Recent Trends in the Application of Cellulose-Based Hemostatic and Wound Healing Dressings
Abstract
1. Introduction
1.1. Stages of the Hemostasis Process
1.2. Phases of Wound Healing
2. Cellulose-Based Composites as the Best Candidate for Hemostasis and Wound Healing
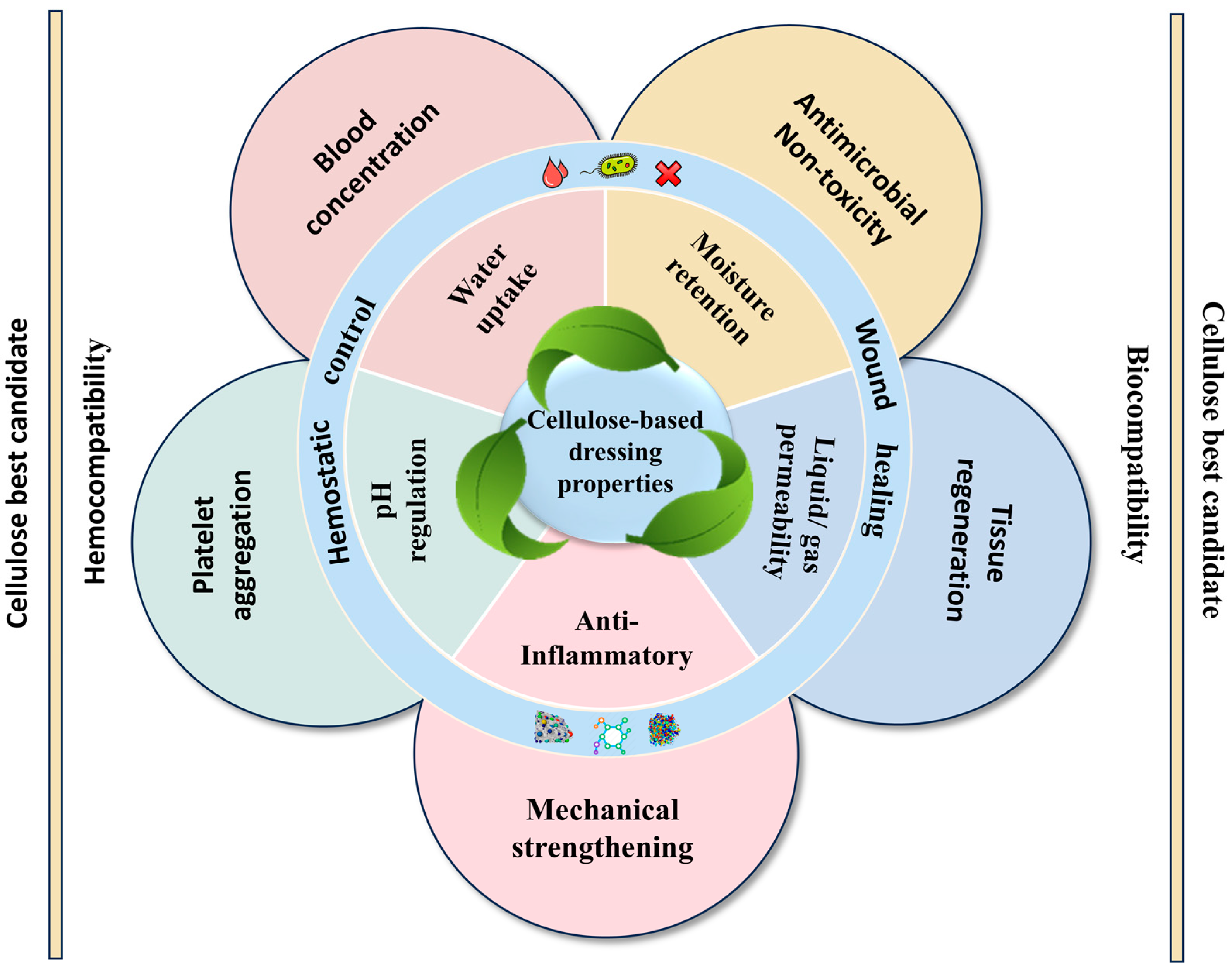
3. Mechanisms of Cellulose-Based Dressings for Hemostasis and Wound Healing
3.1. Hemostasis Mechanisms of Cellulose-Based Dressing Materials
3.1.1. External Mechanisms
3.1.2. Intrinsic Mechanisms
3.2. Wound Repair Mechanisms of Cellulose-Based Dressing Materials
3.2.1. Physical Mechanisms
3.2.2. Chemical Mechanisms
3.2.3. Biological Mechanisms
| Mechanism | Description | Ref. |
|---|---|---|
| Exterior mechanisms | Large surface area of fibers forms a dense matrix Absorbs fluid and exudate | [65,66] |
| Interior mechanisms | Physical barrier Potential activation of FXII Tigered intrinsic pathway | [67,70] |
| Physical mechanisms | Fluid absorbance, desiccation, crust formation, and necrotic tissue removal | [78,79] |
| Mechanical mechanisms | Release bioactive (growth factors, cytokines) Provide bactericide effect | [61,80,81] |
| Biological mechanisms | Interact at cellular level Modulate inflammation, migration, and proliferation of key cells (keratinocytes, fibroblasts, endothelial cells). | [77,82,83] |
4. Fabrication Design Strategies for Novel Cellulose-Based Hemostatic and Wound Dressing Materials
4.1. Fabrication Design Strategies for Hemostatic Cellulose Dressings
4.1.1. Material Selection and Chemical Modifications

4.1.2. Structural Engineering and Microstructure Optimization
4.1.3. Bioactive Coatings and Functional Enhancements
4.1.4. Mechanical Properties and Biocompatibility
4.2. Fabrication Design Strategies for Cellulose-Based Wound Dressings
4.2.1. Material Selection and Chemical Modification
4.2.2. Microstructure Engineering and Fabrication Techniques
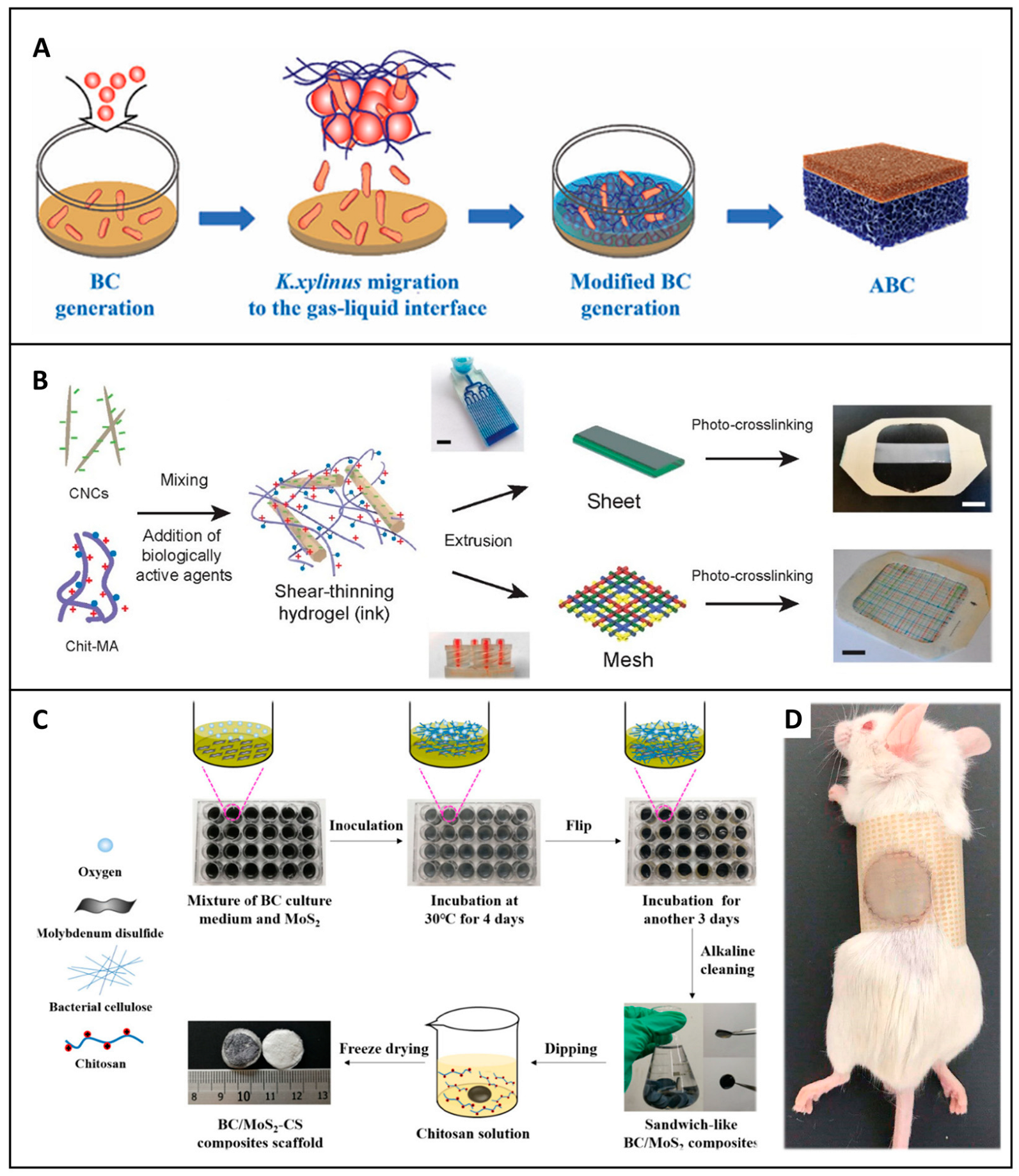
5. Application of Cellulose-Based Dressings for Hemostasis and Wound Healing
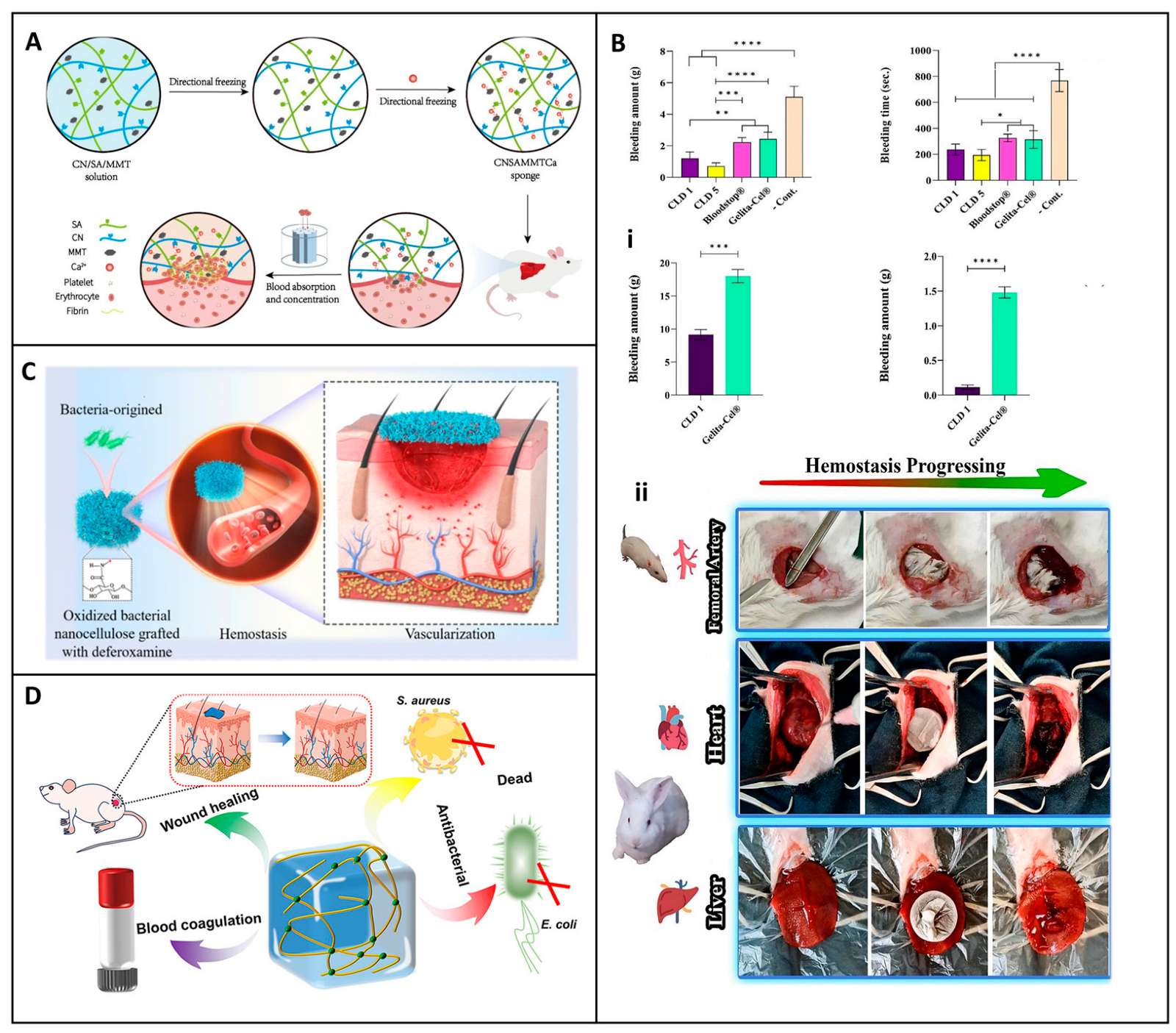
5.1. Hemostatic Applications
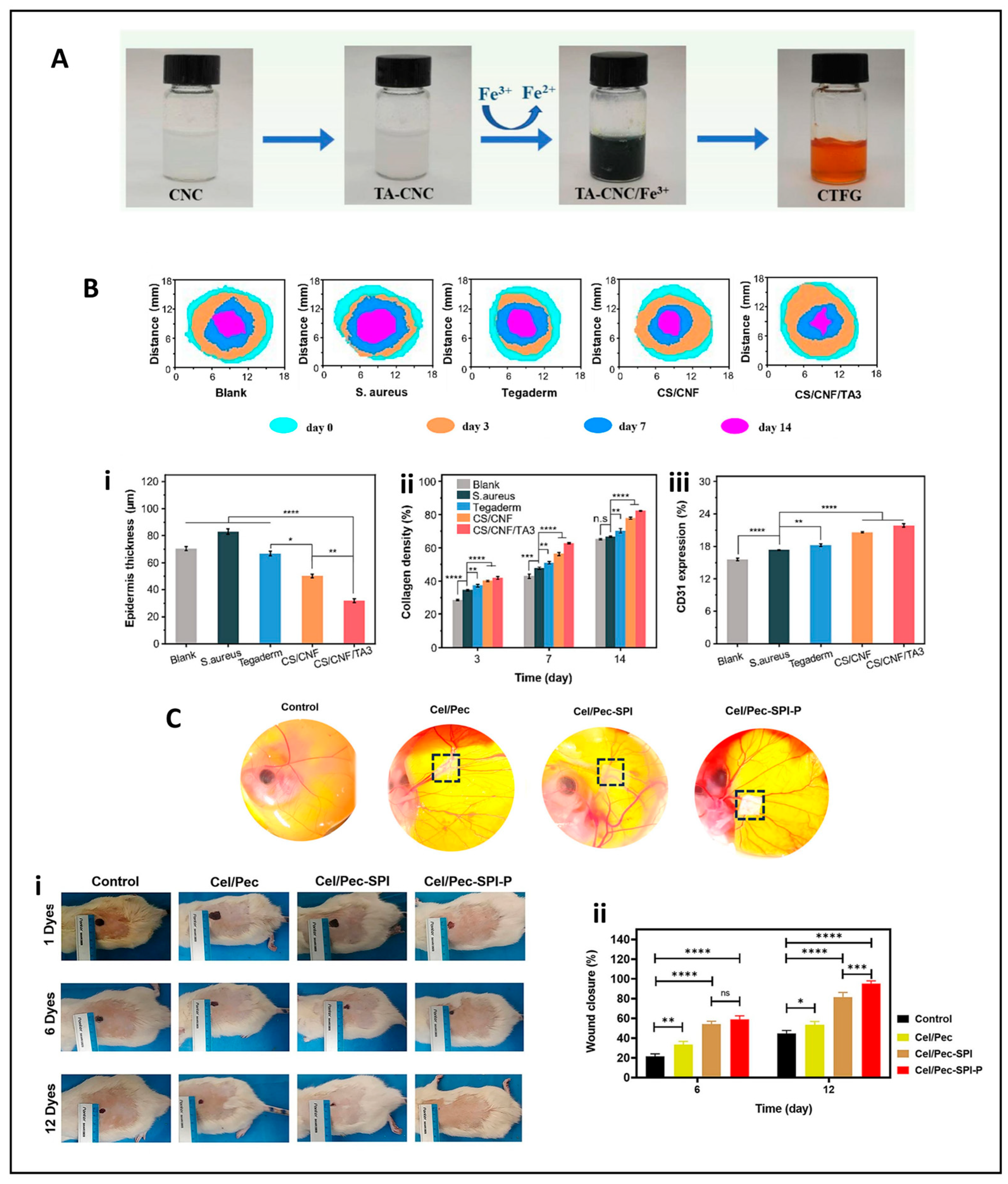
5.2. Wound Healing Applications
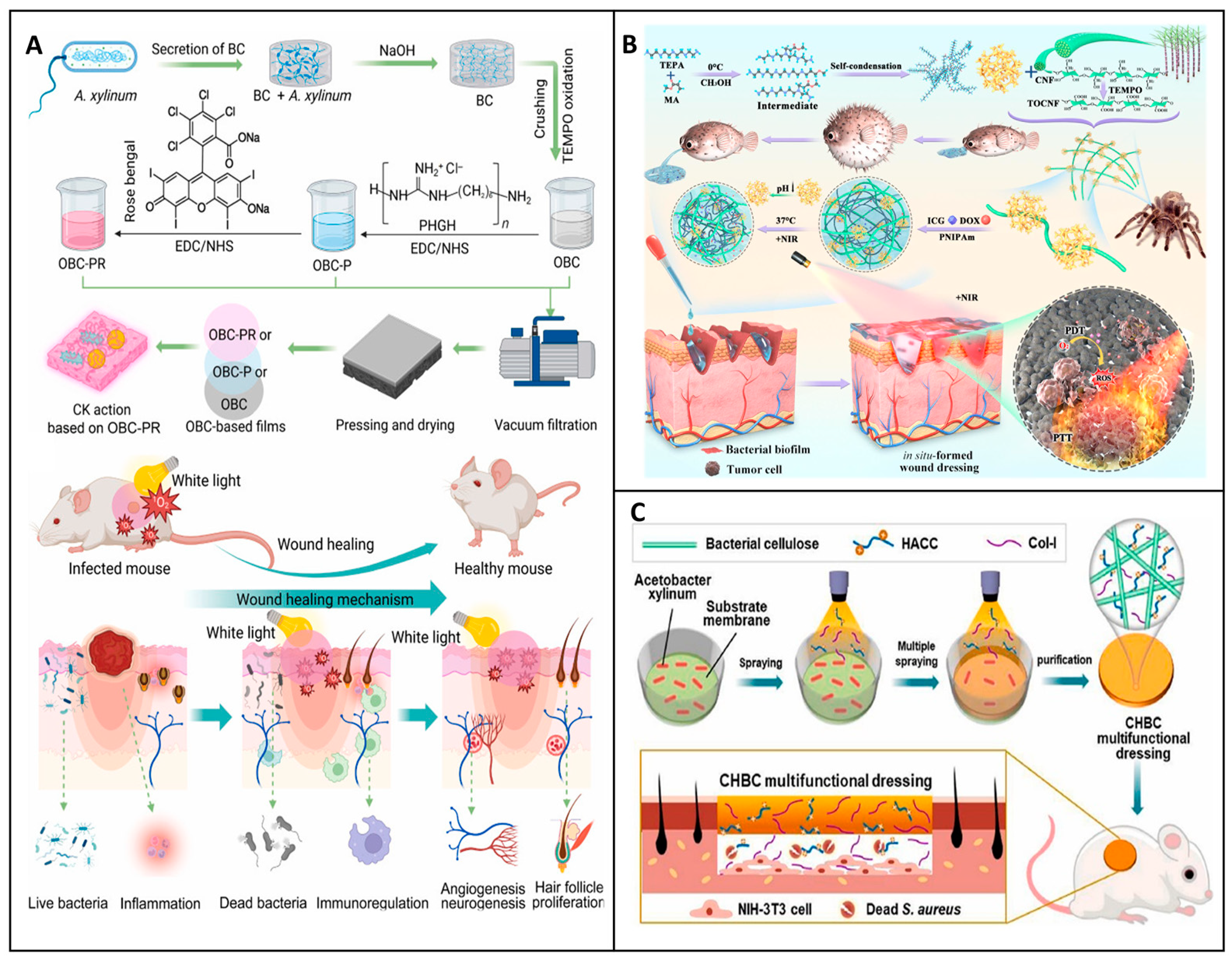
6. Clinical Efficacy of Commercial Cellulose-Based Dressings
6.1. Cellulose-Based Dressings for Hemostasis Available in the Market
6.2. Cellulose-Based Wound Dressings Available on the Market
| Formulation | Properties | Examples | Ref. |
|---|---|---|---|
| Oxidized Cellulose | High water absorption Clot promotion Antimicrobial | Surgicel® | [149] |
| Oxidized Regenerated Cellulose | High mechanical strength Low hemolysis Rapid clot formation | Traumastem®, Interceed® | [153] |
| Bacterial Cellulose | Moisture retention Biocompatible | Biofill® XCell® | [163] |
| Bacterial Cellulose with Active Ingredients | Moisturizing Sterile environment Skin hydration | Moisturizer BC mask | [164] |
| Regenerated Cotton Cellulose | Rapid clot initiation Optimal moist environment Gel-like subtract | Bloodstop® | [165] |
| Carboxymethyl Cellulose (CMC) based | High absorbency Manage high moderate Chronic wound | Fiber 3M™, MedVance® AquaRite Extra CMC™ Hcel® NaT | [25,156] |
| Sodium Carboxymethyl Cellulose Blend | Antimicrobial management of exudate High absorbency Promotes angiogenesis and autolytic debridement | Suprasorb X®, Granugel® Aquacel® | [157,166] |
6.3. Clinical Trials and Therapeutic Evidence
6.4. Comparison with Synthetic, Hydrocolloid, Hydrogel, Foam, Alginate, and Protein-Based Dressings
| Category | Composition | Mechanism of Action | Clinical Application | Benefit | Limitation | Commercial Product | Ref. |
|---|---|---|---|---|---|---|---|
| Synthetic based | PEG, PVA, PU Polyesters α-cyanoacrylates | Sealant formation Vessel occlusion Crosslinking | Trauma, surgical bleeding controlled drug delivery | Customizable Scalable Fast hemostasis | Brittle gels, inflammation risk, poor biodegradability | Dermabond®, Omnex®, Glubran®, Glubran2®, IFABond®, PVA-Chitosan pad | [176,177,178] |
| Hydrocolloid based | Sodium CMC, gelatin, pectin, sodium alginate | Gel formation, moisture retention, autolysis stimulation | Low–moderate, exudating wounds, ulcers, pressure injuries | Moist environment, self-adherent, bacterial barrier | Not for infected wounds, trauma during removal | DuoDERM®, Comfeel®, Tegasorb®, Granuflex®, Hydrocoll® | [184,185] |
| Hydrogel based | PEG-based hydrogels natural/synthetic blends | Hydration, cooling, autolytic debridement | Burns, dry wounds, necrotic wounds | High moisture, soothing, drug- loaded capacity | Poor absorbency, not ideal for heavy exudate | Clearsite®, Intrasite®, DermaSyn®, NuGel®, AquaClear®, SOLOSITE® | [180,191,192] |
| Foam based | Polyurethane Silicone-coated | Exudate absorption Mechanical protection | Moderate–heavy, post-op wounds, pressure injuries | High absorbency, cushioning, non- adherent top layers | Risk of maceration, May require secondary dressing | Mepilex®, Allevyn®, Lyofoam®, PolyMem®, Biatain® | [182,183] |
| Protein based | Collagen, Fibrin, Keratin, Silk fibrin/sericin | Cell migration Growth factor delivery Enzymatic degradation | Chronic wound Burns wound Diabetes ulcers | Biocompatible, Promotes granulation,re-epithelialization | Costly, Sometimes antigenic | Promogran®, EpiFix®, FIBRACOL®, OASIS® Biostep®, MatriStem®, | [186,187,188] |
| Alginate based | Calcium/sodium Alginate from brown seaweed | Ion exchange (Ca2+ for Na⁺) Gel forming matrix | Moderate– heavy wounds, surgical wounds, bleeding ulcers | High absorbency, Promotes clotting | Not for dry wounds, can leave residue | Kaltostat®, Algisite® Tegaderm Alginate®, Sorbsan®, Melgisorb® | [170,189] |
| Cellulose based | Bacterial cellulose | Moisture retention biocompatibility | Diverse burns, chronic wounds, ulcers, | Abundant, non-toxic, customizable, porosity, | Slower bio resorption | Biofill®, XCell®, | [168] |
| Oxidized cellulose (OC) | Hemostasis | bleeding wounds Surgical intervention | sterile, non-toxic, biodegradable | Some need Secondary dressings | Surgicel®, Traumastem® | [7] | |
| Oxidized regenerated cellulose (ORC) | Hemostasis, promotes granulation | bleeding wounds Surgical bleeding | biodegradable, drug delivery | Some allergy reaction | Surgicel®, Traumastem® | [149,171] | |
| CMC derivatives | Physical protection | Trauma wound, surgical sites, outpatient and trauma care | diverse product types | Variable clinical performance depending on formulate | Aquacel®, | ||
| AquaRite | [193] | ||||||
| Extra CMC™ | |||||||
| Hcel® NaT | [25] |
6.5. Cost-Effectiveness and Sustainability Analysis
7. Emerging Trends and Future Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.-F.; Lu, P.; Jia, H.-R.; Li, G.; Zhu, B.; Wang, X.; Wu, F.-G. Emerging materials for hemostasis. Coord. Chem. Rev. 2023, 475, 214823. [Google Scholar] [CrossRef]
- Latif, R.K.; Clifford, S.P.; Baker, J.A.; Lenhardt, R.; Haq, M.Z.; Huang, J.; Farah, I.; Businger, J.R. Traumatic hemorrhage and chain of survival. Scand. J. Trauma Resusc. Emerg. Med. 2023, 31, 25. [Google Scholar] [CrossRef]
