In(Ga)N Nanostructures and Devices Grown by Molecular Beam Epitaxy and Metal-Assisted Photochemical Etching
Abstract
:1. Introduction
2. Synthesis Methods for (In)GaN Nanostructures
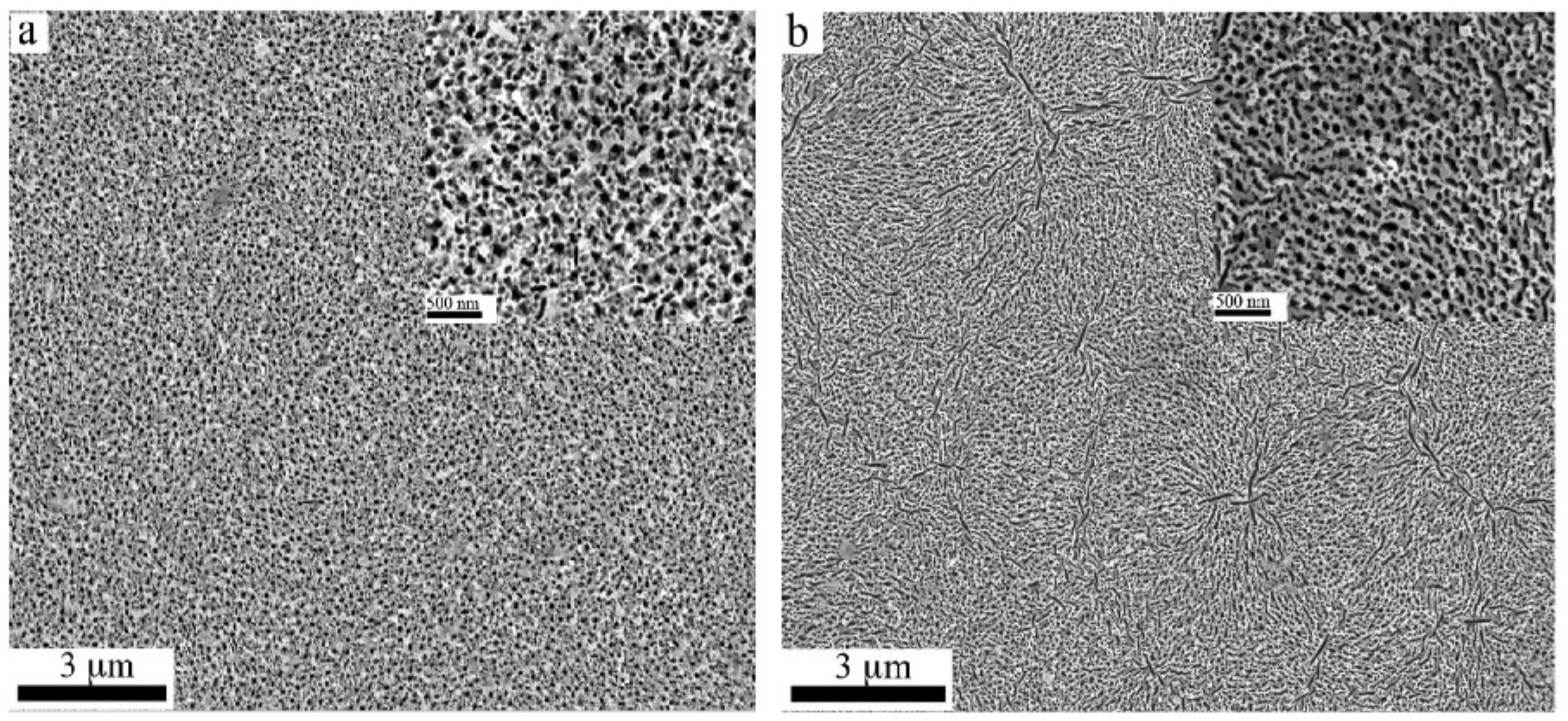
2.1. GaN and InGaN: Porous and Nanowire Structures Prepared via MacEtch
2.2. Exfoliation of Porous Structure and NWs
2.3. Nitride NWs Grown by MBE
3. Applications of (In)GaN Nanostructures
3.1. Application to Gas Sensors
3.2. Application of Nitride Nanostructures in LEDs
3.3. Application of Nitride Nanostructure in Lasers
4. Challenges and Future Directions
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.Q. When group-III nitrides go infrared: New properties and perspectives. J. Appl. Phys. 2009, 106, 28. [Google Scholar] [CrossRef]
- Sirbuly, D.J.; Law, M.; Yan, H.Q.; Yang, P.D. Semiconductor nanowires for subwavelength photonics integration. J. Phys. Chem. B 2005, 109, 15190–15213. [Google Scholar] [CrossRef] [PubMed]
- Ponce, F.; Bour, D. Nitride-based semiconductors for blue and green light-emitting devices. Nature 1997, 386, 351–359. [Google Scholar] [CrossRef]
- Bain, L.E.; Ivanisevic, A.J.S. Engineering the Cell–Semiconductor Interface: A Materials Modification Approach using II–VI and III–V Semiconductor Materials. Small 2015, 11, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Chu, B.H.; Chen, K.H.; Chang, C.Y.; Chen, V.; Pearton, S.J. GaN-Based Sensors. In GaN and ZnO-Based Materials and Devices; Pearton, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 165–207. [Google Scholar] [CrossRef]
- Baek, S.-H.; Lee, H.-J.; Lee, S.-N. High-performance flat-type InGaN-based light-emitting diodes with local breakdown conductive channel. Sci. Rep. 2019, 9, 13654. [Google Scholar] [CrossRef] [Green Version]
- Yngman, S.; Lenrick, F.; Liu, Y.-P.; Ren, Z.; Khalilian, M.; Ohlsson, B.J.; Hessman, D.; Samuelson, L.; Timm, R.; Mikkelsen, A. GaN nanowires as probes for high resolution atomic force and scanning tunneling microscopy. Rev. Sci. Instrum. 2019, 90, 103703. [Google Scholar] [CrossRef]
- Routray, S.R.; Lenka, T.R. InGaN-based solar cells: A wide solar spectrum harvesting technology for twenty-first century. CSI Trans. ICT 2018, 6, 83–96. [Google Scholar] [CrossRef]
- Hardy, M.T.; Feezell, D.F.; DenBaars, S.P.; Nakamura, S. Group III-nitride lasers: A materials perspective. Mater. Today 2011, 14, 408–415. [Google Scholar] [CrossRef]
- Ryu, S.-W.; Zhang, Y.; Leung, B.; Yerino, C.; Han, J. Improved photoelectrochemical water splitting efficiency of nanoporous GaN photoanode. Semicond. Sci. Technol. 2011, 27, 015014. [Google Scholar] [CrossRef]
- Hus, J.-W.; Chen, C.-C.; Lee, M.-J.; Liu, H.-H.; Chyi, J.-I.; Huang, M.R.S.; Liu, C.-P.; Wei, T.-C.; He, J.-H.; Lai, K.-Y. Bottom-Up Nano-heteroepitaxy of Wafer-Scale Semipolar GaN on (001) Si. Adv. Mater. 2015, 27, 4845–4850. [Google Scholar] [CrossRef]
- Wang, D.; Pierre, A.; Kibria, M.G.; Cui, K.; Han, X.; Bevan, K.H.; Guo, H.; Paradis, S.; Hakima, A.-R.; Mi, Z. Wafer-level photocatalytic water splitting on GaN nanowire arrays grown by molecular beam epitaxy. Nano Lett. 2011, 11, 2353–2357. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.D.; Priante, D.; Min, J.W.; Subedi, R.C.; Shakfa, M.K.; Ren, Z.J.; Li, K.H.; Lin, R.H.; Zhao, C.; Ng, T.K.; et al. Graded-Index Separate Confinement Heterostructure AlGaN Nanowires: Toward Ultraviolet Laser Diodes Implementation. ACS Photonics 2018, 5, 3305. [Google Scholar] [CrossRef]
- Huo, Q.; Shao, Y.; Wu, Y.; Zhang, B.; Hu, H.; Hao, X. High quality self-separated GaN crystal grown on a novel nanoporous template by HVPE. Sci. Rep. 2018, 8, 3166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Zhang, H.; Li, H.; Zhang, Y.; Yao, Y.; Palmquist, N.; Iza, M.; Speck, J.S.; Nakamura, S.; DenBaars, S.P. Metalorganic chemical vapor deposition grown n-InGaN/n-GaN tunnel junctions for micro-light-emitting diodes with very low forward voltage. Semicond. Sci. Technol. 2020, 35, 125023. [Google Scholar] [CrossRef]
