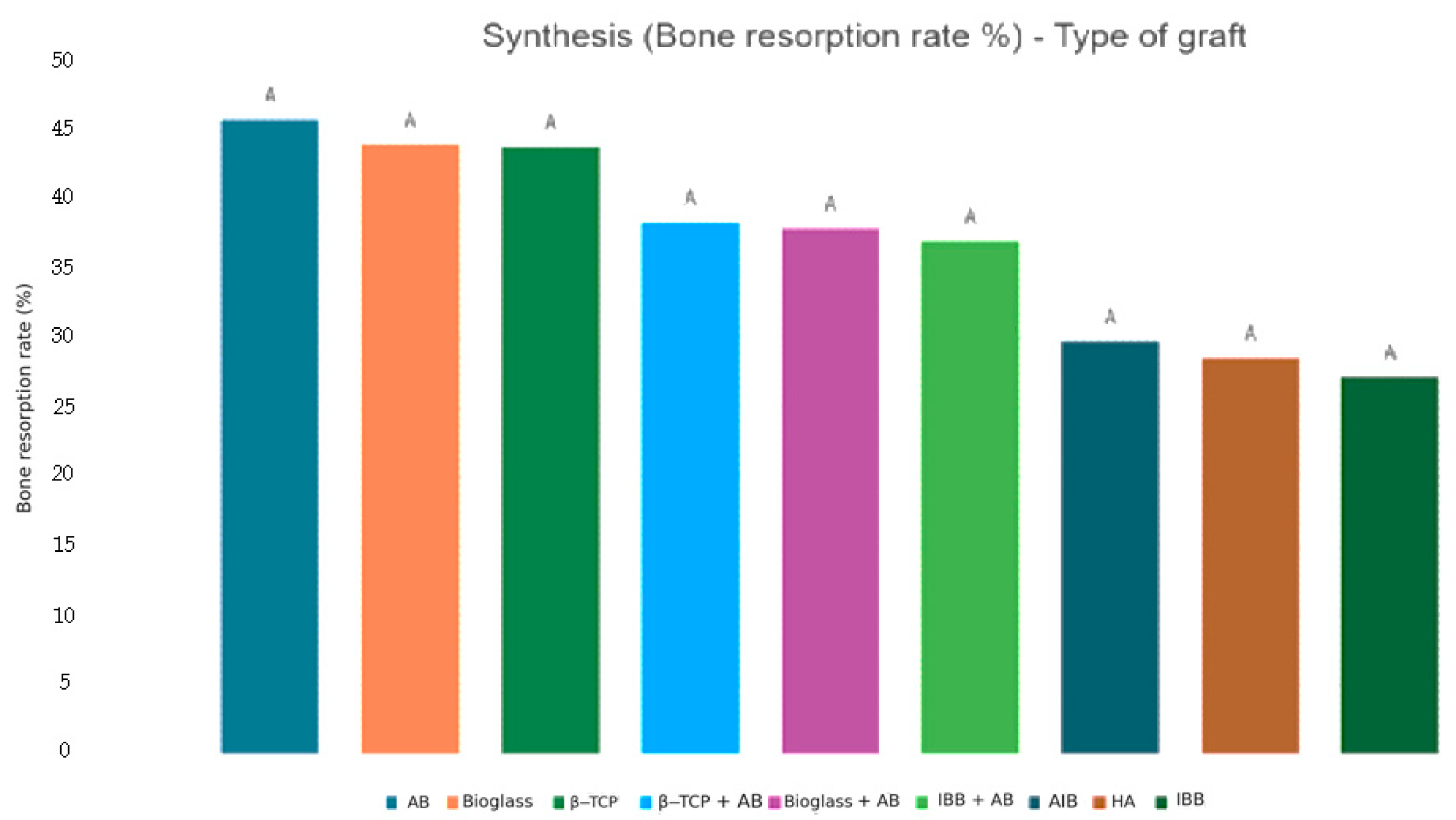
3.3. Bone Resorption Ratio
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riben, C.; Thor, A. The maxillary sinus membrane elevation procedure: Augmentation of bone around dental implants without grafts—A review of a surgical technique. Int. J. Dent. 2012, 2012, 105483. [Google Scholar] [CrossRef]
- Healthjade.net. Available online: https://healthjade.net/sinus-lift/ (accessed on 8 July 2023).
- Themes, U.F.O. The Maxillary Sinus Lift. Pocket Dentistry. 2016. Available online: https://pocketdentistry.com/the-maxillary-sinus-lift/ (accessed on 8 July 2023).
- Elévation Sinusienne par Voie Latérale ou Crestale: Quel Choix Thérapeutique? Le Courrier du Dentiste. 2019. Available online: https://www.lecourrierdudentiste.com/dossiers-du-mois/elevation-sinusienne-par-voie-laterale-ou-crestale-quel-choix-therapeutique.html (accessed on 8 July 2023).