- Zeng, Q.; Qi, X.; Shi, G.; Zhang, M.; Haick, H. Wound dressing: From nanomaterials to diagnostic dressings and healing evaluations. ACS Nano 2022, 16, 1708–1733. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, M.; Liu, Q.; Liu, G.; Wang, S.; Li, J. Recent advances in the medical applications of hemostatic materials. Theranostics 2023, 13, 161. [Google Scholar] [CrossRef]
- Bakadia, B.M.; Boni, B.O.O.; Ahmed, A.A.Q.; Zheng, R.; Shi, Z.; Ullah, M.W.; Lamboni, L.; Yang, G. In Situ Synthesized Porous Bacterial Cellulose/Poly(vinyl alcohol)-Based Silk Sericin and Azithromycin Release System for Treating Chronic Wound Biofilm. Macromol. Biosci. 2022, 22, e2200201. (In English) [Google Scholar] [CrossRef]
- Mao, L.; Wang, L.; Zhang, M.; Ullah, M.W.; Liu, L.; Zhao, W.; Li, Y.; Ahmed, A.A.Q.; Cheng, H.; Shi, Z.; et al. In Situ Synthesized Selenium Nanoparticles-Decorated Bacterial Cellulose/Gelatin Hydrogel with Enhanced Antibacterial, Antioxidant, and Anti-Inflammatory Capabilities for Facilitating Skin Wound Healing. Adv. Healthc. Mater. 2021, 10, 2100402. [Google Scholar] [CrossRef]
- Resch, A.; Staud, C.; Radtke, C. Nanocellulose-based wound dressing for conservative wound management in children with second-degree burns. Int. Wound J. 2021, 18, 478–486. (In English) [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric hydrogel systems as emerging biomaterial platforms to enable hemostasis and wound healing. Adv. Healthc. Mater. 2020, 9, 2000905. [Google Scholar] [CrossRef]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Tyshkunova, I.V.; Poshina, D.N.; Skorik, Y.A. Cellulose cryogels as promising materials for biomedical applications. Int. J. Mol. Sci. 2022, 23, 2037. [Google Scholar] [CrossRef]
- Naomi, R.; Bt Hj Idrus, R.; Fauzi, M.B. Plant-vs. Bacterial-derived cellulose for wound healing: A review. Int. J. Environ. Res. Public Health 2020, 17, 6803. [Google Scholar] [CrossRef]
- Tamahkar, E.; Topçu, A.A.; Perçin, I.; Aslıyüce, S.; Denizli, A. Bacterial cellulose nanofibers for separation, drug delivery, wound dressing, and tissue engineering applications. In Nanocellulose Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–20. [Google Scholar]
- Jose, J.; Pai, A.R.; Gopakumar, D.A.; Dalvi, Y.; Ruby, V.; Bhat, S.G.; Pasquini, D.; Kalarikkal, N.; Thomas, S. Novel 3D porous aerogels engineered at nano scale from cellulose nano fibers and curcumin: An effective treatment for chronic wounds. Carbohydr. Polym. 2022, 287, 119338. [Google Scholar] [CrossRef]
- Leong, M.; Kong, Y.; Harun, M.; Looi, C.; Wong, W. Current advances of nanocellulose application in biomedical field. Carbohydr. Res. 2023, 532, 108899. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, J.; Wang, H. Chronic wound management: A liquid diode-based smart bandage with ultrasensitive pH sensing ability. Microsyst. Nanoeng. 2024, 10, 193. (In English) [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Wei, L.; Ma, S.; Wang, J.; Zhu, W.; Wang, H. Engineering High-Performance Composite Cellulose Materials for Fast Hemostasis. ACS Biomater. Sci. Eng. 2024, 10, 5313–5326. [Google Scholar] [CrossRef]
- Biranje, S.S.; Sun, J.; Cheng, L.; Cheng, Y.; Shi, Y.; Yu, S.; Jiao, H.; Zhang, M.; Lu, X.; Han, W. Development of cellulose nanofibril/casein-based 3D composite hemostasis scaffold for potential wound-healing application. ACS Appl. Mater. Interfaces 2022, 14, 3792–3808. [Google Scholar] [CrossRef]
- Xie, Y.; Qiao, K.; Yue, L.; Tang, T.; Zheng, Y.; Zhu, S.; Yang, H.; Fang, Z. A self-crosslinking, double-functional group modified bacterial cellulose gel used for antibacterial and healing of infected wound. Bioact. Mater. 2022, 17, 248–260. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Qiao, L.; Zou, F.; Xie, Y.; Zheng, Y.; Chao, Y.; Yang, Y.; He, W.; Yang, S. Cellulose fibers-reinforced self-expanding porous composite with multiple hemostatic efficacy and shape adaptability for uncontrollable massive hemorrhage treatment. Bioact. Mater. 2021, 6, 2089–2104. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U.; Al Rashid, A.; Bukhari, S.M.Z.S.; Hossain, M.; Koç, M. Shape-memory and self-healing properties of sustainable cellulosic nanofibers-based hybrid materials for novel applications. Giant 2024, 19, 100299. [Google Scholar] [CrossRef]
- Tripathi, G.; Park, M.; Lim, H.; Lee, B.-T. Natural TEMPO oxidized cellulose nano fiber/alginate/dSECM hybrid aerogel with improved wound healing and hemostatic ability. Int. J. Biol. Macromol. 2023, 243, 125226. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Applewhite, A.J.; Niezgoda, J.; Snyder, R.; Shah, J.; Cullen, B.; Schultz, G.; Harrison, J.; Hill, R.; Howell, M. Oxidized regenerated cellulose/collagen dressings: Review of evidence and recommendations. Adv. Ski. Wound Care 2017, 30, S1–S18. [Google Scholar] [CrossRef]
- Cheng, F.; Xu, L.; Dai, J.; Yi, X.; He, J.; Li, H. N, O-carboxymethyl chitosan/oxidized cellulose composite sponge containing ε-poly-l-lysine as a potential wound dressing for the prevention and treatment of postoperative adhesion. Int. J. Biol. Macromol. 2022, 209, 2151–2164. [Google Scholar] [CrossRef]
- Zhou, M.; Liao, J.; Li, G.; Yu, Z.; Xie, D.; Zhou, H.; Wang, F.; Ren, Y.; Xu, R.; Dai, Y. Expandable carboxymethyl chitosan/cellulose nanofiber composite sponge for traumatic hemostasis. Carbohydr. Polym. 2022, 294, 119805. [Google Scholar] [CrossRef]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef]
- Yadav, C.; Saini, A.; Li, K.; Chawla, S.; Li, X.; Jang, W.-D. Cinnamomum cassia perfused nanocellulose-based biocompatible sponge for hemostatic wound care dressing. Cellulose 2023, 30, 5857–5870. [Google Scholar] [CrossRef]
- Su, L.; Jia, Y.; Fu, L.; Guo, K.; Xie, S. The emerging progress on wound dressings and their application in clinic wound management. Heliyon 2023, 9, e22520. [Google Scholar] [CrossRef]
- Aderibigbe, B. Application of Nanocellulose for Wound Dressings. In Nanocellulose: A Biopolymer for Biomedical Applications; Scrivener Publishing LLC: Beverly, MA, USA, 2024; pp. 193–219. [Google Scholar]
- Horue, M.; Silva, J.M.; Berti, I.R.; Brandão, L.R.; Barud, H.d.S.; Castro, G.R. Bacterial cellulose-based materials as dressings for wound healing. Pharmaceutics 2023, 15, 424. [Google Scholar] [CrossRef]
- Menegasso, J.F.; Moraes, N.A.C.; Vasquez, T.P.; Felipetti, F.A.; Antonio, R.V.; Dutra, R.C. Modified montmorillonite-bacterial cellulose composites as a novel dressing system for pressure injury. Int. J. Biol. Macromol. 2022, 194, 402–411. [Google Scholar] [CrossRef]
- Ndlovu, S.P.; Alven, S.; Hlalisa, K.; Aderibigbe, B.A. Cellulose acetate-based wound dressings loaded with bioactive agents: Potential scaffolds for wound dressing and skin regeneration. Curr. Drug Deliv. 2024, 21, 1226–1240. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Antunes, J.C.; Seabra, C.L.; Tohidi, S.D.; Reis, S.; Amorim, M.T.P.; Felgueiras, H.P. Tiger 17 and pexiganan as antimicrobial and hemostatic boosters of cellulose acetate-containing poly (vinyl alcohol) electrospun mats for potential wound care purposes. Int. J. Biol. Macromol. 2022, 209, 1526–1541. [Google Scholar] [CrossRef]