- Choi, K.; Arita, M.; Arakawa, Y. Selective-area growth of thin GaN nanowires by MOCVD. J. Cryst. Growth 2012, 357, 58–61. [Google Scholar] [CrossRef]
- Gradečak, S.; Qian, F.; Li, Y.; Park, H.-G.; Lieber, C.M. GaN nanowire lasers with low lasing thresholds. Appl. Phys. Lett. 2005, 87, 173111. [Google Scholar] [CrossRef] [Green Version]
- Zettler, J.K.; Corfdir, P.; Geelhaar, L.; Riechert, H.; Brandt, O.; Fernández-Garrido, S. Improved control over spontaneously formed GaN nanowires in molecular beam epitaxy using a two-step growth process. Nanotechnology 2015, 26, 445604. [Google Scholar] [CrossRef]
- Ma, Z.; McDowell, D.; Panaitescu, E.; Davydov, A.V.; Upmanyu, M.; Menon, L. Vapor–liquid–solid growth of serrated GaN nanowires: Shape selection driven by kinetic frustration. J. Mater. Chem. C 2013, 1, 7294–7302. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Duan, X.; Cui, Y.; Lieber, C.M. Gallium Nitride Nanowire Nanodevices. Nano Lett. 2002, 2, 101–104. [Google Scholar] [CrossRef]
- He, B.; Yang, Y.; Yuen, M.F.; Chen, X.; Lee, C.S.; Zhang, W. Vertical nanostructure arrays by plasma etching for applications in biology, energy, and electronics. Nano Today 2013, 8, 265–289. [Google Scholar] [CrossRef]
- Haab, A.; Mikulics, M.; Sutter, E.; Jin, J.; Stoica, T.; Kardynal, B.; Rieger, T.; Gruetzmacher, D.; Hardtdegen, H. Evolution and characteristics of GaN nanowires produced via maskless reactive ion etching. Nanotechnology 2014, 25, 255301. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, Y.H.; Xu, B.; Jin, P.; Wang, Z.G. Evolution of InAs nanostructures grown by droplet epitaxy. Appl. Phys. Lett. 2007, 91. [Google Scholar] [CrossRef]
- Zhao, C.; Ng, T.K.; Wei, N.; Prabaswara, A.; Alias, M.S.; Janjua, B.; Shen, C.; Ooi, B.S. Facile formation of high-quality InGaN/GaN quantum-disks-in-nanowires on bulk-metal substrates for high-power light-emitters. Nano Lett. 2016, 16, 1056–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Ng, T.K.; Prabaswara, A.; Conroy, M.; Jahangir, S.; Frost, T.; O’Connell, J.; Holmes, J.D.; Parbrook, P.J.; Bhattacharya, P.; et al. An enhanced surface passivation effect in InGaN/GaN disk-in-nanowire light emitting diodes for mitigating Shockley-Read-Hall recombination. Nanoscale 2015, 7, 16658–16665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabaswara, A.; Min, J.-W.; Zhao, C.; Janjua, B.; Zhang, D.; Albadri, A.M.; Alyamani, A.Y.; Ng, T.K.; Ooi, B.S. Direct Growth of III-Nitride Nanowire-Based Yellow Light-Emitting Diode on Amorphous Quartz Using Thin Ti Interlayer. Nanoscale Res. Lett. 2018, 13, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebaid, M.; Min, J.W.; Zhao, C.; Ng, T.K.; Idriss, H.; Ooi, B.S. Water splitting to hydrogen over epitaxially grown InGaN nanowires on a metallic titanium/silicon template: Reduced interfacial transfer resistance and improved stability to hydrogen. J. Mater. Chem. A 2018, 6, 6920–6933. [Google Scholar] [CrossRef] [Green Version]
- Janjua, B.; Ng, T.K.; Zhao, C.; Prabaswara, A.; Consiglio, G.B.; Priante, D.; Shen, C.; Elafandy, R.T.; Anjum, D.H.; Alhamoud, A.A.; et al. True Yellow Light-Emitting Diodes as Phosphor for Tunable Color-Rendering Index Laser-Based White Light. ACS Photonics 2016, 3, 2089–2095. [Google Scholar] [CrossRef]
- Zhao, C.; Ng, T.K.; Tseng, C.C.; Li, J.; Shi, Y.M.; Wei, N.N.; Zhang, D.L.; Consiglio, G.B.; Prabaswara, A.; Alhamoud, A.A.; et al. InGaN/GaN nanowires epitaxy on large-area MoS2 for high-performance light-emitters. RSC Adv. 2017, 7, 26665–26672. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, P.; Wen, Y.; Zhao, C.; Zhang, J.W.; Manchon, A.; Mi, W.B.; Peng, Y.; Zhang, X.X. Anomalous Hall effect in Fe/Au multilayers. Phys. Rev. B 2016, 94. [Google Scholar] [CrossRef] [Green Version]
- Li, X. Metal assisted chemical etching for high aspect ratio nanostructures: A review of characteristics and applications in photovoltaics. Curr. Opin. Solid State Mater. Sci. 2012, 16, 71–81. [Google Scholar] [CrossRef]