- Bathla, S.C.; Fry, R.R.; Majumdar, K. Maxillary sinus augmentation. J. Indian Soc. Periodontol. 2018, 22, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Pikos, M.A. Maxillary sinus membrane repair: Report of a technique for large perforations. Implant Dent. 1999, 8, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Shiffler, K.; Lee, D.; Aghaloo, T.; Moy, P.K.; Pi-Anfruns, J. Sinus membrane perforations and the incidence of complications: A retrospective study from a residency program. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 10–14. Available online: https://www.sciencedirect.com/science/article/pii/S221244031500560X (accessed on 8 July 2023). [PubMed]
- Maarek, H. Les Complications Sinusiennes post Implantaires. LEFILDENTAIRE Magazine Dentaire. 2014. Available online: https://www.lefildentaire.com/articles/clinique/implantologie/les-complications-sinusiennes-post-implantaires/ (accessed on 8 July 2023).
- Damlar, İ. Disappearance of a dental implant after migration into the maxillary sinus: An unusual case. J. Korean Assoc. Oral Maxillofac. Surg. 2015, 41, 278–280. [Google Scholar] [CrossRef]
- Alshamrani, A.M.; Mubarki, M.; Alsager, A.S.; Alsharif, H.K.; AlHumaidan, S.A.; Al-Omar, A. Maxillary Sinus Lift Procedures: An Overview of Current Techniques, Presurgical Evaluation, and Complications. Cureus 2023, 15, e49553. [Google Scholar] [CrossRef]
- Journal LS—Implantologie Dentaire Greffe Osseuse Autologue: Les Sites de Prélèvement. Available online: https://journal-stomato-implanto.com/content/greffe-osseuse-autologue%C2%A0-les-sites-de-pr%C3%A9l%C3%A8vement (accessed on 8 July 2023).
- Olivier, S.A.A.D. Les substituts Osseux Allogénique et Xénogénique: Utilisation en Chirurgie pré Implantaire. Postgraduate Ph.D. Thesis, Université de Lorraine, Metz, France, 2018. Available online: https://hal.univ-lorraine.fr/hal-01739102/document (accessed on 8 July 2023).
- Utilisation de Greffes Allogéniques Selon un Protocole Spécifique: Rapport de cas Cliniques. Biobank.fr. Available online: https://www.biobank.fr/Medias/Fichier/utilisation-de-greffes-allogeniques-selon-un-protocole-specifique-dr-mailhes.pdf (accessed on 8 July 2023).
- Jordana, F.; Le Visage, C.; Weiss, P. Bone substitutes. Med. Sci. 2017, 33, 60–65. Available online: https://www.medecinecences.org/en/artcles/medsci/full_html/2017/01/medsci20173301p60/medsci20173301p60.html (accessed on 8 July 2023).
- Augmentation des Tissus Durs—Un Aperçu des Techniques de Régénération en Dentisterie. Straumann.com. Available online: https://www.straumann.com/be/fr/shared/news/bone-substitutes/augmentation-of-hard-tissue-regenerative-techniques-in-dentistry.html (accessed on 8 July 2023).
- Deborah, P. Alternatives aux Sinus Lifts: Comblements Sous Sinusiens par Biomatériaux Collagéniques. Ph.D. Thesis, Université de Paris, Paris, France, 2020. [Google Scholar]
- Verrier, P. Utilisation des Verres Bioactifs dans les Dispositifs Médicaux Implantables. Postgraduate Thesis, Université Nancy, Nancy, France, 2008. Available online: http://www.idverre.net/veille/dostec/coll06-verre-bioactif/verre-bioactif.pdf (accessed on 8 July 2023).
- Gallego, T. Utilisation du PRF en implantologie en 2018. Chirurgie 2019, dumas-02102939. [Google Scholar]
- The Use of Barrier Membranes in Implant Dentistry. Glidewell. Available online: https://glidewelldental.com/education/chairside-magazine/volume-13-issue-1/barrier-membranes-implant-dentistry (accessed on 8 July 2023).
- La Regeneration Osseuse Guide. 3Dcelo. Available online: https://www.3dcelo.com/blog/la-regeneration-osseuse-guidee (accessed on 8 July 2023).
- Antoun, H.; Eid, J. Résultats Cliniques, Histologiques et Histomorphométriques des Greffes D’hydroxyapatite Bovine dans les Comblements Sinusiens. Chirurgiens-dentistes.fr. Available online: https://dr-joseph-eid.chirurgiens-dentistes.fr/wp-content/uploads/Resultats-cliniques.pdf (accessed on 8 July 2023).
- Levaudi, A.-L. Évaluation du volume osseux après implantation: Une revue systématique de la littérature. Chirurgie 2017, dumas-01620355. [Google Scholar]
- Khayat, P.G.; Peuch-Lestrade, G.R. Conical Implants by Means of Resonance Frequency Analysis. Asso.fr. 2004. Available online: https://www.sop.asso.fr/admin/documents/ros/ROS0000166/2010.pdf (accessed on 8 July 2023).
