Anti-Aging Effects and Mechanisms of Cod Collagen Peptides (CCPs) in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
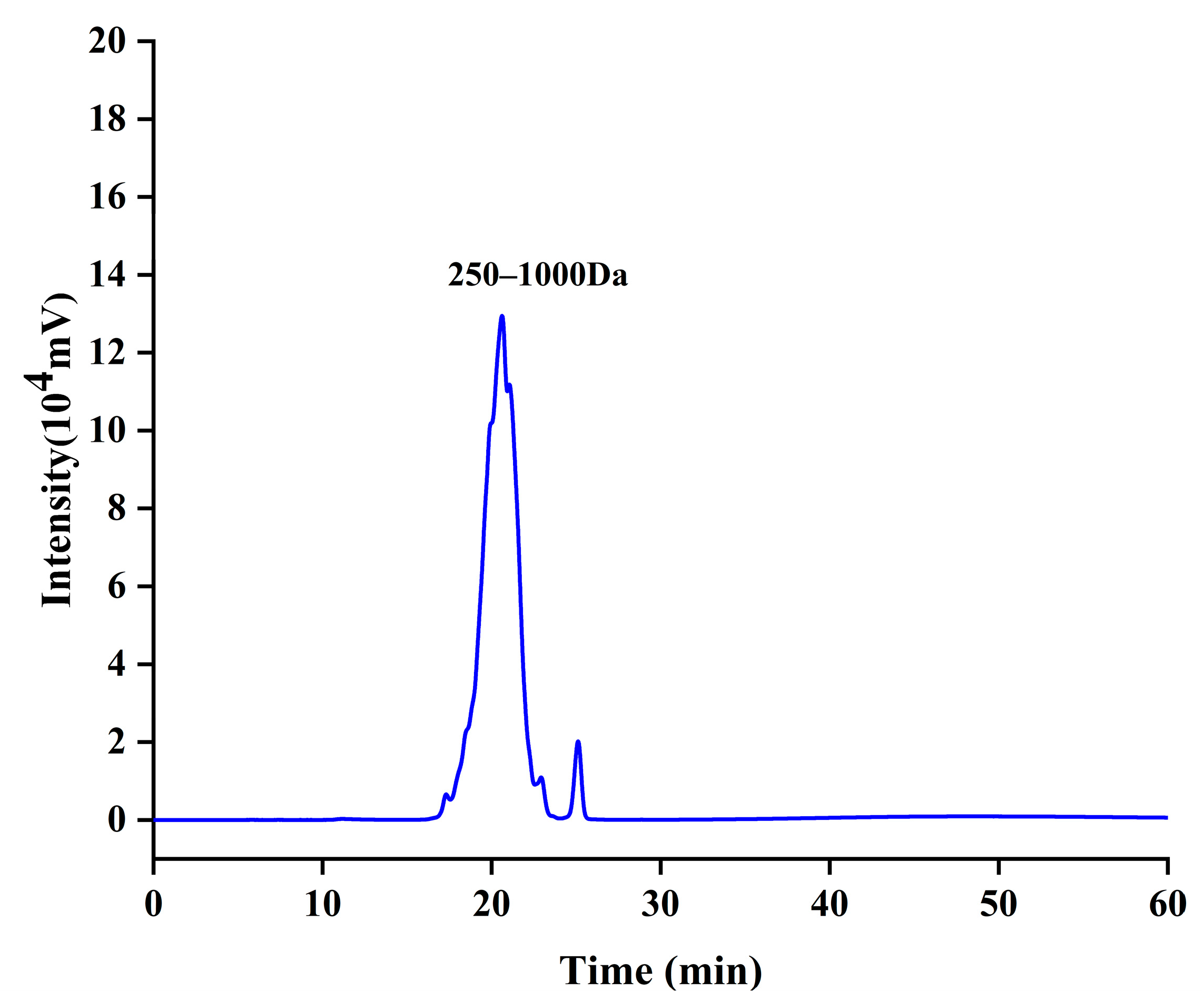
2.2. Molecular Weight Distribution of CCPs
2.3. C. elegans Culture and Synchronization
2.4. C. elegans Drug Delivery
2.5. Lifespan and Body Measurements
2.6. Motor Behavior Measurement
2.7. Determination of Resistance to Adversity
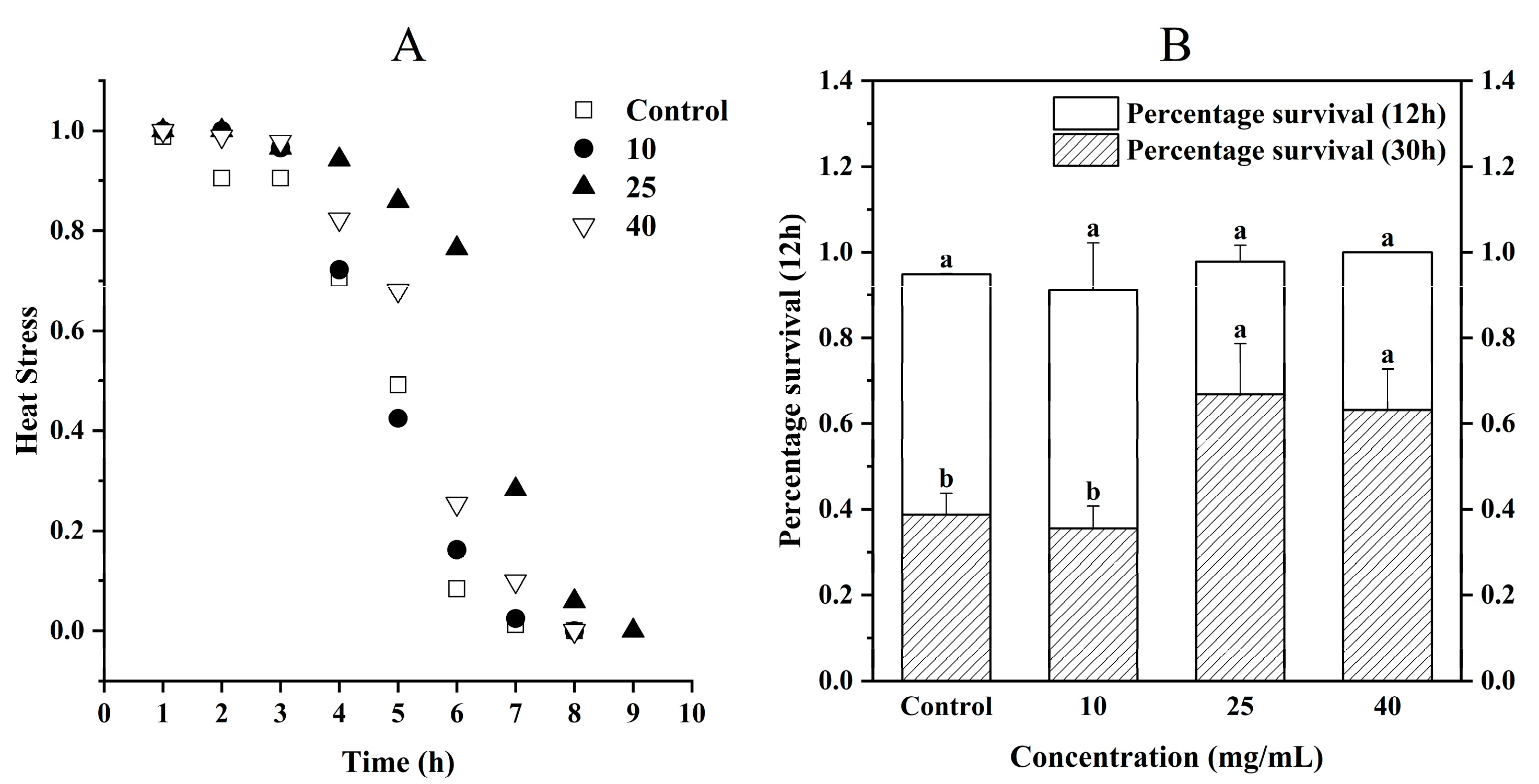
2.7.1. Heat Stress
2.7.2. Hyperosmolar Stress
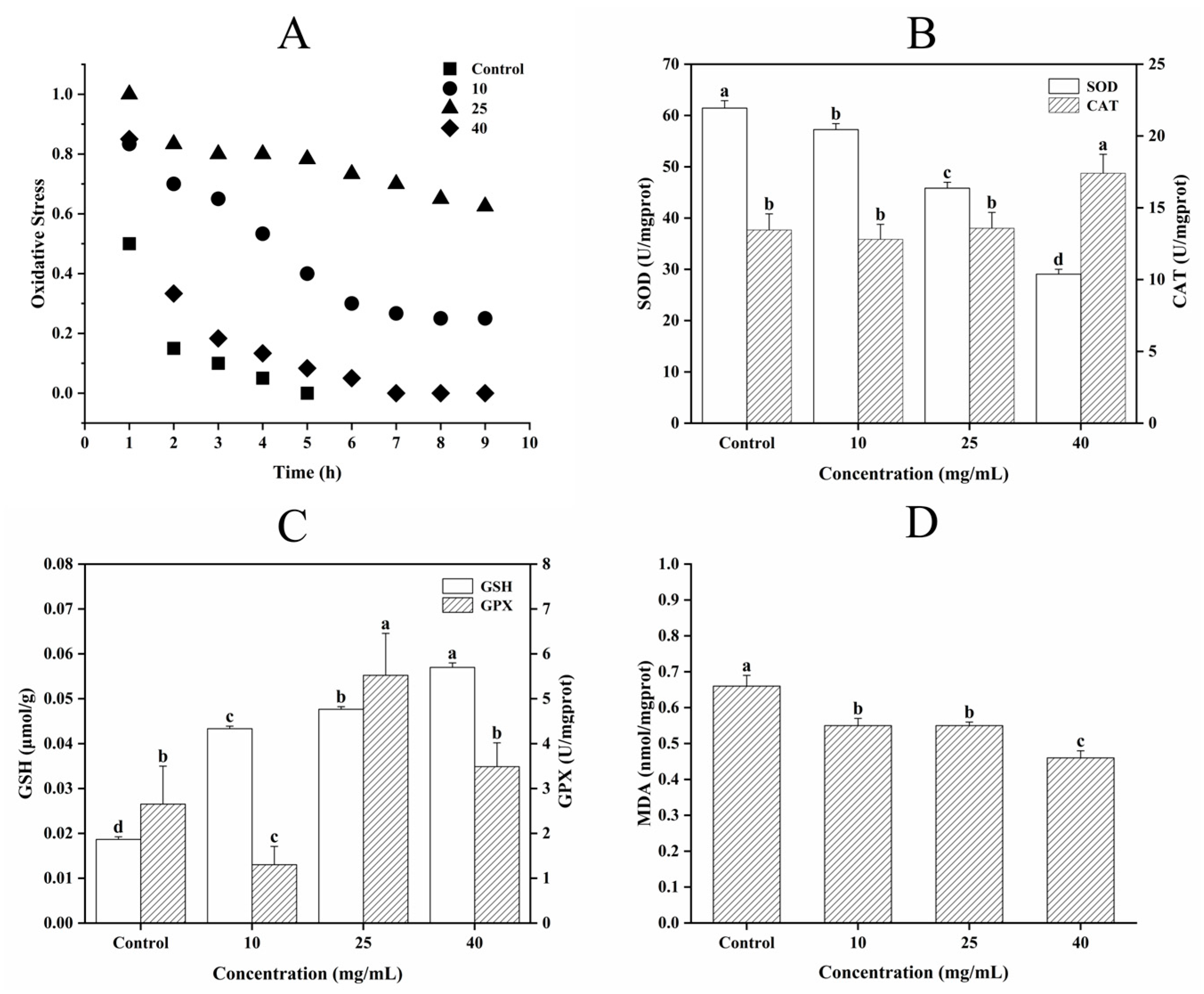
2.7.3. Determination of Oxidative Stress, Antioxidant Enzyme Activity, and MDA Content
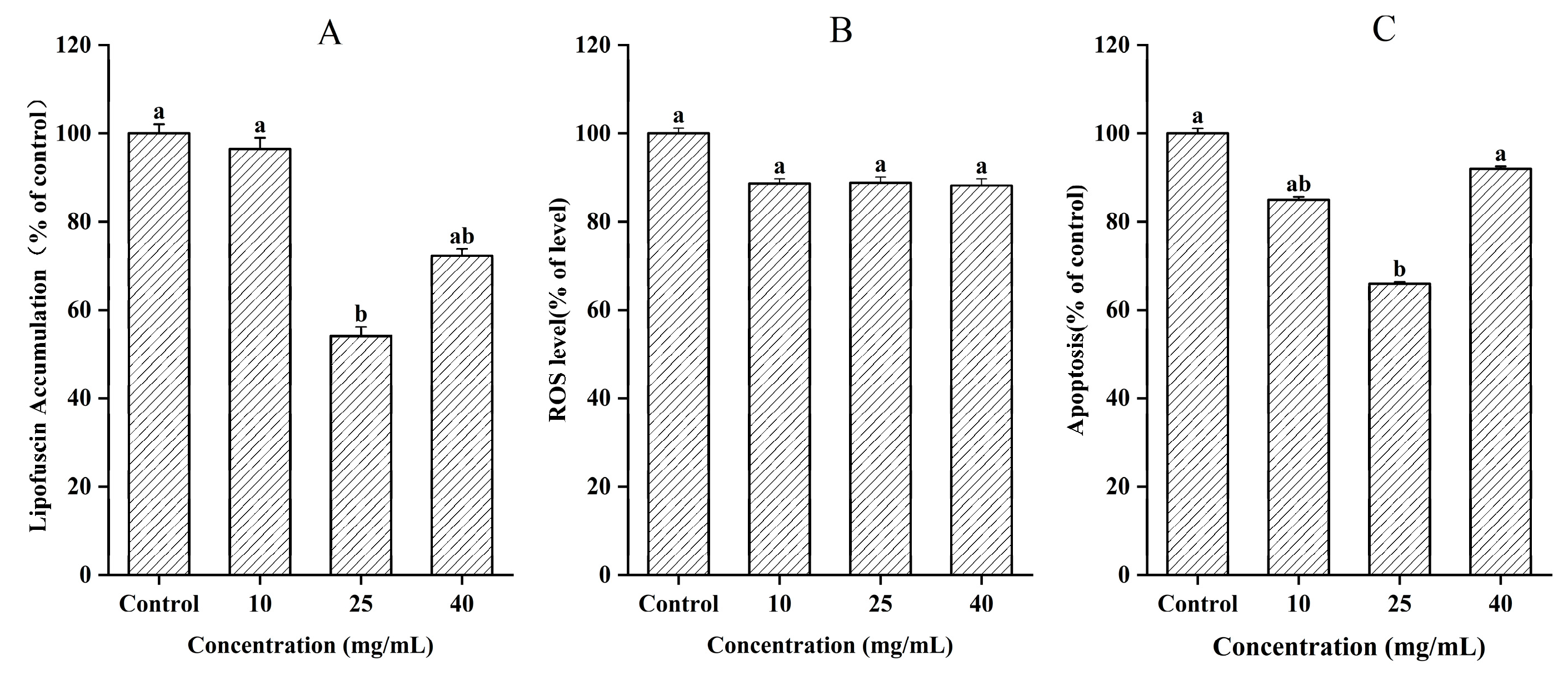
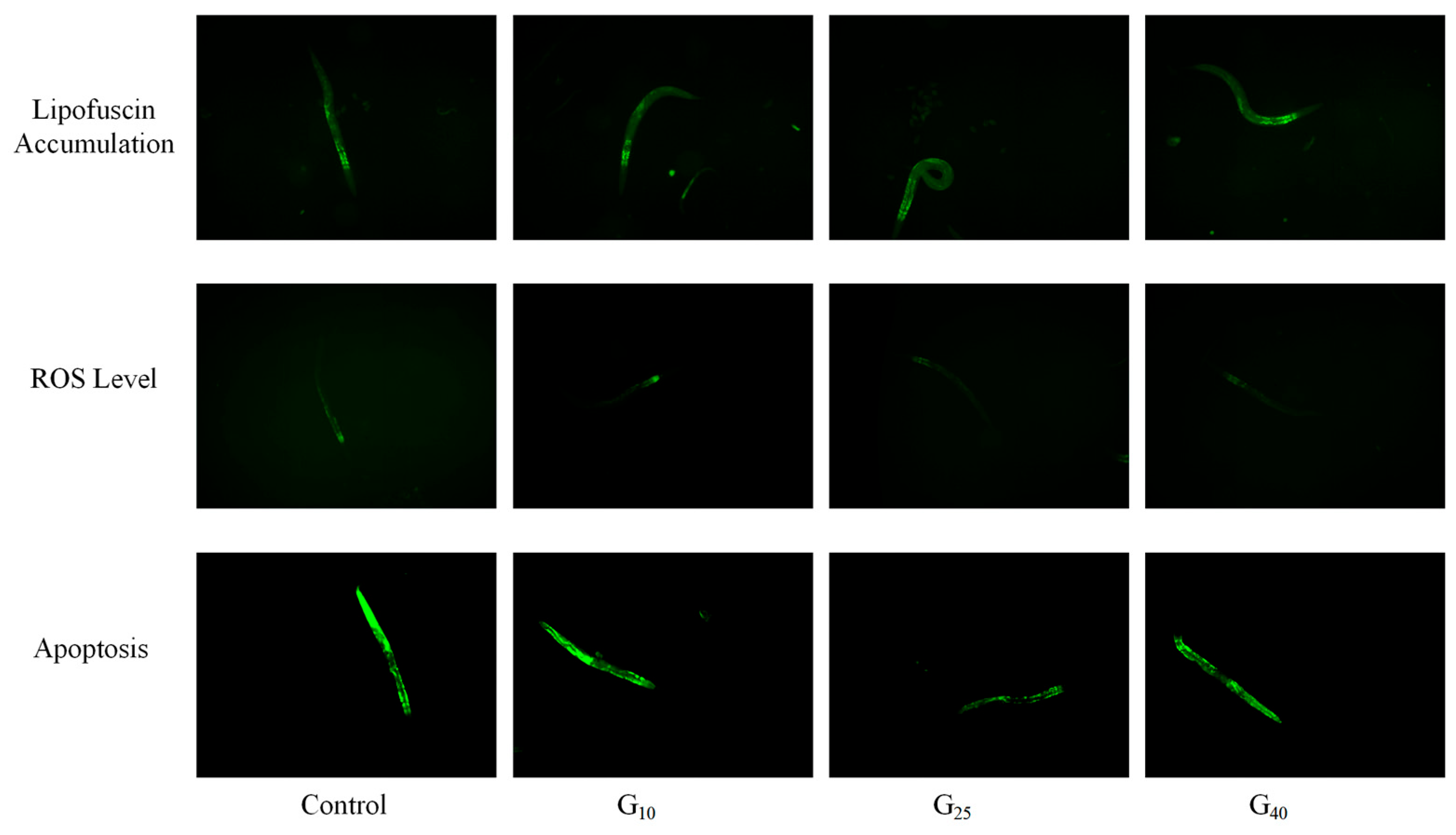
2.8. Measurement of Lipofuscin Accumulation, ROS, and Apoptosis
2.9. Extraction and qRT-PCR of Total RNA from C. elegans
2.10. Data Analysis
3. Results and Discussion
3.1. Molecular Characterization of CCPs
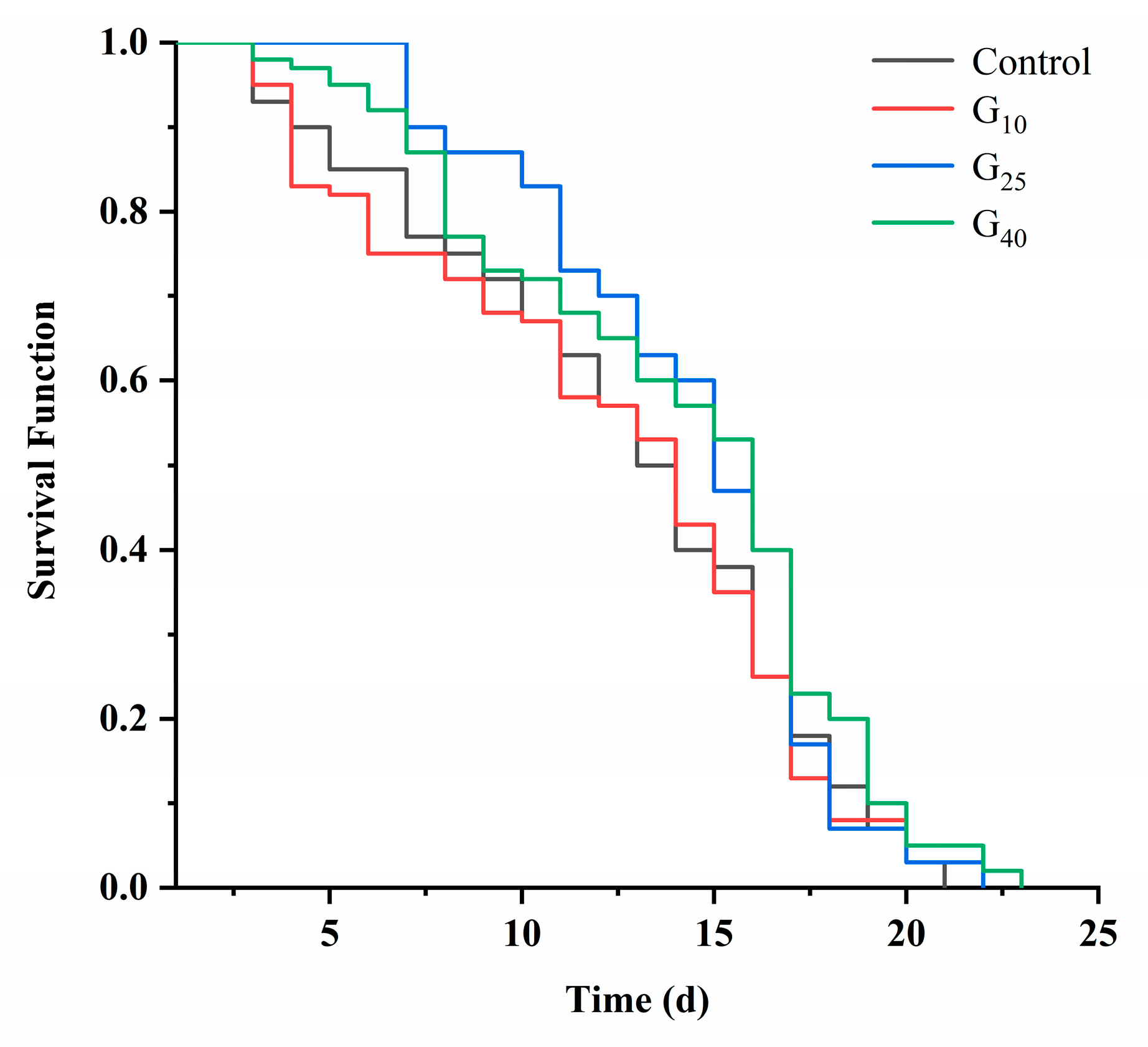
3.2. Lifespan
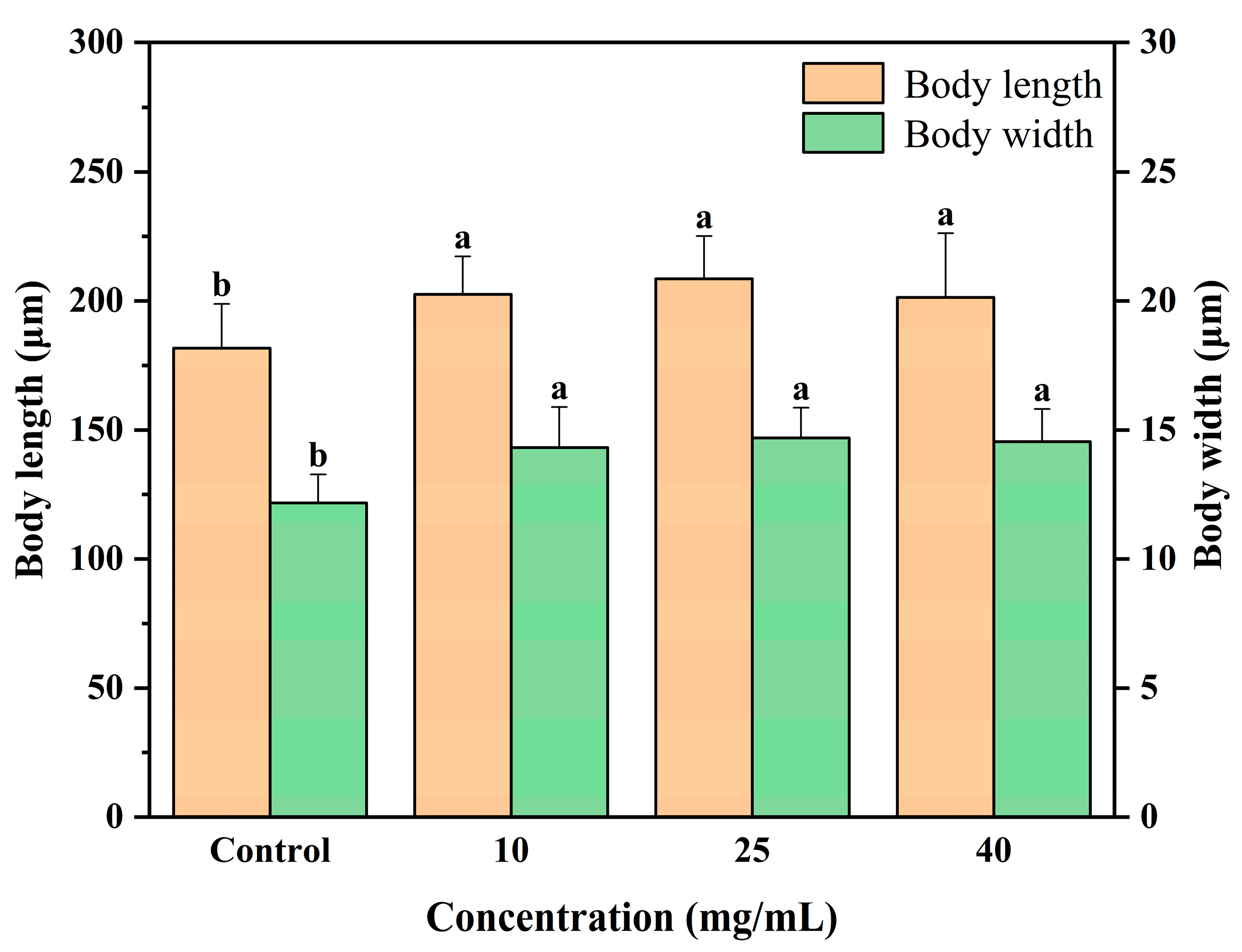
3.3. Body Measurements
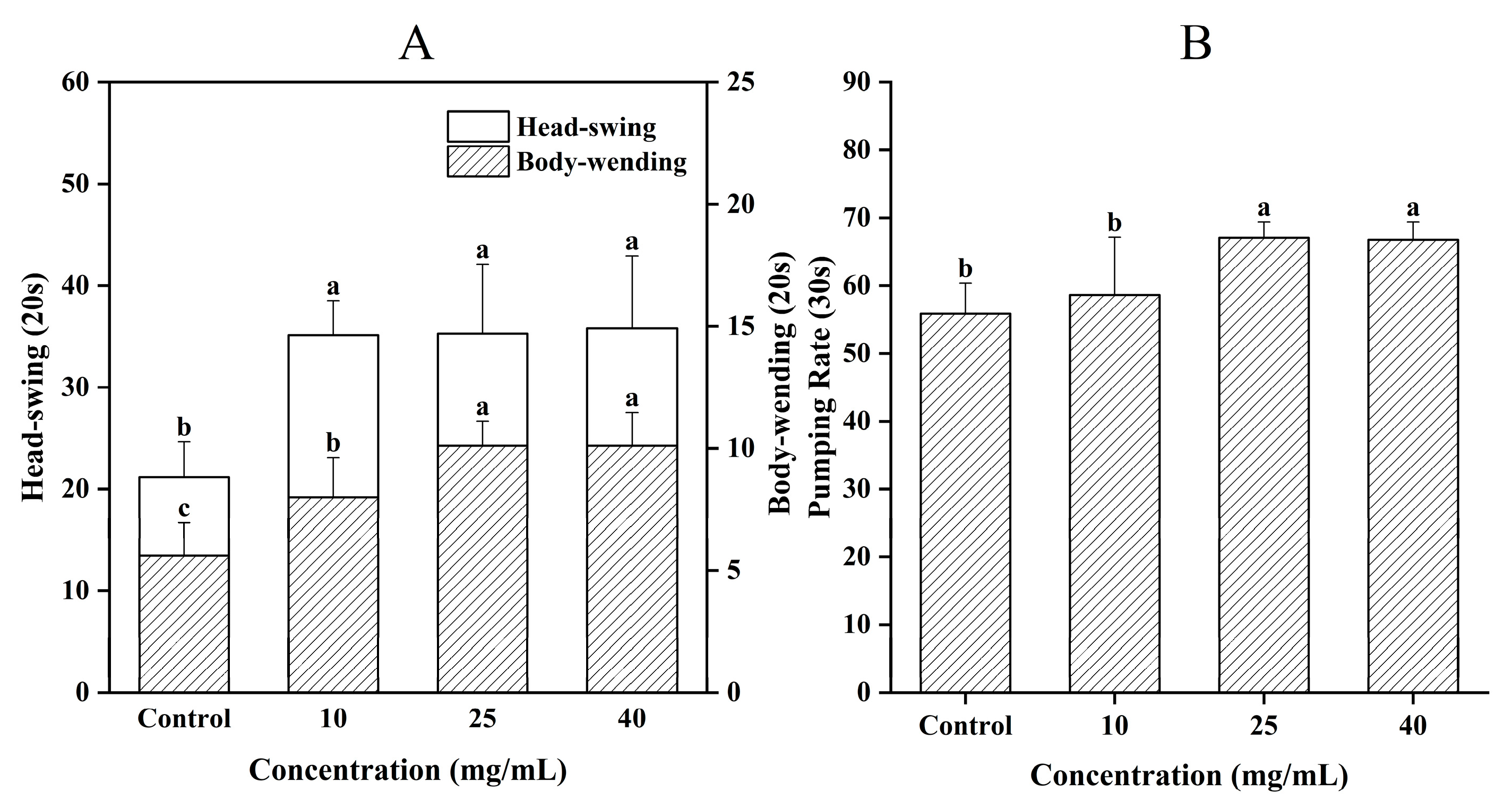
3.4. Effect of CCPs on C. elegans Motility
3.5. Effect of CCPs on Resistance of C. elegans to Adversity
3.5.1. Heat Stress
3.5.2. Hyperosmolar Stress
3.5.3. Determination of Oxidative Stress, Antioxidant Enzyme Activity, and MDA Content
3.6. Measurement of Lipofuscin Accumulation, ROS, and Apoptosis
3.7. Effect of CCPs on Gene Expression of C. elegans
3.7.1. MAPK Pathway
3.7.2. Insulin Signaling Pathway and Protein Aggregation
3.7.3. Lipids Metabolism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCPs | cod collagen peptides |
| C. elegans | Caenorhabditis elegans |
| Escherichia coli OP50 | E. coli OP50 |
| Cat | catalase |
| GPX | glutathione peroxidase |
| GSH | glutathione |
| MDA | malondialdehyde |
| MAPK | mitogen-activated protein kinase |
| IIS | insulin/IGF-1 signaling |
References
- Martemucci, G.; Portincasa, P.; Di Ciaula, A.; Mariano, M.; Centonze, V.; D’Alessandro, A.G. Oxidative stress, aging, antioxidant supplementation and their impact on human health: An overview. Mech. Ageing Dev. 2022, 206, 111707. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, R.C.G.; Peralta, R.M.; Haminiuk, C.W.I.; Maciel, G.M.; Bracht, A.; Ferreira, I. New phytochemicals as potential human anti-aging compounds: Reality, promise, and challenges. Crit. Rev. Food Sci. Nutr. 2018, 58, 942–957. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W.; Crystal, H.A.; Mattiace, L.A.; Masur, D.M.; Blau, A.D.; Davies, P.; Yen, S.H.; Aronson, M.K. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol. Aging 1992, 13, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, X.; Dong, Y.; Xie, W.; Jin, T.; Xu, D.; Liu, L. Cod (Gadus) skin collagen peptide powder reduces inflammation, restores mucosal barrier function, and inhibits fibrosis in dextran sodium sulfate-induced colitis in mice. J. Ethnopharmacol. 2023, 316, 116728. [Google Scholar] [CrossRef]
- Silva, I.; Vaz, B.M.C.; Sousa, S.; Pintado, M.M.; Coscueta, E.R.; Ventura, S.P.M. Gastrointestinal delivery of codfish Skin-Derived collagen Hydrolysates: Deep eutectic solvent extraction and bioactivity analysis. Food Res. Int. 2024, 175, 113729. [Google Scholar] [CrossRef]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef]
- Siahaan, E.A.; Agusman; Pangestuti, R.; Shin, K.H.; Kim, S.K. Potential Cosmetic Active Ingredients Derived from Marine By-Products. Mar. Drugs 2022, 20, 734. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Kim, S.K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, O.; Martins, I.M.; Hou, H.; Zhao, X.; Blumberg, J.B.; Li, B. Collagen peptides ameliorate intestinal epithelial barrier dysfunction in immunostimulatory Caco-2 cell monolayers via enhancing tight junctions. Food Funct. 2017, 8, 1144–1151. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef]
- Wu, L.; Hu, X.; Xu, L.; Zhang, G. Cod Skin Oligopeptide Inhibits Human Gastric Carcinoma Cell Growth by Inducing Apoptosis. Nutr. Cancer 2020, 72, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Wang, Z.; Hou, H.; Zhang, Z.; Li, B. Protective Effect of Cod (Gadus macrocephalus) Skin Collagen Peptides on Acetic Acid-Induced Gastric Ulcer in Rats. J. Food Sci. 2016, 81, H1807–H1815. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xie, J.; Gao, H.; Xia, Y.; Chen, X.; Wang, C. Hepatoprotective effect of collagen peptides from cod skin against liver oxidative damage in vitro and in vivo. Cell Biochem. Biophys. 2015, 71, 1089–1095. [Google Scholar] [CrossRef]