- Vajpeyi, A.; Chua, S.; Tripathy, S.; Fitzgerald, E. Effect of carrier density on the surface morphology and optical properties of nanoporous GaN prepared by UV assisted electrochemical etching. Appl. Phys. Lett. 2007, 91, 083110. [Google Scholar] [CrossRef]
- Vajpeyi, A.P.; Chua, S.J.; Tripathy, S.; Fitzgerald, E.A.; Liu, W.; Chen, P.; Wang, L. High optical quality nanoporous GaN prepared by photoelectrochemical etching. Electrochem. Solid State Lett. 2005, 8, G85. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ryu, S.W.; Yerino, C.; Leung, B.; Sun, Q.; Song, Q.; Cao, H.; Han, J. A conductivity-based selective etching for next generation GaN devices. Phys. Status Solidi (B) 2010, 247, 1713–1716. [Google Scholar] [CrossRef]
- Al-Heuseen, K.; Hashim, M.; Ali, N. Effect of different electrolytes on porous GaN using photo-electrochemical etching. Appl. Surf. Sci. 2011, 257, 6197–6201. [Google Scholar] [CrossRef]
- Cho, H.; Auh, K.; Han, J.; Shul, R.; Donovan, S.; Abernathy, C.; Lambers, E.; Ren, F.; Pearton, S. UV-photoassisted etching of GaN in KOH. J. Electron. Mater. 1999, 28, 290–294. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Geyer, N.; Werner, P.; de Boor, J.; Gösele, U. Metal-Assisted Chemical Etching of Silicon: A Review. Adv. Mater. 2011, 23, 285–308. [Google Scholar] [CrossRef]
- Geng, X.; Duan, B.K.; Grismer, D.A.; Zhao, L.; Bohn, P.W. Catalyst and processing effects on metal-assisted chemical etching for the production of highly porous GaN. Semicond. Sci. Technol. 2013, 28, 065001. [Google Scholar] [CrossRef]
- Duan, B.K.; Bohn, P.W. High sensitivity hydrogen sensing with Pt-decorated porous gallium nitride prepared by metal-assisted electroless etching. Analyst 2010, 135, 902–907. [Google Scholar] [CrossRef]
- Geng, X.; Duan, B.K.; Grismer, D.A.; Zhao, L.; Bohn, P.W. Monodisperse GaN nanowires prepared by metal-assisted chemical etching with in situ catalyst deposition. Electrochem. Commun. 2012, 19, 39–42. [Google Scholar] [CrossRef]
- Qu, Y.; Liao, L.; Li, Y.; Zhang, H.; Huang, Y.; Duan, X. Electrically conductive and optically active porous silicon nanowires. Nano Lett. 2009, 9, 4539–4543. [Google Scholar] [CrossRef] [Green Version]
- Najar, A.; Slimane, A.; Hedhili, M.N.; Anjum, D.; Sougrat, R.; Ng, T.; Ooi, B.S. Effect of hydrofluoric acid concentration on the evolution of photoluminescence characteristics in porous silicon nanowires prepared by Ag-assisted electroless etching method. J. Appl. Phys. 2012, 112, 033502. [Google Scholar] [CrossRef] [Green Version]
- Najar, A.; Charrier, J.; Pirasteh, P.; Sougrat, R. Ultra-low reflection porous silicon nanowires for solar cell applications. Opt. Express 2012, 20, 16861–16870. [Google Scholar] [CrossRef] [Green Version]
- Najar, A.; Charrier, J.; Lorrain, N.; Haji, L.; Oueslati, M. Optical gain measurements in porous silicon planar waveguides codoped by erbium and ytterbium ions at 1.53 μm. Appl. Phys. Lett. 2007, 91, 121120. [Google Scholar] [CrossRef]
- Najar, A.; Charrier, J. Study of Mesoporous Silicon Nanowires. In Comprehensive Guide for Mesoporous Materials. Volume 2: Analysis and Functionalization; Nova Science Publishers: Harpak, NY, USA, 2015; pp. 201–224. ISBN 978-1-63463-982-8. [Google Scholar]
- Najar, A.; Shafa, M.; Anjum, D. Synthesis, optical properties and residual strain effect of GaN nanowires generated via metal-assisted photochemical electroless etching. RSC Adv. 2017, 7, 21697–21702. [Google Scholar] [CrossRef] [Green Version]
- Benton, J.; Bai, J.; Wang, T. Utilisation of GaN and InGaN/GaN with nanoporous structures for water splitting. Appl. Phys. Lett. 2014, 105, 223902. [Google Scholar] [CrossRef]
- Najar, A.; Gerland, M.; Jouiad, M. Porosity-induced relaxation of strains in GaN layers studied by means of micro-indentation and optical spectroscopy. J. Appl. Phys. 2012, 111, 093513. [Google Scholar] [CrossRef] [Green Version]
- Slimane, A.B.; Najar, A.; Ng, T.K.; Ooi, B.S. Thermal annealing induced relaxation of compressive strain in porous GaN structures. In Proceedings of the IEEE Photonics Conference, Burlingame, CA, USA, 23–27 September 2012; pp. 921–922. [Google Scholar] [CrossRef] [Green Version]