- Levin, B.P. The Correlation Between Immediate Implant Insertion Torque and Implant Stability Quotient. Int. J. Periodontics Restor. Dent. 2016, 36, 833–840. [Google Scholar] [CrossRef]
- Guthmann, M. Évaluation des taux de survie et des taux de succès implantaires chez des patients atteints de maladies rares traités dans le centre de références “O-rares” de l’hôpital Rothschild Marion Guthmann. Sciences du Vivant 2020, dumas-03420590. [Google Scholar]
- Pereira, R.S.; Gorla, L.F.; Boos, F.B.J.D.; Okamoto, R.; Garcia Júnior, I.R.; Hochuli-Vieira, E. Use of autogenous bone and beta-tricalcium phosphate in maxillary sinus lifting: Histomorphometric study and immunohistochemical assessment of RUNX2 and VEGF. Int. J. Oral Maxillofac. Surg. 2017, 46, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Traini, T.; Piattelli, A.; Caputi, S.; Degidi, M.; Angano, C.; Scarano, A.; Perrotti, V.; Iezzi, G. Regeneration of human bone using different bone substitute biomaterials. Clin. Implant Dent. Relat. Res. 2015, 17, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Taschieri, S.; Testori, T.; Corbella, S.; Weinstein, R.; Francetti, L.; di Giancamillo, A.; del Fabbro, M. Platelet-rich plasma and deproteinized bovine bone matrix in maxillary sinus lift surgery: A split-mouth histomorphometric evaluation. Implant Dent. 2015, 24, 592–597. [Google Scholar] [CrossRef]
- Taschieri, S.; Corbella, S.; Weinstein, R.; di Giancamillo, A.; Mortellaro, C.; del Fabbro, M. Maxillary sinus floor elevation using platelet-rich plasma combined with either biphasic calcium phosphate or deproteinized bovine bone. J. Craniofac. Surg. 2016, 27, 702–707. [Google Scholar] [CrossRef]
- dos Santos Pereira, R.; Boos, F.; Gorla, L.; Garcia, I.; Okamoto, R.; Hochuli-Vieira, E. Maxillary Sinus Elevation Surgery with ChronOS and Autogenous Bone Graft: Immunohistochemical Assessment of RUNX2, VEGF, TRAP, and Osteocalcin. Int. J. Periodontics Restor. Dent. 2017, 37, e321–e327. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Valente, N.A.; Iezzi, G.; Petrini, M.; Derchi, G.; Barone, A. Maxillary sinus augmentation with three different biomaterials: Histological, histomorphometric, clinical, and patient-reported outcomes from a randomized controlled trial. Clin. Implant Dent. Relat. Res. 2021, 23, 86–95. [Google Scholar] [CrossRef]
- Nizam, N.; Eren, G.; Acalı, A.; Donos, N. Maxillary sinus augmentation with leukocyte and platelet-rich fibrin and deproteinized bovine bone mineral: A split-mouth histological and histomorphometric study. Clin. Oral Implants Res. 2018, 29, 67–75. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; de Buitrago, J.G.; Padial-Molina, M.; Fernández-Barbero, J.E.; Ata-Ali, J.; O’Valle, F. Histopathological comparison of healing after maxillary sinus augmentation using xenograft mixed with autogenous bone versus allograft mixed with autogenous bone. Clin. Oral Implants Res. 2018, 29, 192–201. [Google Scholar] [CrossRef]
- dos Santos Pereira, R.; Menezes, J.D.; Bonardi, J.P.; Griza, G.L.; Okamoto, R.; Hochuli-Vieira, E. Histomorphometric and immunohistochemical assessment of RUNX2 and VEGF of BiogranTM and autogenous bone graft in human maxillary sinus bone augmentation: A prospective and randomized study. Clin. Implant Dent. Relat. Res. 2017, 19, 867–875. [Google Scholar] [CrossRef]
- Annibali, S.; Iezzi, G.; Sfasciotti, G.L.; Cristalli, M.P.; Vozza, I.; Mangano, C.; la Monaca, G.; Polimeni, A. Histological and histomorphometric human results of HA-Beta-TCP 30/70 compared to three different biomaterials in maxillary sinus augmentation at 6 months: A preliminary report. BioMed Res. Int. 2015, 2015, 156850. [Google Scholar] [CrossRef]