- Xin, X.; Jie, Y.; Gaoge, L.; Wang, Y. Effects of Collagen Peptides from Cod (Gadus) Skin on Delaying H2O2-Induced Senescence of 2BS Cells. J. Aquat. Food Prod. Technol. 2024, 33, 291–302. [Google Scholar] [CrossRef]
- Herndon, L.A.; Wolkow, C.A.; Driscoll, M.; Hall, D.H. Effects of Ageing on the Basic Biology and Anatomy of C. elegans. In Ageing: Lessons from C. elegans; Olsen, A., Gill, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 9–39. [Google Scholar]
- Li, Y.; Wang, Y.; Li, P.; Zhou, Q.; Zheng, X.; Gu, Q. Caenorhabditis elegans: A nature present for advanced food science. Curr. Opin. Food Sci. 2023, 49, 100971. [Google Scholar] [CrossRef]
- Zheng, P.; Hao, G.; Weng, W.; Ren, H. Antioxidant Activities of Hydrolysates from Abalone Viscera Using Subcritical Water-Assisted Enzymatic Hydrolysis. Food Bioprocess Technol. 2019, 12, 910–918. [Google Scholar] [CrossRef]
- Farias-Pereira, R.; Oshiro, J.; Kim, K.-H.; Park, Y. Green coffee bean extract and 5-O-caffeoylquinic acid regulate fat metabolism in Caenorhabditis elegans. J. Funct. Foods 2018, 48, 586–593. [Google Scholar] [CrossRef]
- Chen, K.; Shi, L.; Ren, Z.; Weng, W. Antioxidant characteristics of hydrolysate from low-value sea cucumber: In vitro and in vivo activities of Caenorhabditis elegans. Food Chem. X 2023, 19, 100836. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Ye, D.; Wei, C.; Pang, X.; Kong, C.; Fang, Y.; Yang, H.; Zhang, Y.; Liu, Y. Exploring the anti-aging effects of chlorogenic acid and the underlying mechanisms based on a Caenorhabditis elegans model. J. Tradit. Chin. Med. Sci. 2023, 10, 208–217. [Google Scholar] [CrossRef]
- Zhang, N.; Jiao, S.; Jing, P. Red Cabbage Rather Than Green Cabbage Increases Stress Resistance and Extends the Lifespan of Caenorhabditis elegans. Antioxidants 2021, 10, 930. [Google Scholar] [CrossRef]