- Ooi, B.S.; ElAfandy, R.T.; Gasim, A.A.; Li, Q.; Najar, A.; Ng, T.K.; Ben Slimane, A. GaN-Based Nano-Pores and Nano-Wires Fabricated Using Electroless Chemical Etching Process; The Electrochemical Society: Pennington, NJ, USA, 2012; Volume MA2012-01, p. 822. [Google Scholar] [CrossRef]
- Najar, A.; Slimane, A.; Anjum, D.; Ng, T.; Ooi, B. GaN Nanowires synthesized by electroless etching method. In Proceedings of the Quantum Electronics and Laser Science Conference, San Jose, CA, USA, 6–11 May 2012; p. JTh2A-101. [Google Scholar] [CrossRef] [Green Version]
- Perumal, R.; Hassan, Z. Nanoporous Gallium Nitride Through Anisotropic Metal-Assisted Electroless Photochemical Wet Etching Technique. Surf. Rev. Lett. 2016, 23, 1550106. [Google Scholar] [CrossRef]
- Zhang, M.-R.; Jiang, Q.-M.; Zhang, S.-H.; Wang, Z.-G.; Hou, F.; Pan, G.-B. Fabrication of gallium nitride nanowires by metal-assisted photochemical etching. Appl. Surf. Sci. 2017, 422, 216–220. [Google Scholar] [CrossRef]
- Wang, Q.; Yuan, G.; Zhao, S.; Liu, W.; Liu, Z.; Wang, J.; Li, J. Metal-assisted photochemical etching of GaN nanowires: The role of metal distribution. Electrochem. Commun. 2019, 103, 66–71. [Google Scholar] [CrossRef]
- Wang, K.; Yuan, G.; Wu, R.; Lu, H.; Liu, Z.; Wei, T.; Wang, J.; Li, J.; Zhang, W. GaN nanowire arrays by a patterned metal-assisted chemical etching. J. Cryst. Growth 2016, 440, 96–101. [Google Scholar] [CrossRef]
- Yuan, G.; Mitdank, R.d.; Mogilatenko, A.; Fischer, S.F. Porous nanostructures and thermoelectric power measurement of electro-less etched black silicon. J. Phys. Chem. C 2012, 116, 13767–13773. [Google Scholar] [CrossRef]
- Li, X.; Bohn, P. Metal-assisted chemical etching in HF/H2O2 produces porous silicon. Appl. Phys. Lett. 2000, 77, 2572–2574. [Google Scholar] [CrossRef]
- Li, X.; Kim, Y.-W.; Bohn, P.W.; Adesida, I. In-plane bandgap control in porous GaN through electroless wet chemical etching. Appl. Phys. Lett. 2002, 80, 980–982. [Google Scholar] [CrossRef]
- DeJarld, M.; Shin, J.C.; Chern, W.; Chanda, D.; Balasundaram, K.; Rogers, J.A.; Li, X. Formation of High Aspect Ratio GaAs Nanostructures with Metal-Assisted Chemical Etching. Nano Lett. 2011, 11, 5259–5263. [Google Scholar] [CrossRef] [PubMed]
- Radzali, R.; Zainal, N.; Yam, F.K.; Hassan, Z. Nanoporous InGaN of high In composition prepared by KOH electrochemical etching. Mater. Sci. Semicond. Process. 2013, 16, 2051–2057. [Google Scholar] [CrossRef]
- Najar, A.; Jouiad, M. Synthesis of InGaN nanowires via metal-assisted photochemical electroless etching for solar cell application. Sol. Energy Mater. Sol. Cells 2018, 180, 243–246. [Google Scholar] [CrossRef]
- Dorogan, V.; Vieru, T.; Tigyneanu, I.; Hartnagel, H.; Mutamba, C.; Sturza, R. Photoelectrochemical etching of GaN-AlGaN heterostructures formed on sapphire substrates. Mold. J. Phys. Sci. 2002, 1, 156–159. [Google Scholar]
- Chen, D.; Xiao, H.; Han, J. Nanopores in GaN by electrochemical anodization in hydrofluoric acid: Formation and mechanism. J. Appl. Phys. 2012, 112, 064303. [Google Scholar] [CrossRef]
- Senapati, B.; Samanta, S.; Maikap, S.; Bera, L.; Maiti, C. Effects of nitric-oxide-plasma treatment on the electrical properties of tetraethylorthosilicate-deposited silicon dioxides on strained-Si 1− x Ge x layers. Appl. Phys. Lett. 2000, 77, 1840–1842. [Google Scholar] [CrossRef]
- Leung, K.; Wright, A.; Stechel, E. Charge accumulation at a threading edge dislocation in gallium nitride. Appl. Phys. Lett. 1999, 74, 2495–2497. [Google Scholar] [CrossRef] [Green Version]
- Varley, J.; Weber, J.; Janotti, A.; Van de Walle, C. Erratum: Oxygen vacancies and donor impurities in β-Ga2O3. Appl. Phys. Lett. 2016, 108, 039901. [Google Scholar] [CrossRef] [Green Version]
- Vajpeyi, A.P.; Tripathy, S.; Chua, S.J.; Fitzgerald, E.A. Investigation of optical properties of nanoporous GaN films. Phys. E Low Dimens. Syst. Nanostruct. 2005, 28, 141–149. [Google Scholar] [CrossRef]