- Sehn, F.P.; Dias, R.R.; de Santana Santos, T.; Silva, E.R.; Salata, L.A.; Chaushu, G.; Xavier, S.P. Fresh-frozen allografts combined with bovine bone mineral enhance bone formation in sinus augmentation. J. Biomater. Appl. 2015, 29, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- la Monaca, G.; Iezzi, G.; Cristalli, M.P.; Pranno, N.; Sfasciotti, G.L.; Vozza, I. Comparative Histological and Histomorphometric Results of Six Biomaterials Used in Two-Stage Maxillary Sinus Augmentation Model after 6-Month Healing. BioMed Res. Int. 2018, 2018, 9430989. [Google Scholar] [CrossRef]
- Stacchi, C.; Lombardi, T.; Oreglia, F.; Maltoni, A.A.; Traini, T. Histologic and Histomorphometric Comparison between Sintered Nanohydroxyapatite and Anorganic Bovine Xenograft in Maxillary Sinus Grafting: A Split-Mouth Randomized Controlled Clinical Trial. BioMed Res. Int. 2017, 2017, 9489825. [Google Scholar] [CrossRef]
- de Oliveira, T.A.; Aloise, A.C.; Orosz, J.E.; Oliveira, R.D.M.E.; de Carvalho, P.; Pelegrine, A.A. Double Centrifugation Versus Single Centrifugation of Bone Marrow Aspirate Concentrate in Sinus Floor Elevation: A Pilot Study. Int. J. Oral Maxillofac. Implants 2016, 31, 216–222. [Google Scholar] [CrossRef]
- Pasquali, P.J.; Teixeira, M.L.; de Oliveira, T.A.; de Macedo, L.G.S.; Aloise, A.C.; Pelegrine, A.A. Maxillary Sinus Augmentation Combining Bio-Oss with the Bone Marrow Aspirate Concentrate: A Histomorphometric Study in Humans. Int. J. Biomater. 2015, 2015, 121286. [Google Scholar] [CrossRef]
- Wildburger, A.; Payer, M.; Jakse, N.; Strunk, D.; Etchard-Liechtenstein, N.; Sauerbier, S. Impact of autogenous concentrated bone marrow aspirate on bone regeneration after sinus floor augmentation with a bovine bone substitute—A split-mouth pilot study. Clin. Oral Implants Res. 2014, 25, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Batas, L.; Tsalikis, L.; Stavropoulos, A. PRGF as adjunct to DBB in maxillary sinus floor augmentation: Histological results of a pilot split-mouth study. Int. J. Implant Dent. 2019, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.S.; Seo, Y.S.; Lee, G.J.; You, J.S.; Kim, S.G. A Comparative Study with Biphasic Calcium Phosphate to Deproteinized Bovine Bone in Maxillary Sinus Augmentation: A Prospective Randomized and Controlled Clinical Trial. Int. J. Oral Maxillofac. Implants 2019, 34, 233–242. [Google Scholar] [CrossRef]
- Kivovics, M.; Szabó, B.T.; Németh, O.; Czinkóczky, B.; Dőri, F.; Nagy, P.; Szabo, G. Microarchitecture of the Augmented Bone Following Sinus Elevation with an Albumin Impregnated Demineralized Freeze-Dried Bone Allograft (BoneAlbumin) versus Anorganic Bovine Bone Mineral: A Randomized Prospective Clinical, Histomorphometric, and Micro-Computed Tomography Study. Materials 2018, 11, 202. [Google Scholar] [CrossRef]
- Menezes, J.D.; Pereira, R.D.S.; Bonardi, J.P.; Griza, L.G.; Okamoto, R.; Hochuli-Vieira, E. Bioactive glass added to autogenous bone graft in maxillary sinus augmentation: A prospective histomorphometric, immunohistochemical, and bone graft resorption assessment. J. Appl. Oral Sci. Rev. FOB 2018, 26, e20170296. [Google Scholar] [CrossRef]
- Payer, M.; Lohberger, B.; Strunk, D.; Reich, K.M.; Acham, S.; Jakse, N. Effects of directly autotransplanted tibial bone marrow aspirates on bone regeneration and osseointegration of dental implants. Clin. Oral Implants Res. 2014, 25, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Jelusic, D.; Zirk, M.L.; Fienitz, T.; Plancak, D.; Puhar, I.; Rothamel, D. Monophasic ß-TCP vs. biphasic HA/ß-TCP in two-stage sinus floor augmentation procedures—A prospective randomized clinical trial. Clin. Oral Implants Res. 2017, 28, e175–e183. [Google Scholar] [CrossRef]