- Solomon, A.; Bandhakavi, S.; Jabbar, S.; Shah, R.; Beitel, G.J.; Morimoto, R.I. Caenorhabditis elegans OSR-1 regulates behavioral and physiological responses to hyperosmotic environments. Genetics 2004, 167, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Su, Z.; Luo, J.; Jiang, L.; Shen, S.; Zheng, W.; Gu, W.; Cao, Y.; Chen, Y. Polysaccharide extracted from the leaves of Cyclocarya paliurus (Batal.) Iljinskaja enhanced stress resistance in Caenorhabditis elegans via skn-1 and hsf-1. Int. J. Biol. Macromol. 2020, 143, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, P.; Zheng, F.; Zhu, Z.; Bai, F.; Gao, R. Collagen peptides from sturgeon swim bladder prolong the lifespan and healthspan in Caenorhabditis elegans. J. Sci. Food Agric. 2024, 104, 5244–5251. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, P.; Ren, Y.; Bai, F.; Wang, J.; Zhang, Y.; Jin, W.; El-Seedi, H.; Gao, R. A novel extraction approach and unique physicochemical properties of gelatin from the swim bladder of sturgeon. J. Sci. Food Agric. 2021, 101, 2912–2919. [Google Scholar] [CrossRef]
- Antebi, A. Genetics of aging in Caenorhabditis elegans. PLoS Genet. 2007, 3, 1565–1571. [Google Scholar] [CrossRef]
- Sabeena Farvin, K.H.; Andersen, L.L.; Otte, J.; Nielsen, H.H.; Jessen, F.; Jacobsen, C. Antioxidant activity of cod (Gadus morhua) protein hydrolysates: Fractionation and characterisation of peptide fractions. Food Chem. 2016, 204, 409–419. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, H.Q.; Chu, Z.W.; Zuo, X.; Wang, L.; Ren, X.L.; Ma, H.; Du, R.Y.; Ju, J.J.; Ye, X.L.; et al. Arsenic induces transgenerational behavior disorders in Caenorhabditis elegans and its underlying mechanisms. Chemosphere 2020, 252, 126510. [Google Scholar] [CrossRef]
- Yue, Y.; Liang, L.; Zhang, H.; Li, C.; Zhao, M.; Yang, M.; Cao, X.; Zhong, L.; Du, J.; Shi, R.; et al. Antioxidant and anti-aging effects of purified Rehmannia glutinosa polysaccharide in Caenorhabditis elegans. Process Biochem. 2024, 137, 41–53. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S.; Zhou, A.; Miao, J.; Liu, J.; Benjakul, S. A novel antioxidant peptide purified from defatted round scad (Decapterus maruadsi) protein hydrolysate extends lifespan in Caenorhabditis elegans. J. Funct. Foods 2020, 68, 103907. [Google Scholar] [CrossRef]
- Spadaro, O.; Youm, Y.; Shchukina, I.; Ryu, S.; Sidorov, S.; Ravussin, A.; Nguyen, K.; Aladyeva, E.; Predeus, A.N.; Smith, S.R.; et al. Caloric restriction in humans reveals immunometabolic regulators of health span. Science 2022, 375, 671–677. [Google Scholar] [CrossRef]