- LaPelusa, A.; Dave, H.D. Physiology, hemostasis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Scridon, A. Platelets and their role in hemostasis and thrombosis—From physiology to pathophysiology and therapeutic implications. Int. J. Mol. Sci. 2022, 23, 12772. [Google Scholar] [CrossRef]
- Sang, Y.; Roest, M.; de Laat, B.; de Groot, P.G.; Huskens, D. Interplay between platelets and coagulation. Blood Rev. 2021, 46, 100733. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, J.; Zhu, Y.; Wu, C. Inorganic-based biomaterials for rapid hemostasis and wound healing. Chem. Sci. 2023, 14, 29–53. [Google Scholar] [CrossRef]
- Hsu, B.B.; Conway, W.; Tschabrunn, C.M.; Mehta, M.; Perez-Cuevas, M.B.; Zhang, S.; Hammond, P.T. Clotting mimicry from robust hemostatic bandages based on self-assembling peptides. ACS Nano 2015, 9, 9394–9406. [Google Scholar] [CrossRef]
- Hickman, D.A.; Pawlowski, C.L.; Sekhon, U.D.; Marks, J.; Gupta, A.S. Biomaterials and advanced technologies for hemostatic management of bleeding. Adv. Mater. 2018, 30, 1700859. [Google Scholar] [CrossRef]
- Periayah, M.H.; Halim, A.S.; Saad, A.Z.M. Mechanism action of platelets and crucial blood coagulation pathways in hemostasis. Int. J. Hematol.-Oncol. Stem Cell Res. 2017, 11, 319. [Google Scholar]
- Nurden, A.T. Molecular basis of clot retraction and its role in wound healing. Thromb. Res. 2023, 231, 159–169. [Google Scholar] [CrossRef]
- Jansen, E.E.; Hartmann, M. Clot retraction: Cellular mechanisms and inhibitors, measuring methods, and clinical implications. Biomedicines 2021, 9, 1064. [Google Scholar] [CrossRef]
- Chapin, J.C.; Hajjar, K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015, 29, 17–24. [Google Scholar] [CrossRef]
- Yang, Y.; Du, Y.; Zhang, J.; Zhang, H.; Guo, B. Structural and functional design of electrospun nanofibers for hemostasis and wound healing. Adv. Fiber Mater. 2022, 4, 1027–1057. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF-β family in wound healing, burns and scarring: A review. Int. J. Burn. Trauma 2012, 2, 18. [Google Scholar]
- Spielman, A.F.; Griffin, M.F.; Parker, J.; Cotterell, A.C.; Wan, D.C.; Longaker, M.T. Beyond the scar: A basic science review of wound remodeling. Adv. Wound Care 2023, 12, 57–67. [Google Scholar] [CrossRef]
- Guo, S.a.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Meng, S.; Wu, H.; Xiao, D.; Lan, S.; Dong, A. Recent advances in bacterial cellulose-based antibacterial composites for infected wound therapy. Carbohydr. Polym. 2023, 316, 121082. [Google Scholar] [CrossRef]
- Heinze, T. Cellulose: Structure and properties. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–52. [Google Scholar]
- Xiang, Z.; Liu, Q.; Chen, Y.; Lu, F. Effects of physical and chemical structures of bacterial cellulose on its enhancement to paper physical properties. Cellulose 2017, 24, 3513–3523. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Z.; Liu, K.; Ji, X.; Fatehi, P.; Chen, J. A cellulose nanofibril-reinforced hydrogel with robust mechanical, self-healing, pH-responsive and antibacterial characteristics for wound dressing applications. J. Nanobiotechnol. 2022, 20, 312. [Google Scholar] [CrossRef]
- Mahmoodzadeh, A.; Moghaddas, J.; Jarolmasjed, S.; Kalan, A.E.; Edalati, M.; Salehi, R. Biodegradable cellulose-based superabsorbent as potent hemostatic agent. Chem. Eng. J. 2021, 418, 129252. [Google Scholar] [CrossRef]
- Te Grotenhuis, R.; van Grunsven, P.M.; Heutz, W.M.; Tan, E.C. Prehospital use of hemostatic dressings in emergency medical services in the Netherlands: A prospective study of 66 cases. Injury 2016, 47, 1007–1011. [Google Scholar] [CrossRef]
- Jayabal, P.; Kannan Sampathkumar, V.; Vinothkumar, A.; Mathapati, S.; Pannerselvam, B.; Achiraman, S.; Venkatasubbu, G.D. Fabrication of a chitosan-based wound dressing patch for enhanced antimicrobial, hemostatic, and wound healing application. ACS Appl. Bio Mater. 2023, 6, 615–627. [Google Scholar] [CrossRef]
- Ouyang, X.-k.; Zhao, L.; Jiang, F.; Ling, J.; Yang, L.-Y.; Wang, N. Cellulose nanocrystal/calcium alginate-based porous microspheres for rapid hemostasis and wound healing. Carbohydr. Polym. 2022, 293, 119688. [Google Scholar] [CrossRef]
- Sena, M.J.; Douglas, G.; Gerlach, T.; Grayson, J.K.; Pichakron, K.O.; Zierold, D. A pilot study of the use of kaolin-impregnated gauze (Combat Gauze) for packing high-grade hepatic injuries in a hypothermic coagulopathic swine model. J. Surg. Res. 2013, 183, 704–709. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, W.; Wang, C.; Xiao, L.; Yu, L.; Fan, J. Hemostatic and antibacterial calcium–copper zeolite gauze for infected wound healing. RSC Adv. 2024, 14, 878–888. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Fertuzinhos, A.; Freitas, D.S.; Silva, C.; Ferreira, D.P.; Felgueiras, H.P. Thermo-mechanical performance of nanostructured electrospun composites produced from poly (vinyl alcohol) and cellulosic compounds for potential uses as wound dressings. Polymer 2023, 281, 126131. [Google Scholar] [CrossRef]
- Tudoroiu, E.-E.; Kaya, M.G.A.; Titorencu, I.; Dinu-Pîrvu, C.E.; Marin, M.M.; Roșca, A.-M.; Popa, L.; Anuța, V.; Antoniac, A.; Chelaru, C. Design and evaluation of new wound dressings based on collagen-cellulose derivatives. Mater. Des. 2023, 236, 112469. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Mendiola-Garza, G.; de Melo, E.M.; Dugmore, T.I.; Matharu, A.S.; Morones-Ramirez, J.R. Antimicrobial activity of a silver-microfibrillated cellulose biocomposite against susceptible and resistant bacteria. Sci. Rep. 2020, 10, 7281. [Google Scholar] [CrossRef]
- Li, Y.; Chu, C.; Chen, C.; Sun, B.; Wu, J.; Wang, S.; Ding, W.; Sun, D. Quaternized chitosan/oxidized bacterial cellulose cryogels with shape recovery for noncompressible hemorrhage and wound healing. Carbohydr. Polym. 2024, 327, 121679. [Google Scholar] [CrossRef]
- Yoon, H.S.; Na, Y.C.; Choi, K.H.; Huh, W.H.; Kim, J.M. Wound healing effect of regenerated oxidized cellulose versus fibrin sealant patch: An in vivo study. Arch. Craniofacial Surg. 2019, 20, 289. [Google Scholar] [CrossRef]
- Chiara, O.; Cimbanassi, S.; Bellanova, G.; Chiarugi, M.; Mingoli, A.; Olivero, G.; Ribaldi, S.; Tugnoli, G.; Basilicò, S.; Bindi, F. A systematic review on the use of topical hemostats in trauma and emergency surgery. BMC Surg. 2018, 18, 68. [Google Scholar] [CrossRef]
- Yin, X.; Hu, Y.; Kang, M.; Hu, J.; Wu, B.; Liu, Y.; Liu, X.; Bai, M.; Wei, Y.; Huang, D. Cellulose based composite sponges with oriented porous structure and superabsorptive capacity for quick hemostasis. Int. J. Biol. Macromol. 2023, 253, 127295. [Google Scholar] [CrossRef]
- Ryšavá, J.; Dyr, J.; Homola, J.; Dostálek, J.; Křížová, P.; Mášová, L.; Suttnar, J.; Briestenský, J.; Santar, I.; Myška, K. Surface interactions of oxidized cellulose with fibrin (ogen) and blood platelets. Sens. Actuators B Chem. 2003, 90, 243–249. [Google Scholar] [CrossRef]