- Meimandi, M.; Moghaddam, A.A.; Gholami, A.G.; Abbass, F.M.; Solati, M. Histomorphometric and Histologic Evaluation of Nano-HA with and without PRGF in Bilateral Sinus Lift Augmentation: A Randomized Clinical Trial. J. Res. Med. Dent. Sci. 2017, 5, 69–81. [Google Scholar]
- Kılıç, S.C.; Güngörmüş, M.; Parlak, S.N. Histologic and histomorphometric assessment of sinus-floor augmentation with beta-tricalcium phosphate alone or in combination with pure-platelet-rich plasma or platelet-rich fibrin: A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2017, 19, 959–967. [Google Scholar] [CrossRef]
- Mordenfeld, A.; Johansson, C.B.; Albrektsson, T.; Hallman, M. A randomized and controlled clinical trial of two different compositions of deproteinized bovine bone and autogenous bone used for lateral ridge augmentation. Clin. Oral Implants Res. 2014, 25, 310–320. [Google Scholar] [CrossRef]
- Correia, F.; Pozza, D.H.; Gouveia, S.; Felino, A.C.; Faria-Almeida, R. Advantages of porcine xenograft over autograft in sinus lift: A randomised clinical trial. Materials 2021, 14, 3439. [Google Scholar] [CrossRef]
- Starch-Jensen, T.; Mordenfeld, A.; Becktor, J.P.; Jensen, S.S. Maxillary sinus floor augmentation with synthetic bone substitutes compared with other grafting materials: A systematic review and meta-analysis. Implant Dent. 2018, 27, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Sadi, A.; Engebretson, S.P.; Janal, M.N. Histomorphometric results of different grafting materials and effect of healing time on bone maturation after sinus floor augmentation: A systematic review and meta-analysis. J. Periodontal Res. 2017, 52, 301–312. [Google Scholar] [CrossRef]
- Pereira, R.S.; Menezes, J.D.; Bonardi, J.P.; Griza, G.L.; Okamoto, R.; Hochuli-Vieira, E. Comparative study of volumetric changes and trabecular microarchitecture in human maxillary sinus bone augmentation with bioactive glass and autogenous bone graft: A prospective and randomized assessment. Int. J. Oral Maxillofac. Surg. 2018, 47, 665–671. [Google Scholar] [CrossRef]
- Xavier, S.P.; Santos, T.D.S.; Sehn, F.P.; Silva, E.R.; Garcez-Filho, J.D.A.; Martins-Filho, P.R.S. Maxillary sinus grafting with fresh frozen allograft versus bovine bone mineral: A tomographic and histological study. J. Cranio-Maxillofac. Surg. 2016, 44, 708–714. [Google Scholar] [CrossRef]
- Trimmel, B.; Gede, N.; Hegyi, P.; Szakács, Z.; Mezey, G.A.; Varga, E.; Kivovics, M.; Hanák, L.; Rumbus, Z.; Szabó, G. Relative performance of various biomaterials used for maxillary sinus augmentation: A Bayesian network meta-analysis. Clin. Oral Implants Res. 2021, 32, 135–153. [Google Scholar] [CrossRef]
- Papageorgiou, S.N.; Papageorgiou, P.N.; Deschner, J.; Götz, W. Comparative effectiveness of natural and synthetic bone grafts in oral and maxillofacial surgery prior to insertion of dental implants: Systematic review and network meta-analysis of parallel and cluster randomized controlled trials. J. Dent. 2016, 48, 1–8. [Google Scholar] [CrossRef]
- Lemos, C.A.A.; Mello, C.C.; dos Santos, D.M.; Verri, F.R.; Goiato, M.C.; Pellizzer, E.P. Effects of platelet-rich plasma in association with bone grafts in maxillary sinus augmentation: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2016, 45, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Mejia, H.; Estrugo-Devesa, A.; Saka-Herrán, C.; Ayuso- Montero, R.; López-López, J.; Velasco-Ortega, E. Platelet-rich plasma in maxillary sinus augmentation: Systematic review. Materials 2020, 13, 622. [Google Scholar] [CrossRef] [PubMed]