- Chuah, L.; Hassan, Z.; Hassan, H.A. Optical characterization of nanoporous GaN through electroless wet chemical etching. Mater. Sci. Pol. 2008, 26, 609–615. [Google Scholar]
- Díaz, D.J.; Williamson, T.L.; Adesida, I.; Bohn, P.W.; Molnar, R.J. Morphology and luminescence of porous GaN generated via Pt-assisted electroless etching. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2002, 20, 2375–2383. [Google Scholar] [CrossRef]
- Chuah, L.; Hassan, Z.; Chin, C.; Hassan, H.A. Surface morphology and formation of nanostructured porous GaN by UV-assisted electrochemical etching. World Acad. Sci. Eng. Technol. 2009, 55, 16. [Google Scholar] [CrossRef]
- Nie, B.; Duan, B.K.; Bohn, P.W. Nanoporous GaN–Ag composite materials prepared by metal-assisted electroless etching for direct laser desorption-ionization mass spectrometry. ACS Appl. Mater. Interfaces 2013, 5, 6208–6215. [Google Scholar] [CrossRef]
- Shah, A.Z.; Noor, N.H.M.; Hassan, Z.; Mahmood, A.; Kwong, Y.F. Effect of porosity on GaN for hydrogen gas sensing. Malays. J. Fundam. Appl. Sci. 2012, 8. [Google Scholar] [CrossRef] [Green Version]
- Nie, B.; Duan, B.K.; Bohn, P.W. Nanoporous Ag–GaN thin films prepared by metal-assisted electroless etching and deposition as three-dimensional substrates for surface-enhanced Raman scattering. J. Raman Spectrosc. 2012, 43, 1347–1353. [Google Scholar] [CrossRef]
- Mahmood, A.; Hassan, Z.; Ahmed, N.M.; Yam, F.K.; Chuah, L.S.; Mokhtar, M.; Mohd Noor, N.H.; Rosli, S.A. Structural and Optical Studies of Undoped Porous GaN Prepared by Pt-Assisted Electroless Etching. Mater. Sci. Forum 2016, 846, 358–365. [Google Scholar] [CrossRef]
- Williamson, T.L.; Díaz, D.J.; Bohn, P.W.; Molnar, R.J. Structure–Property relationships in porous GaN generated by Pt-assisted electroless etching studied by Raman spectroscopy. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2004, 22, 925–931. [Google Scholar] [CrossRef]
- Kim, B.-M.; Reddy, M.S.P.; Lee, Y.-W.; Park, C. Enhanced surface and optical properties of colloidal silver nano-particles on GaN-based light-emitting diodes by a localized surface plasmon resonance effect using a low-cost metal-assisted chemical etching method. Opt. Commun. 2019, 450, 276–281. [Google Scholar] [CrossRef]
- Zhang, M.-R.; Wang, F.-X.; Pan, G.-B. Metal-assisted photochemical etching of gallium nitride using electrodeposited noble metal nanoparticles as catalysts. Electrochem. Commun. 2017, 76, 59–62. [Google Scholar] [CrossRef]
- Aravindh, S.A.; Xin, B.; Mitra, S.; Roqan, I.S.; Najar, A. GaN and InGaN nanowires prepared by metal-assisted electroless etching: Experimental and theoretical studies. Results Phys. 2020, 19, 103428. [Google Scholar] [CrossRef]
- Al-Jumaili, B.E.; Talib, Z.A.; Paiman, S.B.; Ahmed, N.M.; Al-Jumaily, A.H.; Ramizy, A.; Abdulateef, S.A.; Muh’d, I.B.; Mofdal, M.E. The Correlation of Blue Shift of Photoluminescence and Morphology of Silicon Nanoporous. AIP Conf. Proc. 2016, 1733, 020019. [Google Scholar] [CrossRef]
- Livneh, T.; Zhang, J.; Cheng, G.; Moskovits, M. Polarized Raman scattering from single GaN nanowires. Phys. Rev. B 2006, 74, 035320. [Google Scholar] [CrossRef]
- Xi, X.; Li, J.; Ma, Z.; Li, X.; Zhao, L. Enhanced water splitting performance of GaN nanowires fabricated using anode aluminum oxide templates. RSC Adv. 2019, 9, 14937–14943. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Xu, S.; Gao, B.; Pan, L.J.N. PL Tunable GaN Nanoparticles Synthesis through Femtosecond Pulsed Laser Ablation in Different Environments. Nanomaterials 2020, 10, 439. [Google Scholar] [CrossRef] [Green Version]
- Syed, N.; Zavabeti, A.; Messalea, K.A.; Della Gaspera, E.; Elbourne, A.; Jannat, A.; Mohiuddin, M.; Zhang, B.Y.; Zheng, G.; Wang, L. Wafer-sized ultrathin gallium and indium nitride nanosheets through the ammonolysis of liquid metal derived oxides. J. Am. Chem. Soc. 2018, 141, 104–108. [Google Scholar] [CrossRef]
- Jayaprakash, R.; Kalaitzakis, F.; Kayambaki, M.; Tsagaraki, K.; Monroy, E.; Pelekanos, N.T. Ultra-smooth GaN membranes by photo-electrochemical etching for photonic applications. J. Mater. Sci. 2014, 49, 4018–4024. [Google Scholar] [CrossRef]