- Devarajan, S.; Muthuchamy, M.; Muthukumar, A.; Rengaswami, G.V. Development of oxidized cellulose fabrics for hemostat applications. Mater. Lett. 2022, 308, 131160. [Google Scholar] [CrossRef]
- Yi, X.; He, J.; Wei, X.; Li, H.; Liu, X.; Cheng, F. A mussel-inspired multifunctional hydrogel reinforced by bacterial cellulose for wound healing: Sustained drug release, enhanced adhesion and self-healing property. Cellulose 2023, 30, 6523–6538. [Google Scholar] [CrossRef]
- Sasmal, P.K.; Ganguly, S. Polymer in hemostasis and follow-up wound healing. J. Appl. Polym. Sci. 2023, 140, e53559. [Google Scholar] [CrossRef]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef]
- Sibbald, R.G.; Williamson, D.; Orsted, H.L.; Campbell, K.; Keast, D.; Krasner, D.; Sibbald, D. Preparing the wound bed--debridement, bacterial balance, and moisture balance. Ostomy Wound Manag. 2000, 46, 14–22, 24–28, 30–35. quiz 36–37(In English) [Google Scholar]
- Wolcott, R.; Rhoads, D. A study of biofilm-based wound management in subjects with critical limb ischaemia. J. Wound Care 2008, 17, 145–155. [Google Scholar] [CrossRef]
- Rigo, C.; Ferroni, L.; Tocco, I.; Roman, M.; Munivrana, I.; Gardin, C.; Cairns, W.R.; Vindigni, V.; Azzena, B.; Barbante, C. Active silver nanoparticles for wound healing. Int. J. Mol. Sci. 2013, 14, 4817–4840. [Google Scholar] [CrossRef]
- Gheorghita, D.; Grosu, E.; Robu, A.; Ditu, L.M.; Deleanu, I.M.; Gradisteanu Pircalabioru, G.; Raiciu, A.-D.; Bita, A.-I.; Antoniac, A.; Antoniac, V.I. Essential oils as antimicrobial active substances in wound dressings. Materials 2022, 15, 6923. [Google Scholar] [CrossRef]
- He, W.; Xu, J.; Zheng, Y.; Chen, J.; Yin, Y.; Mosselhy, D.A.; Zou, F.; Ma, M.; Liu, X. Bacterial cellulose/soybean protein isolate composites with promoted inflammation inhibition, angiogenesis and hair follicle regeneration for wound healing. Int. J. Biol. Macromol. 2022, 211, 754–766. [Google Scholar] [CrossRef]
- Napavichayanun, S.; Ampawong, S.; Harnsilpong, T.; Angspatt, A.; Aramwit, P. Inflammatory reaction, clinical efficacy, and safety of bacterial cellulose wound dressing containing silk sericin and polyhexamethylene biguanide for wound treatment. Arch. Dermatol. Res. 2018, 310, 795–805. [Google Scholar] [CrossRef]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical role of transforming growth factor beta in different phases of wound healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.-G.; Lee, B.-T. Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Mahmood, S.; Khan, N.R.; Razaque, G.; Shah, S.U.; Shahid, M.G.; Albarqi, H.A.; Alqahtani, A.A.; Alasiri, A.; Basit, H.M. Microwave-Treated Physically Cross-Linked Sodium Alginate and Sodium Carboxymethyl Cellulose Blend Polymer Film for Open Incision Wound Healing in Diabetic Animals-A Novel Perspective for Skin Tissue Regeneration Application. Pharmaceutics 2023, 15, 418. (In English) [Google Scholar] [CrossRef]
- Elangwe, C.N.; Morozkina, S.N.; Olekhnovich, R.O.; Krasichkov, A.; Polyakova, V.O.; Uspenskaya, M.V. A review on chitosan and cellulose hydrogels for wound dressings. Polymers 2022, 14, 5163. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- He, W.; Wu, J.; Xu, J.; Mosselhy, D.A.; Zheng, Y.; Yang, S. Bacterial cellulose: Functional modification and wound healing applications. Adv. Wound Care 2021, 10, 623–640. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Wen, X.; Lin, Q.; Chen, X.; Wu, Z. Silver nanoparticle/bacterial cellulose gel membranes for antibacterial wound dressing: Investigation in vitro and in vivo. Biomed. Mater. 2014, 9, 035005. [Google Scholar] [CrossRef]
- Arulprasanna, A.; Omkumar, M. A review on composites: Selection and its applications. Mater. Today Proc. 2024. [Google Scholar] [CrossRef]
- Zhang, M.; Han, F.; Duan, X.; Zheng, D.; Cui, Q.; Liao, W. Advances of biological macromolecules hemostatic materials: A review. Int. J. Biol. Macromol. 2024, 269, 131772. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L. Applications of injectable hemostatic materials in wound healing: Principles, strategies, performance requirements, and future perspectives. Theranostics 2023, 13, 4615–4635. (In English) [Google Scholar] [CrossRef]
- Cheng, F.; Liu, C.; Li, H.; Wei, X.; Yan, T.; Wang, Y.; Song, Y.; He, J.; Huang, Y. Carbon nanotube-modified oxidized regenerated cellulose gauzes for hemostatic applications. Carbohydr. Polym. 2018, 183, 246–253. [Google Scholar] [CrossRef]
- Li, S.; Wu, X.; Bai, N.; Ni, J.; Liu, X.; Mao, W.; Jin, L.; Xiang, H.; Fu, H.; Shou, Q. Fabricating oxidized cellulose sponge for hemorrhage control and wound healing. ACS Biomater. Sci. Eng. 2023, 9, 6398–6408. [Google Scholar] [CrossRef]
- Liu, L.; Liu, L.; Chen, L.; Chen, G.; Wei, Y.; Hong, F.F. Synthesis of hemostatic aerogel of TEMPO-oxidized cellulose nanofibers/collagen/chitosan and in vivo/vitro evaluation. Mater. Today Bio 2024, 28, 101204. [Google Scholar] [CrossRef]
- Li, S.; Gong, L.; Chen, J.; Wu, X.; Liu, X.; Fu, H.; Shou, Q. Fabricating the multibranch carboxyl-modified cellulose for hemorrhage control. Mater. Today Bio 2024, 24, 100878. [Google Scholar] [CrossRef]
- Kędzierska, M.; Blilid, S.; Miłowska, K.; Kołodziejczyk-Czepas, J.; Katir, N.; Lahcini, M.; El Kadib, A.; Bryszewska, M. Insight into Factors Influencing Wound Healing Using Phosphorylated Cellulose-Filled-Chitosan Nanocomposite Films. Int. J. Mol. Sci. 2021, 22, 11386. [Google Scholar] [CrossRef]
- Zeynep, B.D.; Umran, A.S.; Dilek, U.K.; Serdar, S. Development of Metal Ion Binded Oxidized Regenerated Cellulose Powder as Hemostatic Agent: A Comparative Study with in Vivo Performance. Ind. Eng. Chem. Res. 2015, 54, 4906–4914. [Google Scholar]
- Chen, J.; Zhao, L.; Ling, J.; Yang, L.-Y.; Ouyang, X.-k. A quaternized chitosan and carboxylated cellulose nanofiber-based sponge with a microchannel structure for rapid hemostasis and wound healing. Int. J. Biol. Macromol. 2023, 233, 123631. [Google Scholar] [CrossRef]
- Fan, X.; Li, Y.; Li, N.; Wan, G.; Ali, M.A.; Tang, K. Rapid hemostatic chitosan/cellulose composite sponge by alkali/urea method for massive haemorrhage. Int. J. Biol. Macromol. 2020, 164, 2769–2778. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, G.; Dong, Y.; Su, W.; Zhang, P.; Li, Y.; Wan, G.; Tang, K.; Fan, X. Mussel-inspired oxidized sodium alginate/cellulose composite sponge with excellent shape recovery and antibacterial properties for the efficient control of non-compressible hemorrhage. Int. J. Biol. Macromol. 2024, 283, 137800. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, Y.; Shen, Z.; Ni, M.; Xu, J.; Wang, T. Enhancing Wound Recovery: A Self-Gelling Powder for Improved Hemostasis and Healing. Polymers 2024, 16, 1795. [Google Scholar] [CrossRef]