- Aludden, H.C.; Mordenfeld, A.; Hallman, M.; Dahlin, C.; Jensen, T. Lateral ridge augmentation with Bio-Oss alone or Bio-Oss mixed with particulate autogenous bone graft: A systematic review. Int. J. Oral Maxillofac. Surg. 2017, 46, 1030–1038. [Google Scholar] [CrossRef]
- Bouwman, W.F.; Bravenboer, N.; ten Bruggenkate, C.M.; Eijsackers, F.A.; Stringa, N.; Schulten, E.A.J.M. Tissue level changes after maxillary sinus floor elevation with three types of calcium phosphate ceramics: A radiological study with a 5-year follow-up. Materials 2021, 14, 1471. [Google Scholar] [CrossRef]
- Maddalone, M.; Mirabelli, L.; Venino, P.M.; Karanxha, L.; Porcaro, G.; del Fabbro, M. Long-term stability of autologous bone graft of intraoral origin after lateral sinus floor elevation with simultaneous implant placement. Clin. Implant Dent. Relat. Res. 2018, 20, 713–721. [Google Scholar] [CrossRef]
- Shin, S.Y.; Hwang, Y.J.; Kim, J.H.; Seol, Y.J. Longterm results of new deproteinized bovine bone material in a maxillary sinus graft procedure. J. Periodontal Implant Sci. 2014, 44, 259–264. [Google Scholar] [CrossRef]
- Murata, M.; Hirose, Y.; Ochi, M.; Tazaki, J.; Okubo, N.; Akazawa, T. Twenty Years-Passed Case of Demineralized Dentin Matrix Autograft for Sinus Bone Augmentation—A First Case of Dentin Graft in Human. J. Clin. Exp. Dent. 2023, 15, e861–e865. [Google Scholar]




| Author’s Information | Sample | Intervention | Histomorphological Results | Healing Time | |||
|---|---|---|---|---|---|---|---|
| First Author | Publication Year | Number of SL | Biomaterials | Subgroups | New Bone (%) | Months | |
| Average | Standard Deviation (SD) | ||||||
| Pereira R. [26] | 2017 | 12 | Beta tricalcium phosphate | β-TCP | 46.3 | 11.6 | 6 |
| 9 | Beta tricalcium phosphate + Autologous (1:1) | β-TCP + AB | 35.0 | 15.8 | 6 | ||
| 12 | Autologous bone | AB | 46.1 | 16.3 | 6 | ||
| Traini T. [27] | 2015 | 32 | Inorganic bovine bone | IBB | 34.5 | 0.9 | 6 |
| 7 | Inorganic bovine bone + Autologous (1:1) | IBB + AB | 38.7 | 3.2 | 6 | ||
| 22 | Hydroxyapatite | HA | 34.2 | 2.4 | 6 | ||
| 16 | Autologous bone | AB | 40.1 | 3.2 | 6 | ||
| Taschier S. [28] | 2015 | 6 | Inorganic bovine bone | IBB | 22.7 | 11.3 | 6 |
| 6 | Inorganic bovine bone + PRF | IBB + PRF | 30.7 | 12.4 | 6 | ||
| Taschieri S. [29] | 2016 | 10 | Biphasic calcium phosphate (40HA-60β-TCP) + PRP | BCP + PRF | 18.6 | 3.3 | 6 |
| 10 | Inorganic bovine bone + PRF | IBB + PRF | 21.9 | 4.9 | 6 | ||
| dos Santos Pereira R. [30] | 2017 | 9 | Β-tricalcium phosphate + Autologous (1:1) | β-TCP + AB | 25.4 | 6.4 | 6 |
| 12 | Autologous | AB | 38.6 | 10.5 | 6 | ||
| Velasco-Ortega E. [31] | 2021 | 8 | Biphasic calcium phosphate (40HA-60β-TCP) | BCP | 23.8 | 3.3 | 6 |
| 8 | Inorganic bovine bone | IBB | 25.9 | 2.7 | 6 | ||
| Nizam N. [32] | 2018 | 13 | Inorganic bovine bone + PRF | IBB + PRF | 21.4 | 8.8 | 6 |
| 13 | Inorganic bovine bone | IBB | 21.2 | 5.6 | 6 | ||
| Galindo-Moreno P. [33] | 2018 | 7 | Inorganic bovine bone + Autologous (1:1) | IBB + AB | 34.5 | 13.1 | 6 |
| 7 | Allogenous + Autologous (1:1) | AlB + AB | 41.0 | 12.9 | 6 | ||
| Pereira R. [34] | 2017 | 10 | Bioactive glass ceramic | Bioglass | 42.0 | 7.3 | 6 |
| 10 | Bioactive glass ceramic + Autologous | Bioglass + AB | 33.2 | 13.3 | 6 | ||
| 10 | Autologous | AB | 35.3 | 14.7 | 6 | ||
| Annibali S. [35] | 2015 | 1 | Biphasic calcium phosphate (30HA-70β-TCP) | BCP | 30.2 | NR | 6 |
| 1 | Inorganic bovine bone | IBB | 20.1 | NR | 6 | ||
| 1 | Allogenous bone | AlB | 16.4 | NR | 6 | ||
| 1 | Inorganic equine bone | EB | 21.9 | NR | 6 | ||
| Sehn F. [36] | 2015 | 17 | Allogenous bone | AlB | 12.5 | 2.5 | 6 |
| 17 | Allogenous + Inorganic bovine bone (2:1) | AlB + IBB | 24.4 | 7.2 | 6 | ||
| La Monaca G. [37] | 2018 | 1 | Allogenous bone | AlB | 26.4 | NR | 6 |