- Schubert, T.; Steinhoff, G.; von Ribbeck, H.-G.; Stutzmannn, M.; Eickhoff, M.; Tanaka, M. Gallium nitride electrodes for membrane-based electrochemical biosensors. Eur. Phys. J. E 2009, 30, 233–238. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lin, D.-W.; Liu, C.-Y.; Hsu, S.-C.; Kuo, H.-C.; Wang, S.-C.; Chang, C.-Y. A study of mechanical lift-off technology for high-efficiency vertical LEDs using Micro-Porous GaN template. In Proceedings of the CLEO: 2013, San Jose, CA, USA, 9–14 June 2013; pp. 1–2. [Google Scholar] [CrossRef]
- Zhang, Y.; Leung, B.; Han, J. A liftoff process of GaN layers and devices through nanoporous transformation. Appl. Phys. Lett. 2012, 100, 181908. [Google Scholar] [CrossRef]
- Seo, J.-H.; Swinnich, E.; Zhang, Y.-Y.; Kim, M. Low dimensional freestanding semiconductors for flexible optoelectronics: Materials, synthesis, process, and applications. Mater. Res. Lett. 2020, 8, 123–144. [Google Scholar] [CrossRef]
- Doan, M.; Kim, S.; Lee, J.; Lim, H.; Rotermund, F.; Kim, K. Influence of laser lift-off on optical and structural properties of InGaN/GaN vertical blue light emitting diodes. AIP Adv. 2012, 2, 022122. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.; Lee, C.-H.; Yi, G.-C. Transferable GaN layers grown on ZnO-coated graphene layers for optoelectronic devices. Science 2010, 330, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Kumakura, K.; Akasaka, T.; Makimoto, T. Layered boron nitride as a release layer for mechanical transfer of GaN-based devices. Nature 2012, 484, 223–227. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Huang, T.-W.; Wu, C.-L.; Chen, M.K.; Chu, C.H.; Wu, Y.-R.; Shih, M.-H.; Lee, C.-K.; Kuo, H.-C.; Tsai, D.P. Transferring the bendable substrateless GaN LED grown on a thin C-rich SiC buffer layer to flexible dielectric and metallic plates. J. Mater. Chem. C 2017, 5, 607–617. [Google Scholar] [CrossRef]
- Voronenkov, V.; Bochkareva, N.; Gorbunov, R.; Zubrilov, A.; Kogotkov, V.; Latyshev, P.; Lelikov, Y.; Leonidov, A.; Shreter, Y. Laser slicing: A thin film lift-off method for GaN-on-GaN technology. Results Phys. 2019, 13, 102233. [Google Scholar] [CrossRef]
- Xiao, H.; Cui, J.; Cao, D.; Gao, Q.; Liu, J.; Ma, J. Self-standing nanoporous GaN membranes fabricated by UV-assisted electrochemical anodization. Mater. Lett. 2015, 145, 304–307. [Google Scholar] [CrossRef]
- Yoo, H.G.; Park, K.-I.; Koo, M.; Kim, S.; Lee, S.Y.; Lee, S.H.; Lee, K.J. Flexible GaN LED on a polyimide substrate for display applications. In Proceedings of the Quantum Sensing and Nanophotonic Devices IX, San Francisco, CA, USA, 20 January 2012; p. 82681Y. [Google Scholar] [CrossRef]
- Chun, J.; Hwang, Y.; Choi, Y.-S.; Jeong, T.; Baek, J.H.; Ko, H.C.; Park, S.-J. Transfer of GaN LEDs from sapphire to flexible substrates by laser lift-off and contact printing. IEEE Photonics Technol. Lett. 2012, 24, 2115–2118. [Google Scholar] [CrossRef]
- Bharrat, D.; Hosalli, A.; Van Den Broeck, D.; Samberg, J.; Bedair, S.; El-Masry, N. Gallium nitride nanowires by maskless hot phosphoric wet etching. Appl. Phys. Lett. 2013, 103, 082106. [Google Scholar] [CrossRef]
- ElAfandy, R.T.; Majid, M.A.; Ng, T.K.; Zhao, L.; Cha, D.; Ooi, B.S. Exfoliation of Threading Dislocation-Free, Single-Crystalline, Ultrathin Gallium Nitride Nanomembranes. Adv. Funct. Mater. 2014, 24, 2305–2311. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, S.N.; Kim, W.; Salvador, A.; Morkoc, H. Reactive molecular-beam epitaxy for Wurtzite GaN. MRS Bull. 1997, 22, 22–28. [Google Scholar] [CrossRef]
- Zulkifli, N.A.A.; Park, K.; Min, J.-W.; Ooi, B.S.; Zakaria, R.; Kim, J.; Tan, C.L. A highly sensitive, large area, and self-powered UV photodetector based on coalesced gallium nitride nanorods/graphene/silicon (111) heterostructure. Appl. Phys. Lett. 2020, 117, 191103. [Google Scholar] [CrossRef]
- NSM Archive—Gallium Nitride (GaN). Available online: http://www.ioffe.ru/SVA/NSM/Semicond/GaN/index.html (accessed on 17 September 2020).