- Cai, B.; Fan, Y.; Yang, S.; Che, C.; Li, X.; Wang, X. A highly compressible and expandable cellulose sponge with arch-like lamellar structures for non-compressible hemorrhage. Carbohydr. Polym. 2025, 353, 123255. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, C.; Dong, Y.; Su, W.; Xue, R.; Zhang, P.; Li, Y.; Wan, G.; Tang, K.; Fan, X. Self-expanding cellulose sponge with enhanced hemostatic ability by tannic acid/metal ion composite coating for highly effective hemostasis of difficult-to-control bleeding wounds. Biomater. Adv. 2025, 166, 214025. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Feng, F.; Jiang, W.; Ma, L.; Liu, M.; Li, K.; Wan, Y.; Ao, H. Designment of polydopamine/bacterial cellulose incorporating copper (II) sulfate as an antibacterial wound dressing. Biomater. Adv. 2022, 134, 112591. [Google Scholar] [CrossRef]
- Li, Z.; Milionis, A.; Zheng, Y.; Yee, M.; Codispoti, L.; Tan, F.; Poulikakos, D.; Yap, C.H. Superhydrophobic hemostatic nanofiber composites for fast clotting and minimal adhesion. Nat. Commun. 2019, 10, 5562. [Google Scholar] [CrossRef]
- Sedlář, M.; Kacvinská, K.; Fohlerová, Z.; Izsák, D.; Chalupová, M.; Suchý, P.; Dohnalová, M.; Sopuch, T.; Vojtová, L. A synergistic effect of fibrous carboxymethyl cellulose with equine collagen improved the hemostatic properties of freeze-dried wound dressings. Cellulose 2023, 30, 11113–11131. [Google Scholar] [CrossRef]
- Shakya, K.R.; Nigam, K.; Sharma, A.; Jahan, K.; Tyagi, A.K.; Verma, V. Preparation and assessment of agar/TEMPO-oxidized bacterial cellulose cryogels for hemostatic applications. J. Mater. Chem. B 2024, 12, 3453–3468. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. (In English) [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, Y.; Liu, Y.; Zhao, X.; Huang, Y.; Zhang, X.; Hu, X.; Mequanint, K.; Luo, G.; Xing, M. Platelet Vesicles Synergetic with Biosynthetic Cellulose Aerogels for Ultra-Fast Hemostasis and Wound Healing. Adv. Healthc. Mater. 2024, 13, e2304523. (In English) [Google Scholar] [CrossRef]
- Yuan, H.; Chen, L.; Hong, F.F. A Biodegradable Antibacterial Nanocomposite Based on Oxidized Bacterial Nanocellulose for Rapid Hemostasis and Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 3382–3392. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Zhao, X.-J.; Zhao, X.-Q.; Ma, X.-F.; Xue, N.; Liu, X.-Z.; Wang, F.-P.; Jia, S.-R.; Zhong, C. Fabrication of Bacterial Cellulose-Based Dressings for Promoting Infected Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 32716–32728. [Google Scholar] [CrossRef]
- Deng, L.; Huang, Y.; Chen, S.; Han, Z.; Han, Z.; Jin, M.; Qu, X.; Wang, B.; Wang, H.; Gu, S. Bacterial cellulose-based hydrogel with antibacterial activity and vascularization for wound healing. Carbohydr. Polym. 2023, 308, 120647. [Google Scholar] [CrossRef]
- Wang, F.; Sun, M.; Li, D.; Qin, X.; Liao, Y.; Liu, X.; Jia, S.; Xie, Y.; Zhong, C. Multifunctional Asymmetric Bacterial Cellulose Membrane with Enhanced Anti-Bacterial and Anti-Inflammatory Activities for Promoting Infected Wound Healing. Small 2023, 19, 2303591. [Google Scholar] [CrossRef]
- Pita-Vilar, M.; Concheiro, A.; Alvarez-Lorenzo, C.; Diaz-Gomez, L. Recent advances in 3D printed cellulose-based wound dressings: A review on in vitro and in vivo achievements. Carbohydr. Polym. 2023, 321, 121298. [Google Scholar] [CrossRef] [PubMed]
- Fourmann, O.; Hausmann, M.K.; Neels, A.; Schubert, M.; Nyström, G.; Zimmermann, T.; Siqueira, G. 3D printing of shape-morphing and antibacterial anisotropic nanocellulose hydrogels. Carbohydr. Polym. 2021, 259, 117716. [Google Scholar] [CrossRef]
- Alizadehgiashi, M.; Nemr, C.R.; Chekini, M.; Pinto Ramos, D.; Mittal, N.; Ahmed, S.U.; Khuu, N.; Kelley, S.O.; Kumacheva, E. Multifunctional 3D-printed wound dressings. ACS Nano 2021, 15, 12375–12387. [Google Scholar] [CrossRef]
- Kerwald, J.; de Moura Junior, C.F.; Freitas, E.D.; de Moraes Segundo, J.d.D.P.; Vieira, R.S.; Beppu, M.M. Cellulose-based electrospun nanofibers: A review. Cellulose 2022, 29, 25–54. [Google Scholar]
- Kazeminava, F.; Javanbakht, S.; Nouri, M.; Adibkia, K.; Ganbarov, K.; Yousefi, M.; Ahmadi, M.; Gholizadeh, P.; Kafil, H.S. Electrospun nanofibers based on carboxymethyl cellulose/polyvinyl alcohol as a potential antimicrobial wound dressing. Int. J. Biol. Macromol. 2022, 214, 111–119. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Costa, S.M.; Ferreira, D.P.; Calhelha, R.C.; Barros, L.; Stojković, D.; Soković, M.; Ferreira, I.C.; Fangueiro, R. Chitosan/nanocellulose electrospun fibers with enhanced antibacterial and antifungal activity for wound dressing applications. React. Funct. Polym. 2021, 159, 104808. [Google Scholar] [CrossRef]
- Shen, H.; Jiang, C.; Li, W.; Wei, Q.; Reza, A.; Wang, Q. Synergistic photodynamic and photothermal antibacterial activity of in situ grown bacterial cellulose/MoS2-chitosan nanocomposite materials with visible light illumination. ACS Appl. Mater. Interfaces 2021, 13, 31193–31205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Lu, B.; Yu, K.; Xie, R.; Lan, G.; Xie, J.; Hu, E.; Lu, F. A sandwich-like silk fibroin/polysaccharide composite dressing with continual biofluid draining for wound exudate management. Int. J. Biol. Macromol. 2023, 253, 127000. [Google Scholar] [CrossRef] [PubMed]
- Raisi, A.; Asefnejad, A.; Shahali, M.; Doozandeh, Z.; Kamyab Moghadas, B.; Saber-Samandari, S.; Khandan, A. A soft tissue fabricated using a freeze-drying technique with carboxymethyl chitosan and nanoparticles for promoting effects on wound healing. J. Nanoanalysis 2020, 7, 262–274. [Google Scholar]
- Bi, Z.; Teng, H.; Li, Q.; Zhang, S. Enhanced carboxymethylcellulose sponge for hemostasis and wound repair. Front. Mater. 2022, 9, 944274. [Google Scholar] [CrossRef]
- Wei, X.; Cai, J.; Wang, C.; Yang, K.; Ding, S.; Tian, F.; Lin, S. Quaternized chitosan/cellulose composites as enhanced hemostatic and antibacterial sponges for wound healing. Int. J. Biol. Macromol. 2022, 210, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ma, W.; Yang, Z.; Zhang, H.; Ma, J.; Li, T.; Niu, H.; Zhou, Y.; Yao, Q.; Chang, J. An ultralong hydroxyapatite nanowire aerogel for rapid hemostasis and wound healing. Chem. Eng. J. 2022, 430, 132912. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, S.; Wang, J.; Gan, D.; Gama, M.; Yang, Z.; Zhu, Y.; Yao, F.; Luo, H. Scalable synthesis of robust and stretchable composite wound dressings by dispersing silver nanowires in continuous bacterial cellulose. Compos. Part B Eng. 2020, 199, 108259. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Zayed, M.; Salama, H.S. Antibacterial potential of bacterial cellulose impregnated with green synthesized silver nanoparticle against S. aureus and P. aeruginosa. Curr. Microbiol. 2023, 80, 75. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.; Hu, Y.; Kang, M.; Hu, J.; Wei, Y.; Huang, D.; Wang, Y. Water-triggered shape memory cellulose / sodium alginate / montmorillonite composite sponges for rapid hemostasis. Int. J. Biol. Macromol. 2024, 271, 132679. [Google Scholar] [CrossRef]
- Mahmoodzadeh, A.; Valizadeh, N.; Edalati, M.; Khordadmehr, M.; Zakeri, Z.; Salehi, R.; Jarolmasjed, S. Robust adhesive nanocomposite sponge composed of citric acid and nano clays modified cellulose for rapid hemostasis of lethal non-compressible hemorrhage. Carbohydr. Polym. 2024, 326, 121614. [Google Scholar] [CrossRef]