| 1 | Inorganic bovine bone | IBB | 16.1 | NR | 6 | ||
| 1 | Inorganic equine bone | EB | 22.8 | NR | 6 | ||
| 1 | Biphasic calcium phosphate (30HA-70β-TCP) | BCP | 20.3 | NR | 6 | ||
| 1 | Bioapatite collagen | BC | 21.4 | NR | 6 | ||
| Stacchi F. [38] | 2017 | 26 | Hydroxyapatite | HA | 34.9 | 15 | 6 |
| 26 | Inorganic bovine bone | IBB | 38.5 | 17 | 6 | ||
| de Oliveira P. [39] | 2016 | 7 | Inorganic bovine bone | IBB | 27.3 | 5.5 | 6 |
| 7 | Inorganic bovine bone + Bone marrow concentrate | IBB + BMC | 38.4 | 12.3 | 6 | ||
| Pasquali L. [40] | 2015 | 8 | Inorganic bovine bone | IBB | 27.3 | 5.5 | 6 |
| 8 | Inorganic bovine bone + Bone marrow concentrate | IBB + BMC | 55.1 | 20.9 | 6 | ||
| Wildburger S. [41] | 2014 | 7 | Inorganic bovine bone | IBB | 13.9 | 8.5 | 6 |
| 6 | Inorganic bovine bone + Bone marrow concentrate | IBB + BMC | 13.5 | 5.4 | 6 | ||
| Batas C. [42] | 2019 | 6 | Inorganic bovine bone | IBB | 37.8 | 3.1 | 6 |
| 6 | Inorganic bovine bone + PRF | IBB + PRF | 35.6 | 8.3 | 6 | ||
| Oh J. [43] | 2019 | 25 | Inorganic bovine bone | IBB | 25.1 | 9.6 | 6 |
| 27 | Biphasic calcium phosphate (60HA-40β-TCP) | BCP | 28.8 | 7.9 | 6 | ||
| Kivovics K. [44] | 2018 | 11 | Inorganic bovine bone | IBB | 50.2 | 10.8 | 6 |
| 12 | Allogenous bone | AlB | 36.3 | 8.0 | 6 | ||
| Menezes D. [45] | 2018 | 12 | Bioactive glass ceramic + Autologous bone | Bioglass + AB | 45.8 | 13.8 | 6 |
| 9 | Autologous bone | AB | 42.0 | 16.6 | 6 | ||
| Payer M. [46] | 2014 | 5 | Inorganic bovine bone | IBB | 10.4 | 5.3 | 6 |
| 6 | Inorganic bovine bone + bone marrow concentrate | IBB + BMC | 14.2 | 3.6 | 6 | ||
| Jelusic A. [47] | 2017 | 30 | Biphasic calcium phosphate (60HA-40β-TCP) | BCP | 38.4 | 19.4 | 6 |
| 30 | Beta tricalcium phosphate | β-TCP | 36.2 | 10.4 | 6 | ||
| Meimandi G. [48] | 2017 | 10 | Hydroxyapatite + PRF | HA + PRF | 30.3 | 8.5 | 6 |
| 10 | Hydroxyapatite | HA | 25.3 | 7.3 | 6 | ||
| Kilic C. [49] | 2017 | 9 | Beta tricalcium phosphate | β-TCP | 32 | 6.3 | 6 |
| 8 | Beta tricalcium phosphate + Autologous bone | β-TCP + AB | 33.4 | 10.4 | 6 | ||
| Author’s Information | Sample | Intervention | Histomorphological Results | Healing Time | |||||
|---|---|---|---|---|---|---|---|---|---|
| First Author | Publication Year | SL Number | Biomaterials | Subgroups | Connective Tissue Quantity (%) | Standard Deviation (SD) | Residual Graft Quantity (%) | Standard Deviation (SD) | Months |
| Mordenfeld A. [50] | 2014 | 14 | Inorganic bovine bone | IBB | 42.0 | 5.5 | NR | NR | 7.5 |
| 14 | Inorganic bovine bone + Autogenous | IBB + AB | 46.0 | 6.5 | NR | NR | 7.5 | ||
| La Monaca G. [47] | 2018 | 1 | Allogenic | AlB | 47.8 | NR | 20.1 | NR | 6 |
| 1 | Inorganic bovine bone | IBB | 46.7 | NR | 37.2 | NR | 6 | ||
| 1 | Inorganic equine bone | EB | 47.1 | NR | 30.1 | NR | 6 | ||
| 1 | Biphasic calcium phosphate (30HA/70β-TCP) | BCP | 41.8 | NR | 37.9 | NR | 6 | ||
| 1 | Hydroxyapatite | HA | 53.3 | NR | 25.3 | NR | 6 | ||
| Kilic C. [49] | 2017 | 9 | Beta tricalcium phosphate | Β-TCP | 36.2 | 10.6 | 30.4 | 10.3 | 6 |
| 9 | Beta tricalcium phosphate + PRF | β-TCP + PRF | 35.3 | 10.8 | 32.7 | 7.5 | 6 | ||
| Correia F. [51] | 2021 | 6 | Autogenous | AB | 42.7 | 2.9 | NR | NR | 6 |
| 6 | Inorganic porcine bone | IPB | 44.0 | 2.9 | NR | NR | 6 | ||
| Traini T. [25] | 2015 | 32 | Inorganic bovine bone | IBB | 36.4 | 2.3 | 32.8 | 2.1 | 6 |
| 22 | Hydroxyapatite | HA | 35.6 | 2.3 | 37.1 | 3.8 | 6 | ||
| 16 | Autogenous | AB | 40.0 | 2.1 | 18.3 | 2.3 | 6 | ||
| 7 | Inorganic bovine bone + Autogenous (1:1) | IBB + AB | 45.6 | 5.0 | 14.4 | 2.1 | 6 | ||
| 16 | Bioactive glass ceramic | Bioglass | 49.0 | 1.8 | 20.0 | 2.4 | 6 | ||