- Sun, J.; Zhong, X.; Sun, D.; Xu, L.; Shi, L.; Sui, J.; Liu, Y. Anti-aging effects of polysaccharides from ginseng extract residues in Caenorhabditis elegans. Int. J. Biol. Macromol. 2023, 225, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Zhu, D.; Gao, X.; Zhao, L.; Yang, H.; Wang, Q.; Zhao, Y.; Wang, N. Antiaging and antioxidative effects of water extract of Zizyphus jujuba Mill on Caenorhabditis elegans. J. Funct. Foods 2023, 110, 105829. [Google Scholar] [CrossRef]
- Rubio-Tomás, T.; Alegre-Cortés, E.; Lionaki, E.; Fuentes, J.M.; Tavernarakis, N. Heat shock and thermotolerance in Caenorhabditis elegans: An overview of laboratory techniques. Methods Cell Biol. 2024, 185, 1–17. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, J.; Xu, C.; Li, Q.; Ma, Y.; Zhao, S.; Zhuang, J.; Shen, F.; Wang, Q.; Feng, F. Sea cucumber (Acaudina leucoprocta) peptides extended the lifespan and enhanced antioxidant capacity via DAF-16/DAF-2/SOD-3/OLD-1/PEPT-1 in Caenorhabditis elegans. Front. Nutr. 2022, 9, 1065145. [Google Scholar] [CrossRef]
- Gami, M.S.; Wolkow, C.A. Studies of Caenorhabditis elegans DAF-2/insulin signaling reveal targets for pharmacological manipulation of lifespan. Aging Cell 2006, 5, 31–37. [Google Scholar] [CrossRef]
- Rea, S.L.; Wu, D.; Cypser, J.R.; Vaupel, J.W.; Johnson, T.E. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat. Genet. 2005, 37, 894–898. [Google Scholar] [CrossRef]
- Urso, S.J.; Lamitina, T. The C. elegans Hypertonic Stress Response: Big Insights from Shrinking Worms. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2021, 55, 89–105. [Google Scholar] [CrossRef]
- Burkewitz, K.; Choe, K.P.; Lee, E.C.; Deonarine, A.; Strange, K. Characterization of the proteostasis roles of glycerol accumulation, protein degradation and protein synthesis during osmotic stress in C. elegans. PLoS ONE 2012, 7, e34153. [Google Scholar] [CrossRef]
- Dodd, W.; Tang, L.; Lone, J.C.; Wimberly, K.; Wu, C.W.; Consalvo, C.; Wright, J.E.; Pujol, N.; Choe, K.P. A Damage Sensor Associated with the Cuticle Coordinates Three Core Environmental Stress Responses in Caenorhabditis elegans. Genetics 2018, 208, 1467–1482. [Google Scholar] [CrossRef]