- Bian, J.; Bao, L.; Gao, X.; Wen, X.; Zhang, Q.; Huang, J.; Xiong, Z.; Hong, F.F.; Ge, Z.; Cui, W. Bacteria-engineered porous sponge for hemostasis and vascularization. J. Nanobiotechnol. 2022, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Cheng, F.; Wei, X.; Li, H.; Qian, J.; He, J. Bioinspired adhesive and self-healing bacterial cellulose hydrogels formed by a multiple dynamic crosslinking strategy for sealing hemostasis. Cellulose 2023, 30, 397–411. [Google Scholar] [CrossRef]
- Dong, X.; Sun, Q.; Xu, J.; Wang, T. Development of a Multifunctional Composite Hydrogel for Enhanced Wound Healing: Hemostasis, Sterilization, and Long-Term Moisturizing Properties. ACS Appl. Mater. Interfaces 2024, 16, 2972–2983. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lv, X.; Hou, Y.; Yu, H.; Sun, Y.; Cui, R.; Pan, P.; Chen, J. Multifunctional hemostatic polysaccharide-based sponge enhanced by tunicate cellulose: A promising approach for photothermal antibacterial activity and accelerated wound healing. Int. J. Biol. Macromol. 2023, 251, 126386. [Google Scholar] [CrossRef]
- Fan, X.; Li, M.; Yang, Q.; Wan, G.; Li, Y.; Li, N.; Tang, K. Morphology-controllable cellulose/chitosan sponge for deep wound hemostasis with surfactant and pore-foaming agent. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111408. (In English) [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, D.; Yu, H.; Zhong, Y.; Zhang, L.; Sui, X.; Wang, B.; Feng, X.; Xu, H.; Mao, Z. Composite hydrogel based oxidated sodium carboxymethyl cellulose and gelatin loaded carboxymethylated cotton fabric for hemostasis and infected wound treatment. Int. J. Biol. Macromol. 2023, 224, 1382–1394. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, K.; Li, Q.; Zhang, S.; Chen, J. Injectable and photothermal antibacterial bacterial cellulose cryogel for rapid hemostasis and repair of irregular and deep skin wounds. Carbohydr. Polym. 2023, 320, 121239. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, Z.; He, Y.; Lu, Q.; Chen, R.; Zhao, C.; Dong, D.; Sun, Y.; He, H. Dual light-responsive cellulose nanofibril-based in situ hydrogel for drug-resistant bacteria infected wound healing. Carbohydr. Polym. 2022, 297, 120042. [Google Scholar] [CrossRef]
- Liu, T.; Liu, S.; Shi, Y.; Zhang, Z.; Ding, S.; Hou, K.; Zhang, W.; Meng, X.; Li, F. Electrospun nanofiber membranes for rapid liver hemostasis via N-alkylated chitosan doped chitosan/PEO. Int. J. Biol. Macromol. 2024, 258, 128948. [Google Scholar] [CrossRef]
- Guamba, E.; Vispo, N.S.; Whitehead, D.C.; Singh, A.K.; Santos-Oliveira, R.; Niebieskikwiat, D.; Zamora-Ledezma, C.; Alexis, F. Cellulose-based hydrogels towards an antibacterial wound dressing. Biomater. Sci. 2023, 11, 3461–3468. [Google Scholar] [CrossRef]
- Roberts, E.L.; Abdollahi, S.; Oustadi, F.; Stephens, E.D.; Badv, M. Bacterial-Nanocellulose-Based Biointerfaces and Biomimetic Constructs for Blood-Contacting Medical Applications. ACS Mater. Au 2023, 3, 418–441. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, X.; Hang, T.; Chen, J.; Wang, Z.; Mosselhy, D.A.; Xu, J.; Wang, S.; Zheng, Y. Fabrication of Cu2+-loaded phase-transited lysozyme nanofilm on bacterial cellulose: Antibacterial, anti-inflammatory, and pro-angiogenesis for bacteria-infected wound healing. Carbohydr. Polym. 2023, 309, 120681. [Google Scholar] [CrossRef]
- Deng, L.; Li, F.; Han, Z.; Qu, X.; Li, J.; Zhou, Z.; Chen, S.; Wang, H.; Lv, X. Bacterial cellulose-based hydrogel with regulated rehydration and enhanced antibacterial activity for wound healing. Int. J. Biol. Macromol. 2024, 267, 131291. [Google Scholar] [CrossRef]
- Yang, M.; Huang, C.; Yu, H.-Y.; Dong, Y.; Abdalkarim, S.Y.H.; Qin, C.C.; Wu, M.; Shen, Y. Next-generation self-adhesive dressings: Highly stretchable, antibacterial, and UV-shielding properties enabled by tannic acid-coated cellulose nanocrystals. Int. J. Biol. Macromol. 2024, 257, 128715. [Google Scholar] [CrossRef]
- Li, D.; Dong, X.; Liu, X.; Lin, H.; Yang, D.; Shi, X.; Chen, C.; Tao, F.; Jiang, L.; Deng, H. Cellulose nanofibers embedded chitosan/tannin hydrogel with high antibacterial activity and hemostatic ability for drug-resistant bacterial infected wound healing. Carbohydr. Polym. 2024, 329, 121687. [Google Scholar] [CrossRef]
- Mirhaj, M.; Varshosaz, J.; Nasab, P.M.; Al-Musawi, M.H.; Almajidi, Y.Q.; Shahriari-Khalaji, M.; Tavakoli, M.; Alizadeh, M.; Sharifianjazi, F.; Mehrjoo, M. A double-layer cellulose/pectin-soy protein isolate-pomegranate peel extract micro/nanofiber dressing for acceleration of wound healing. Int. J. Biol. Macromol. 2024, 255, 128198. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, Y.-X.; Yang, Y.; Miao, W.; Shi, X.; Xu, K.-F.; Li, Z.-H.; Xiao, H.; Wu, F.-G. Bioinspired multifunctional cellulose film: In situ bacterial capturing and killing for managing infected wounds. Bioact. Mater. 2024, 36, 595–612. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, R.; Chen, Z.; Lu, Q.; Zhu, H.; Bu, Q.; Yin, J.; He, H. Bioinspired multifunctional cellulose nanofibril-based in situ liquid wound dressing for multiple synergistic therapy of the postoperative infected wound. ACS Appl. Mater. Interfaces 2021, 13, 51578–51591. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, Z.; Xun, X.; Ma, L.; Chen, Z.; Hu, X.; Wu, X.; Wan, Y.; Ao, H. De novo strategy with engineering a multifunctional bacterial cellulose-based dressing for rapid healing of infected wounds. Bioact. Mater. 2022, 13, 212–222. [Google Scholar] [CrossRef]
- Moradpoor, H.; Mohammadi, H.; Safaei, M.; Mozaffari, H.R.; Sharifi, R.; Gorji, P.; Sulong, A.B.; Muhamad, N.; Ebadi, M. Recent Advances on Bacterial Cellulose-Based Wound Management: Promises and Challenges. Int. J. Polym. Sci. 2022, 2022, 1214734. [Google Scholar] [CrossRef]
- Pouraghaei, M.; Shafiee, S.; Mesrian, F.; Bakhtavar, H.E.; Rahmani, F. Traumastem powder in treatment of non-traumatic anterior epistaxis in emergency department; a randomized clinical trial. Arch. Acad. Emerg. Med. 2020, 8, e78. [Google Scholar] [PubMed]
- Yu, P.; Zhong, W. Hemostatic materials in wound care. Burn. Trauma 2021, 9, tkab019. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.M.; Bortoto, J.B.; Fraga, G.P. Topical hemostatic agents in surgery: Review and prospects. Rev. Col. Bras. Cir. 2018, 45, e1900. [Google Scholar]
- Naito, M.; Ogura, N.; Yamanashi, T.; Sato, T.; Nakamura, T.; Miura, H.; Tsutsui, A.; Sakamoto, Y.; Tanaka, R.; Kumagai, Y. Prospective randomized controlled study on the validity and safety of an absorbable adhesion barrier (Interceed®) made of oxidized regenerated cellulose for laparoscopic colorectal surgery. Asian J. Endosc. Surg. 2017, 10, 7–11. [Google Scholar] [CrossRef]
- Ghimire, S.; Sarkar, P.; Rigby, K.; Maan, A.; Mukherjee, S.; Crawford, K.E.; Mukhopadhyay, K. Polymeric materials for hemostatic wound healing. Pharmaceutics 2021, 13, 2127. [Google Scholar] [CrossRef]