- NSM Archive—Aluminium Nitride (AlN). Available online: http://www.ioffe.ru/SVA/NSM/Semicond/AlN/index.html (accessed on 17 September 2020).
- NSM Archive—Indium Nitride (InN). Available online: http://www.ioffe.ru/SVA/NSM/Semicond/InN/index.html (accessed on 17 September 2020).
- Liu, L.; Edgar, J.H. Substrates for gallium nitride epitaxy. Mater. Sci. Eng. R Rep. 2002, 37, 61–127. [Google Scholar] [CrossRef]
- NSM Archive—Silicon Carbide (SiC). Available online: http://www.ioffe.ru/SVA/NSM/Semicond/SiC/index.html (accessed on 17 September 2020).
- Physical Properties of Silicon (Si). Available online: http://www.ioffe.ru/SVA/NSM/Semicond/Si/index.html (accessed on 13 October 2020).
- Yang, P.; Yan, R.; Fardy, M. Semiconductor Nanowire: What’s Next? Nano Lett. 2010, 10, 1529–1536. [Google Scholar] [CrossRef]
- Kuykendall, T.; Ulrich, P.; Aloni, S.; Yang, P. Complete composition tunability of InGaN nanowires using a combinatorial approach. Nat. Mater. 2007, 6, 951–956. [Google Scholar] [CrossRef]
- Calarco, R.; Meijers, R.J.; Debnath, R.K.; Stoica, T.; Sutter, E.; Lüth, H. Nucleation and Growth of GaN Nanowires on Si(111) Performed by Molecular Beam Epitaxy. Nano Lett. 2007, 7, 2248–2251. [Google Scholar] [CrossRef]
- Chang, Y.L.; Wang, J.L.; Li, F.; Mi, Z. High efficiency green, yellow, and amber emission from InGaN/GaN dot-in-a-wire heterostructures on Si(111). Appl. Phys. Lett. 2010, 96, 013106. [Google Scholar] [CrossRef]
- Li, S.; Waag, A. GaN based nanorods for solid state lighting. J. Appl. Phys. 2012, 111, 5. [Google Scholar] [CrossRef]
- Ristić, J.; Calleja, E.; Fernández-Garrido, S.; Cerutti, L.; Trampert, A.; Jahn, U.; Ploog, K.H. On the mechanisms of spontaneous growth of III-nitride nanocolumns by plasma-assisted molecular beam epitaxy. J. Cryst Growth 2008, 310, 4035–4045. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Kikuchi, A.; Mori, M.; Fujita, N.F.N.; Kishino, K.K.K. Growth of Self-Organized GaN Nanostructures on Al2O3(0001) by RF-Radical Source Molecular Beam Epitaxy. Jpn. J. Appl. Phys. 1997, 36, L459. [Google Scholar] [CrossRef]
- Araki, T.; Chiba, Y.; Nobata, M.; Nishioka, Y.; Nanishi, Y. Structural characterization of GaN grown by hydrogen-assisted ECR-MBE using electron microscopy. J. Cryst. Growth 2000, 209, 368–372. [Google Scholar] [CrossRef]
- Namkoong, G.; Doolittle, W.A.; Brown, A.S.; Losurdo, M.; Capezzuto, P.; Bruno, G. Role of sapphire nitridation temperature on GaN growth by plasma assisted molecular beam epitaxy: Part I. Impact of the nitridation chemistry on material characteristics. J. Appl. Phys. 2002, 91, 2499–2507. [Google Scholar] [CrossRef]
- Kikuchi, A.; Yamano, K.; Tada, M.; Kishino, K. Stimulated emission from GaN nanocolumns. Phys. Status Solidi (B) 2004, 241, 2754–2758. [Google Scholar] [CrossRef]
- Ramesh, C.; Tyagi, P.; Yadav, B.S.; Ojha, S.; Maurya, K.K.; Senthil Kumar, M.; Kushvaha, S.S. Effect of nitridation temperature on formation and properties of GaN nanowall networks on sapphire (0001) grown by laser MBE. Mater. Sci. Eng. B 2018, 231, 105–114. [Google Scholar] [CrossRef]
- Kikuchi, A.; Kawai, M.; Tada, M.; Kishino, K. InGaN/GaN Multiple Quantum Disk Nanocolumn Light-Emitting Diodes Grown on (111) Si Substrate. Jpn. J. Appl. Phys. 2004, 43, L1524. [Google Scholar] [CrossRef]
- Bertness, K.A.; Sanford, N.A.; Barker, J.; Schlager, J.B.; Roshko, A.; Davydov, A.; Levin, I. Catalyst-free growth of GaN nanowires. J. Electron. Mater. 2006, 35, 576–580. [Google Scholar] [CrossRef]
- Meijers, R.; Richter, T.; Calarco, R.; Stoica, T.; Bochem, H.-P.; Marso, M.; Lüth, H. GaN-nanowhiskers: MBE-growth conditions and optical properties. J. Cryst. Growth 2006, 289, 381–386. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, M.; Banerjee, A.; Bhattacharya, P. Catalyst-free InGaN/GaN nanowire light emitting diodes grown on (001) silicon by molecular beam epitaxy. Nano Lett. 2010, 10, 3355–3359. [Google Scholar] [CrossRef]