- Enhui, Z.; Na, C.; MengYun, L.; Jia, L.; Dan, L.; Yongsheng, Y.; Ying, Z.; DeFu, H. Isomers and their metabolites of endosulfan induced cytotoxicity and oxidative damage in SH-SY5Y cells. Environ. Toxicol. 2016, 31, 496–504. [Google Scholar] [CrossRef]
- Kamaladevi, A.; Ganguli, A.; Kumar, M.; Balamurugan, K. Lactobacillus casei protects malathion induced oxidative stress and macromolecular changes in Caenorhabditis elegans. Pestic. Biochem. Physiol. 2013, 105, 213–223. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, X.; Ge, Q.; Li, J.; Wang, D.; Wei, Y.; Ouyang, Z. Antioxidant and anti-aging activities of polysaccharides from Cordyceps cicadae. Int. J. Biol. Macromol. 2020, 157, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Deng, N.; Zheng, B.; Li, T.; Liu, R.H. HSF-1 and SIR-2.1 linked insulin-like signaling is involved in goji berry (Lycium spp.) extracts promoting lifespan extension of Caenorhabditis elegans. Food Funct. 2021, 12, 7851–7866. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.V.; Oury, T.D.; Ostergaard, L.; Valnickova, Z.; Wegrzyn, J.; Thøgersen, I.B.; Jacobsen, C.; Bowler, R.P.; Fattman, C.L.; Crapo, J.D.; et al. Extracellular superoxide dismutase (EC-SOD) binds to type I collagen and protects against oxidative fragmentation. J. Biol. Chem. 2004, 279, 13705–13710. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [CrossRef]
- Hou, H.; Li, B.; Zhao, X.; Zhuang, Y.; Ren, G.; Yan, M.; Cai, Y.; Zhang, X.; Chen, L. The effect of pacific cod (Gadus macrocephalus) skin gelatin polypeptides on UV radiation-induced skin photoaging in ICR mice. Food Chem. 2009, 115, 945–950. [Google Scholar] [CrossRef]
- Burns, A.R.; Kwok, T.C.; Howard, A.; Houston, E.; Johanson, K.; Chan, A.; Cutler, S.R.; McCourt, P.; Roy, P.J. High-throughput screening of small molecules for bioactivity and target identification in Caenorhabditis elegans. Nat. Protoc. 2006, 1, 1906–1914. [Google Scholar] [CrossRef]
- Cannizzo, E.S.; Clement, C.C.; Sahu, R.; Follo, C.; Santambrogio, L. Oxidative stress, inflamm-aging and immunosenescence. J. Proteom. 2011, 74, 2313–2323. [Google Scholar] [CrossRef]