- Hupe, M.C.; Büttner, M.; Tabrizi, P.F.; Merseburger, A.S.; Kuczyk, M.A.; Imkamp, F. Hemopatch® as a hemostatic agent is safe in partial nephrectomy: A large, single-surgeon retrospective evaluation. Adv. Ther. 2021, 38, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Al-Attar, N.; de Jonge, E.; Kocharian, R.; Ilie, B.; Barnett, E.; Berrevoet, F. Safety and hemostatic effectiveness of SURGICEL® powder in mild and moderate intraoperative bleeding. Clin. Appl. Thromb./Hemost. 2023, 29, 10760296231190376. [Google Scholar] [CrossRef]
- Roshkovan, L.; Singhal, S.; Katz, S.I.; Galperin-Aizenberg, M. Multimodality imaging of Surgicel®, an important mimic of post-operative complication in the thorax. BJR Open 2021, 3, 20210031. [Google Scholar] [CrossRef]
- Uranues, S.; Fingerhut, A.; Levin, E.; Spazierer, D.; Rahimi, N.; Baumgartner, B. Effectiveness of Hemopatch® versus Surgicel® Original to control mild and moderate liver bleeding. BMC Surg. 2022, 22, 316. [Google Scholar] [CrossRef]
- Okubo, S.; Shindoh, J.; Kobayashi, Y.; Hashimoto, M. Safety of use of a sheet-type adhesion barrier (Interceed®) during liver surgery. World J. Surg. 2020, 44, 4214–4220. [Google Scholar] [CrossRef]
- Sanz-Horta, R.; Matesanz, A.; Gallardo, A.; Reinecke, H.; Jorcano, J.L.; Acedo, P.; Velasco, D.; Elvira, C. Technological advances in fibrin for tissue engineering. J. Tissue Eng. 2023, 14, 20417314231190288. [Google Scholar] [CrossRef]
- Bacakova, M.; Pajorova, J.; Sopuch, T.; Bacakova, L. Fibrin-modified cellulose as a promising dressing for accelerated wound healing. Materials 2018, 11, 2314. [Google Scholar] [CrossRef]
- Maver, T.; Hribernik, S.; Mohan, T.; Smrke, D.M.; Maver, U.; Stana-Kleinschek, K. Functional wound dressing materials with highly tunable drug release properties. RSC Adv. 2015, 5, 77873–77884. [Google Scholar] [CrossRef]
- Metta, V.; Nichkaode, P.B.; Panchbhai, S.V.; Charan, S.; Purohit, A. Clinical study of sterile collagen (Biofil) particles in the management of chronic non healing ulcers and their comparison with saline dressings. Int. Surg. J. 2020, 7, 1148–1152. [Google Scholar] [CrossRef]
- Frankel, V.H.; Serafica, G.C.; Damien, C.J. Development and testing of a novel biosynthesized XCell for treating chronic wounds. Surg. Technol. Int. 2004, 12, 27–33. (In English) [Google Scholar] [PubMed]
- Matsuura, Y.; Itano, Y.; Shoji-Pietraszkiewicz, A.; Terai, I.; Sugimoto, R.; Ishiko, T. Aquacel® Ag Advantage Dressing is Effective in the Wound Management of Extensive Burns After Transplantation of Cultured Epidermal Autograft. Int. J. Surg. Wound Care 2023, 4, 65–69. [Google Scholar] [CrossRef]
- Beele, H.; Van Overschelde, P.; Olivecrona, C.; Smet, S. A prospective randomized controlled clinical investigation comparing two post-operative wound dressings used after elective hip and knee replacement; Mepilex® Border Post-Op versus Aquacel® surgical. Int. J. Orthop. Trauma Nurs. 2020, 38, 100772. [Google Scholar] [CrossRef]
- Mbituyimana, B.; Liu, L.; Ye, W.; Boni, B.O.O.; Zhang, K.; Chen, J.; Thomas, S.; Vasilievich, R.V.; Shi, Z.; Yang, G. Bacterial cellulose-based composites for biomedical and cosmetic applications: Research progress and existing products. Carbohydr. Polym. 2021, 273, 118565. [Google Scholar] [CrossRef] [PubMed]
- Guise, C.; Fangueiro, R. Biomedical applications of nanocellulose. In Natural Fibres: Advances in Science and Technology Towards Industrial Applications: From Science to Market; Springer: Berlin/Heidelberg, Germany, 2016; pp. 155–169. [Google Scholar]
- Kucińska-Lipka, J.; Gubanska, I.; Janik, H. Bacterial cellulose in the field of wound healing and regenerative medicine of skin: Recent trends and future prospectives. Polym. Bull. 2015, 72, 2399–2419. [Google Scholar] [CrossRef]
- Ju, S.; Wang, K.; Qiao, L.; Xu, Y.; Reed-Maldonado, A.; Banie, L.; Peng, D.; Liu, T.; Wang, G.; Xin, Z. Application of BloodSTOP iX Wound Heal Nanocellulose Matrix for Burn Wound Care. J. Surg. Res. 2021, 4, 14–31. [Google Scholar] [CrossRef]
- Finnegan, S.; Percival, S.L. Clinical and Antibiofilm Efficacy of Antimicrobial Hydrogels. Adv. Wound Care 2015, 4, 398–406. (In English) [Google Scholar] [CrossRef] [PubMed]
- Koivuniemi, R.; Hakkarainen, T.; Kiiskinen, J.; Kosonen, M.; Vuola, J.; Valtonen, J.; Luukko, K.; Kavola, H.; Yliperttula, M. Clinical Study of Nanofibrillar Cellulose Hydrogel Dressing for Skin Graft Donor Site Treatment. Adv. Wound Care 2019, 9, 199–210. [Google Scholar] [CrossRef]
- Alvarez, O.M.; Phillips, T.J.; Menzoian, J.O.; Patel, M.; Andriessen, A. An RCT to compare a bio-cellulose wound dressing with a non-adherent dressing in VLUs. J. Wound Care 2012, 21, 448–453. (In English) [Google Scholar] [CrossRef] [PubMed]
- Sanjarnia, P.; Picchio, M.L.; Polegre Solis, A.N.; Schuhladen, K.; Fliss, P.M.; Politakos, N.; Metterhausen, L.; Calderón, M.; Osorio-Blanco, E.R. Bringing innovative wound care polymer materials to the market: Challenges, developments, and new trends. Adv. Drug Deliv. Rev. 2024, 207, 115217. (In English) [Google Scholar] [CrossRef] [PubMed]
- Cidreira, A.C.M.; de Castro, K.C.; Hatami, T.; Linan, L.Z.; Mei, L.H.I. Cellulose nanocrystals-based materials as hemostatic agents for wound dressings: A review. Biomed. Microdevices 2021, 23, 43. (In English) [Google Scholar] [CrossRef]
- Amit, M.; Binenbaum, Y.; Cohen, J.T.; Gil, Z. Effectiveness of an oxidized cellulose patch hemostatic agent in thyroid surgery: A prospective, randomized, controlled study. J. Am. Coll. Surg. 2013, 217, 221–225. (In English) [Google Scholar] [CrossRef]
- Kobylkevich, B.M.; Raihan, M.J.; Uprety, T.; Kaushik, R.S.; Shore, J.S.; Sohn, J.J.; Messerli, M.A. Linear polysaccharides reduce production of inflammatory cytokines by LPS-stimulated bovine fibroblasts. Vet. Immunol. Immunopathol. 2021, 234, 110220. (In English) [Google Scholar] [CrossRef]
- Wei, X.; Ding, S.; Liu, S.; Yang, K.; Cai, J.; Li, F.; Wang, C.; Lin, S.; Tian, F. Polysaccharides-modified chitosan as improved and rapid hemostasis foam sponges. Carbohydr. Polym. 2021, 264, 118028. [Google Scholar] [CrossRef]
- Chowdhry, S.A. Use of oxidized regenerated cellulose (ORC)/collagen/silver-ORC dressings to help manage skin graft donor site wounds. JPRAS Open 2019, 22, 33–40. (In English) [Google Scholar] [CrossRef]
- Lewis, K.M.; Spazierer, D.; Urban, M.D.; Lin, L.; Redl, H.; Goppelt, A. Comparison of regenerated and non-regenerated oxidized cellulose hemostatic agents. Eur. Surg. 2013, 45, 213–220. [Google Scholar] [CrossRef]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef]
- Bremer, L.; Hagemeister, K.; Moss, M.; Ernst, L.; Tolba, R.H.; Jockenhoevel, S.; Apel, C. Long-Term Degradation Assessment of a Polyurethane-Based Surgical Adhesive-Assessment and Critical Consideration of Preclinical In Vitro and In Vivo Testing. J. Funct. Biomater. 2023, 14, 168. (In English) [Google Scholar] [CrossRef] [PubMed]
- Dragu, A.; Unglaub, F.; Schwarz, S.; Beier, J.P.; Kneser, U.; Bach, A.D.; Horch, R.E. Foreign body reaction after usage of tissue adhesives for skin closure: A case report and review of the literature. Arch. Orthop. Trauma Surg. 2009, 129, 167–169. (In English) [Google Scholar] [CrossRef]