| Starch-Jensen T. [52] | 2018 | 10 | Autogenous | AB | 58.4 | NR | 10.8 | NR | 6 |
| 10 | Biphasic calcium phosphate (30HA/70β-TCP) | BCP | 38.9 | NR | 32.9 | NR | 6 | ||
| Danesh Sadi S. [53] | 2017 | NR | Autogenous | AB | 47.6 | NR | 17.7 | NR | 4.5–9 |
| NR | Allogenic | AlB | 50.0 | NR | 25.3 | NR | 4.5–9 | ||
| NR | Inorganic bovine bone | ABB | 44.9 | NR | 29.3 | NR | 4.5–9 | ||
| NR | Inorganic bovine bone + Autogenous (1:1) | IBB + AB | 47.4 | NR | 22.8 | NR | 4.5–9 | ||
| NR | Allogenic + Autogenous (1:1) | AlB + AB | 48.3 | NR | 23.5 | NR | 4.5–9 | ||
| NR | Inorganic bovine bone + Allogenic (1:1) | IBB + AlB | 57.8 | NR | 25.3 | NR | 4.5–9 | ||
| Annibali S. [35] | 2015 | 1 | Biphasic calcium phosphate (30HA/70β-TCP) | BCP | 40.7 | NR | 29.1 | NR | 6 |
| 1 | Inorganic bovine bone | ABB | 60.8 | NR | 19.1 | NR | 6 | ||
| 1 | Inorganic equine bone | EB | 54.9 | NR | 23.2 | NR | 6 | ||
| 1 | Allogenic | AlB | 55.1 | NR | 18.5 | NR | 6 | ||
| Galindo-Moreno P. [33] | 2018 | 7 | Inorganic bovine bone + Autogenous (1:1) | IBB + AB | 43.8 | 19.9 | 21.7 | 17.9 | 6 |
| 7 | Allogenic + Autogenous (1:1) | AlB + AB | 49.0 | 14.3 | 9.3 | 7.7 | 6 | ||
| Nizam N. [32] | 2018 | 13 | Inorganic bovine bone | IBB | 45.9 | 8.4 | 32.8 | 5.9 | 6 |
| 13 | Inorganic bovine bone + PRF | IBB + PRF | 52.7 | 12.5 | 25.9 | 9.5 | 6 | ||
| Author’s Information | Sample | Intervention | Radiological Results | Healing Time | |||
|---|---|---|---|---|---|---|---|
| First author | Publication Year | SL Number | Biomaterials | Subgroups | Bone Resorption Ratio (%) | Standard Deviation (SD) | Months |
| Starch-Jensen T. [52] | 2018 | 14 | Hydroxyapatite | HA | 28.5 | NR | 7 |
| 14 | Inorganic bovine bone | IBB | 22.4 | NR | 7 | ||
| Mordenfeld A. [50] | 2014 | 14 | Inorganic bovine bone + Autogenous | IBB + AB | 37 | 19.9 | 7.5 |
| 14 | Inorganic bovine bone | IBB | 46.9 | 23.5 | 7.5 | ||
| Pereira R. [54] | 2018 | 12 | Bioactive glass ceramic | Bioglass | 44 | 16 | 6 |
| 9 | Bioactive glass ceramic + Autogenous (1:1) | Bioglass + AB | 37.9 | 18.9 | 6 | ||
| 12 | Autogenous | AB | 45.7 | 18,5 | 6 | ||
| Pereira R. [30] | 2017 | 12 | Beta tricalcium phosphate | β-TCP | 43.8 | NR | 6 |
| 12 | Autogenous | AB | 45.7 | NR | 6 | ||
| 9 | Beta tricalcium phosphate + Autogenous (1:1) | β-TCP + AB | 38.3 | NR | 6 | ||
| Sehn F. [36] | 2015 | 17 | Allogenic | AlB | 28.3 | 11.3 | 6 |
| Xavier S. [55] | 2016 | 15 | Allogenic | AlB | 31.2 | 19.9 | 6 |
| 15 | Inorganic bovine bone | IBB | 12.2 | 2.3 | 6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Băbțan, A.-M.; Feurdean, C.N.; Ionel, A.; Uriciuc, W.A.; Chifor, R.; Jaques, C.A.B.; Boșca, B.A.; Ilea, A. Insights into Sinus-Lift Bone Grafting Materials: What’s Changed? J. Funct. Biomater. 2025, 16, 133. https://doi.org/10.3390/jfb16040133
Băbțan A-M, Feurdean CN, Ionel A, Uriciuc WA, Chifor R, Jaques CAB, Boșca BA, Ilea A. Insights into Sinus-Lift Bone Grafting Materials: What’s Changed? Journal of Functional Biomaterials. 2025; 16(4):133. https://doi.org/10.3390/jfb16040133
Chicago/Turabian StyleBăbțan, Anida-Maria, Claudia N. Feurdean, Anca Ionel, Willi A. Uriciuc, Radu Chifor, Chambon Antoine Bernard Jaques, Bianca A. Boșca, and Aranka Ilea. 2025. "Insights into Sinus-Lift Bone Grafting Materials: What’s Changed?" Journal of Functional Biomaterials 16, no. 4: 133. https://doi.org/10.3390/jfb16040133
APA StyleBăbțan, A.-M., Feurdean, C. N., Ionel, A., Uriciuc, W. A., Chifor, R., Jaques, C. A. B., Boșca, B. A., & Ilea, A. (2025). Insights into Sinus-Lift Bone Grafting Materials: What’s Changed? Journal of Functional Biomaterials, 16(4), 133. https://doi.org/10.3390/jfb16040133