- Nath, M.; Vats, M.; Roy, P. Design, spectral characterization, anti-tumor and anti-inflammatory activity of triorganotin(IV) hydroxycarboxylates, apoptosis inducers: In vitro assessment of induction of apoptosis by enzyme, DNA-fragmentation, acridine orange and comet assays. Inorganica Chim. Acta 2014, 423, 70–82. [Google Scholar] [CrossRef]
- Yang, C.; Yan, N.; Parish, J.; Wang, X.; Shi, Y.; Xue, D. RNA aptamers targeting the cell death inhibitor CED-9 induce cell killing in Caenorhabditis elegans. J. Biol. Chem. 2006, 281, 9137–9144. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Berdichevsky, A.; Viswanathan, M.; Horvitz, H.R.; Guarente, L. C. elegans SIR-2.1 interacts with 14-3-3 proteins to activate DAF-16 and extend life span. Cell 2006, 125, 1165–1177. [Google Scholar] [CrossRef]
- Tatar, M.; Bartke, A.; Antebi, A. The endocrine regulation of aging by insulin-like signals. Science 2003, 299, 1346–1351. [Google Scholar] [CrossRef]
- Hsu, A.-L.; Murphy, C.T.; Kenyon, C. Regulation of Aging and Age-Related Disease by DAF-16 and Heat-Shock Factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–283. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Tang, Y.; Lin, C.; Cao, Y.; Chen, Y. Mechanism of Pentagalloyl Glucose in Alleviating Fat Accumulation in Caenorhabditis elegans. J. Agric. Food Chem. 2019, 67, 14110–14120. [Google Scholar] [CrossRef]
- Srinivasan, S. Regulation of body fat in Caenorhabditis elegans. Annu. Rev. Physiol. 2015, 77, 161–178. [Google Scholar] [CrossRef]
- Imanikia, S.; Sheng, M.; Castro, C.; Griffin, J.L.; Taylor, R.C. XBP-1 Remodels Lipid Metabolism to Extend Longevity. Cell Rep. 2019, 28, 581–589.e4. [Google Scholar] [CrossRef]

| Gene Name | Primer Sequences |
|---|---|
| actin | TGGAAGAAGAAATCGCCGC CTGTCCCATACCGACCATCACT |
| JNK-1 | CAGGCATTTACATAGTGCTGGAAT TATCTTGTCACAACGTAAGGAGTCA |
| SEK-1 | GTCACTTGACAAACAGCATGGC TATACCCAAACTCCATACATCAGCT |
| SOD3 | AACTTGGCTAAGGATGGTGGAG AAGTAGTAGGCGTGCTCCCAAAC |
| gst-4 | GATGCTCGTGCTCTTGCTGA GGAGTCGTTGGCTTCAGCTT |
| SKN-1 | TTCCAGTTATGCCAATACTCACC GATTTCTCTGTCAACGTCTGCAA |
| sir-2.1 | CGATTCAGAAGTTGCGGTCAC TTGCCAAGACTCTGAACTGACAC |
| ins-6 | CGACTGAATGCTGCGGAAAT TGATGAGACACGGGTGAAACG |