- Teng, L.; Shao, Z.; Bai, Q.; Zhang, X.; He, Y.-S.; Lu, J.; Zou, D.; Feng, C.; Dong, C.-M. Biomimetic Glycopolypeptide Hydrogels with Tunable Adhesion and Microporous Structure for Fast Hemostasis and Highly Efficient Wound Healing. Adv. Funct. Mater. 2021, 31, 2105628. [Google Scholar] [CrossRef]
- Darkovich, S.L.; Brown-Etris, M.; Spencer, M. Biofilm hydrogel dressing: A clinical evaluation in the treatment of pressure sores. Ostomy Wound Manag. 1990, 29, 47–60. (In English) [Google Scholar]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. (In English) [Google Scholar] [CrossRef] [PubMed]
- Seaman, S. Dressing selection in chronic wound management. J. Am. Podiatr. Med. Assoc. 2002, 92, 24–33. (In English) [Google Scholar] [CrossRef]
- Minsart, M.; Van Vlierberghe, S.; Dubruel, P.; Mignon, A. Commercial wound dressings for the treatment of exuding wounds: An in-depth physico-chemical comparative study. Burn. Trauma 2022, 10, tkac024. [Google Scholar] [CrossRef]
- Chetouani, A.; Elkolli, M.; Haffar, H.; Chader, H.; Riahi, F.; Varacavoudin, T.; Le Cerf, D. Multifunctional hydrogels based on oxidized pectin and gelatin for wound healing improvement. Int. J. Biol. Macromol. 2022, 212, 248–256. [Google Scholar] [CrossRef]
- Laurano, R.; Boffito, M.; Ciardelli, G.; Chiono, V. Wound dressing products: A translational investigation from the bench to the market. Eng. Regen. 2022, 3, 182–200. [Google Scholar] [CrossRef]
- Wang, L.; Hao, F.; Tian, S.; Dong, H.; Nie, J.; Ma, G. Targeting polysaccharides such as chitosan, cellulose, alginate and starch for designing hemostatic dressings. Carbohydr. Polym. 2022, 291, 119574. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.; Cavaco-Paulo, A. Wound dressings for a proteolytic-rich environment. Appl. Microbiol. Biotechnol. 2011, 90, 445–460. [Google Scholar] [CrossRef]
- Kanokpanont, S.; Damrongsakkul, S.; Ratanavaraporn, J.; Aramwit, P. An innovative bi-layered wound dressing made of silk and gelatin for accelerated wound healing. Int. J. Pharm. 2012, 436, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Duciel, L.; Proust, R.; Ponsen, A.C.; Ziarelli, F.; Coudreuse, A.; Jeanmichel, L.; Samardzic, M.; Uzan, G.; des Courtils, C. Are All Alginate Dressings Equivalent? J. Biomed. Mater. Res. B Appl. Biomater. 2025, 113, e35557. (In English) [Google Scholar] [CrossRef]
- Abazari, M.F.; Gholizadeh, S.; Karizi, S.Z.; Birgani, N.H.; Abazari, D.; Paknia, S.; Derakhshankhah, H.; Allahyari, Z.; Amini, S.M.; Hamidi, M.; et al. Recent Advances in Cellulose-Based Structures as the Wound-Healing Biomaterials: A Clinically Oriented Review. Appl. Sci. 2021, 11, 7769. [Google Scholar] [CrossRef]
- Landriscina, A.; Rosen, J.; Friedman, A.J. Systematic Approach to Wound Dressings. J. Drugs Dermatol. 2015, 14, 740–744. (In English) [Google Scholar]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. (In English) [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile application of nanocellulose: From industry to skin tissue engineering and wound healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef]
- Bwatanglang, I.B.; Musa, Y.; Yusof, N.A. Chapter 3—Market analysis and commercially available cellulose and hydrogel-based composites for sustainability, clean environment, and human health. In Sustainable Nanocellulose and Nanohydrogels from Natural Sources; Mohammad, F., Al-Lohedan, H.A., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 65–79. [Google Scholar]
- Peng, N.; Wang, Y.; Ye, Q.; Liang, L.; An, Y.; Li, Q.; Chang, C. Biocompatible cellulose-based superabsorbent hydrogels with antimicrobial activity. Carbohydr. Polym. 2016, 137, 59–64. [Google Scholar] [CrossRef]
- Pandey, A.; Jain, D.S. Poly Lactic-Co-Glycolic Acid (PLGA) copolymer and its pharmaceutical application. In Handbook of Polymers for Pharmaceutical Technologies: Processing and Applications; Scrivener Publishing LLC: Beverly, MA, USA, 2015; Volume 2, pp. 151–172. [Google Scholar]
- Wang, L.; Zhang, C.; Zhao, W.; Li, W.; Wang, G.; Zhou, X.; Zhang, Q. Water-Swellable Cellulose Nanofiber Aerogel for Control of Hemorrhage from Penetrating Wounds. ACS Appl. Bio Mater. 2022, 5, 4886–4895. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Kalita, N.K.; Bhasney, S.M.; Mudenur, C.; Kalamdhad, A.; Katiyar, V. End-of-life evaluation and biodegradation of Poly(lactic acid) (PLA)/Polycaprolactone (PCL)/Microcrystalline cellulose (MCC) polyblends under composting conditions. Chemosphere 2020, 247, 125875. [Google Scholar] [CrossRef] [PubMed]






| Type of Wound | Cellulose-Based Dressing | Mechanism of Action | Therapeutic Role | Ref. |
|---|---|---|---|---|
| Abrasions | Oxidized cellulose | Matrix for fibrin deposition Platelet aggregation | Reduces infection Re-epithelialization | [21,22] |
| Lacerations | Sponge-based hemostatic | Physical barrier Activation platelets Red blood cell activation | Accelerates clotting Tissue repair | [23,24] |
| Surgical Incisions | Carboxymethyl cellulose (CMC) | Formation of gel-like structure upon contact with exudate | Maintains moisture Reduces infection Promotes granulation | [25] |
| Burn Wounds | Nanocellulose-based hydrogels | Mimicking structure of extracellular matrix | Reduces pain and infection Tissue regeneration and recovery | [26,27,28] |
| Pressure Ulcers Chronic Wounds | Bacterial cellulose | Three-dimensional subtract cell fixation Mimicking the extracellular matrix | High fluid retention Tissue regeneration Angiogenesis Collagen formation | [29,30] |
| Chronic Wounds | Cellulose acetate | Modulates immune response Maintains a moist environment | Antibacterial Tissue repair and skin cell proliferation | [31,32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukatuka, C.F.; Mbituyimana, B.; Xiao, L.; Qaed Ahmed, A.A.; Qi, F.; Adhikari, M.; Shi, Z.; Yang, G. Recent Trends in the Application of Cellulose-Based Hemostatic and Wound Healing Dressings. J. Funct. Biomater. 2025, 16, 151. https://doi.org/10.3390/jfb16050151
Bukatuka CF, Mbituyimana B, Xiao L, Qaed Ahmed AA, Qi F, Adhikari M, Shi Z, Yang G. Recent Trends in the Application of Cellulose-Based Hemostatic and Wound Healing Dressings. Journal of Functional Biomaterials. 2025; 16(5):151. https://doi.org/10.3390/jfb16050151
Chicago/Turabian StyleBukatuka, Clemence Futila, Bricard Mbituyimana, Lin Xiao, Abeer Ahmed Qaed Ahmed, Fuyu Qi, Manjilla Adhikari, Zhijun Shi, and Guang Yang. 2025. "Recent Trends in the Application of Cellulose-Based Hemostatic and Wound Healing Dressings" Journal of Functional Biomaterials 16, no. 5: 151. https://doi.org/10.3390/jfb16050151
APA StyleBukatuka, C. F., Mbituyimana, B., Xiao, L., Qaed Ahmed, A. A., Qi, F., Adhikari, M., Shi, Z., & Yang, G. (2025). Recent Trends in the Application of Cellulose-Based Hemostatic and Wound Healing Dressings. Journal of Functional Biomaterials, 16(5), 151. https://doi.org/10.3390/jfb16050151