| ins-7 | ACCAGAAGAGTCCCTGACGA CAGCACTGTTTTCGAATGAAGTC |
| ins-8 | CATTCCGTTTTCTCAACAACACAC CACAAAGTGCCATAACGTAGGATAA |
| DAF-2 | CGTCAATCGTCACCGTTTATCTC GTTATTGGCAATTGACACAGTTCC |
| DAF-16 | GAAGAACTAGCCTATACGGGAGC ATCCGAAGGAAACGATGTCTG |
| AGE-1 | TGATTGCTGTTTGAACCCGTA GCATTGTTTCCGAATCCACTT |
| HSF1 | AATGGGCTCAATGCGTCAA AGATCAGTGGTCCTTCATTATTCGT |
| hsp-16.2 | ATGTCACTTTACCACTATTTCCGTC TTGTTCTCCTTGGATTGATAGCGTA |
| fasn-1 | CTCTATTAGACAGGGTCAATGCG GAGTCTTTGAGCCTTCTTCTTGC |
| fat-5 | GCCCTCTTCCGTTACTGCTT CTCCGACTGCCGCAATAGAT |
| fat-6 | GAGGATTTGGAATAACCGCC CGAGAACTGGGTCACTCAAGAGAT |
| fat-7 | CCATACGATACTTCTGTTTCCGC GCAGTATCAATAAGAACACGGGTC |
| ACS-2 | GACCCATCCGTCGGTTCAT CAGTAGTATTTCTCAGTGTCTGTCCC |
| ACS-22 | CCTCCAGTTATAGACGGACTCAATT CAAATGCTTTTCCTGCTCCC |
| Molecular Weight Range (Da) | Peak Area (1 × 105 mv·min) | Percentage of Peak Area (%) |
|---|---|---|
| >1000 | 1.96 | 1.026 |
| 250–1000 | 181.47 | 95.159 |
| <250 | 7.2 | 3.778 |
| Groups | Average Lifespan (Days) | Average Life Extension (%) |
|---|---|---|
| Control | 12.37 ± 0.27 b | 0 |
| G10 | 12.5 ± 0.50 b | 1.1 |
| G25 | 14 ± 0 a | 13.2 |
| G40 | 13.97 ± 0.01 a | 12.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Zhang, J.; Ding, N.; Liu, Y.; Wu, Y.; Duan, R. Anti-Aging Effects and Mechanisms of Cod Collagen Peptides (CCPs) in Caenorhabditis elegans. J. Funct. Biomater. 2025, 16, 150. https://doi.org/10.3390/jfb16050150
Wei J, Zhang J, Ding N, Liu Y, Wu Y, Duan R. Anti-Aging Effects and Mechanisms of Cod Collagen Peptides (CCPs) in Caenorhabditis elegans. Journal of Functional Biomaterials. 2025; 16(5):150. https://doi.org/10.3390/jfb16050150
Chicago/Turabian StyleWei, Jiale, Junjie Zhang, Nan Ding, Yu Liu, Yuzhen Wu, and Rui Duan. 2025. "Anti-Aging Effects and Mechanisms of Cod Collagen Peptides (CCPs) in Caenorhabditis elegans" Journal of Functional Biomaterials 16, no. 5: 150. https://doi.org/10.3390/jfb16050150
APA StyleWei, J., Zhang, J., Ding, N., Liu, Y., Wu, Y., & Duan, R. (2025). Anti-Aging Effects and Mechanisms of Cod Collagen Peptides (CCPs) in Caenorhabditis elegans. Journal of Functional Biomaterials, 16(5), 150. https://doi.org/10.3390/jfb16050150