- Mi, Z.; Zhao, S.; Woo, S.Y.; Bugnet, M.; Djavid, M.; Liu, X.; Kang, J.; Kong, X.; Ji, W.; Guo, H.; et al. Molecular beam epitaxial growth and characterization of Al(Ga)N nanowire deep ultraviolet light emitting diodes and lasers. J. Phys. D Appl. Phys. 2016, 49, 364006. [Google Scholar] [CrossRef]
- Kruse, J.; Lymperakis, L.; Eftychis, S.; Adikimenakis, A.; Doundoulakis, G.; Tsagaraki, K.; Androulidaki, M.; Olziersky, A.; Dimitrakis, P.; Ioannou-Sougleridis, V. Selective-area growth of GaN nanowires on SiO2-masked Si (111) substrates by molecular beam epitaxy. J. Appl. Phys. 2016, 119, 224305. [Google Scholar] [CrossRef]
- Schuster, F.; Hetzl, M.; Weiszer, S.; Garrido, J.A.; De La Mata, M.; Magen, C.; Arbiol, J.; Stutzmann, M. Position-controlled growth of GaN nanowires and nanotubes on diamond by molecular beam epitaxy. Nano Lett. 2015, 15, 1773–1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reznik, R.; Kotlyar, K.; Ilkiv, I.; Soshnikov, I.; Kukushkin, S.; Osipov, A.; Nikitina, E.; Cirlin, G. MBE growth and optical properties of GaN nanowires on SiC/Si(111) hybrid substrate. AIP Conf. Proc. 2016, 1748, 040003. [Google Scholar] [CrossRef]
- Haouzi, P.; Sonobe, T.; Judenherc-Haouzi, A. Hydrogen sulfide intoxication induced brain injury and methylene blue. Neurobiol. Dis. 2019, 133, 104474. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, S.; Dhakshinamoorthy, J.; Mohan, V.; Ponnuvelu, D.V.; Kallidaikuruchi, V.K.; Thalakkotil, L.M.; Pullithadathil, B. Development of low-cost hybrid multi-walled carbon nanotube-based ammonia gas-sensing strips with an integrated sensor read-out system for clinical breath analyzer applications. J. Breath Res. 2019, 13, 046005. [Google Scholar] [CrossRef]
- Fu, X.; Yang, P.; Xiao, X.; Zhou, D.; Huang, R.; Zhang, X.; Cao, F.; Xiong, J.; Hu, Y.; Tu, Y. Ultra-fast and highly selective room-temperature formaldehyde gas sensing of Pt-decorated MoO3 nanobelts. J. Alloys Compd. 2019, 797, 666–675. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, W.; Gao, N.; Zhao, R.; Ahmed, M.M.; Zhang, T.; Li, J.; Du, J.; Asefa, T. Highly sensitive and selective gas-phase ethanolamine sensor by doping sulfur into nanostructured ZnO. Sens. Actuators B Chem. 2019, 296, 126633. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Wu, N.; Zhang, Y.; Svoronos, S.; Pullammanappallil, P. Low-cost, Arduino-based, portable device for measurement of methane composition in biogas. Renew. Energy 2019, 138, 224–229. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, V.; Singh, R. Formation of ultralong GaN nanowires up to millimeter length scale and photoconduction study in single nanowire. Scr. Mater. 2017, 138, 75–78. [Google Scholar] [CrossRef]
- Song, W.; Wang, X.; Xia, C.; Wang, R.; Zhao, L.; Guo, D.; Chen, H.; Xiao, J.; Su, S.; Li, S. Improved photoresponse of a-axis GaN microwire/p-polymer hybrid photosensor by the piezo-phototronic effect. Nano Energy 2017, 33, 272–279. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, H.; Chen, S.; Li, Z.; Li, S.; Wang, M.; Zhu, H.; Chen, X. In Situ Growth of Leakage-Free Direct-Bridging GaN Nanowires: Application to Gas Sensors for Long-Term Stability, Low Power Consumption, and Sub-ppb Detection Limit. Nano Lett. 2019, 19, 3448–3456. [Google Scholar] [CrossRef] [PubMed]
- Reddeppa, M.; Park, B.-G.; Kim, M.-D.; Peta, K.R.; Chinh, N.D.; Kim, D.; Kim, S.-G.; Murali, G. H2, H2S gas sensing properties of rGO/GaN nanorods at room temperature: Effect of UV illumination. Sens. Actuators B Chem. 2018, 264, 353–362. [Google Scholar] [CrossRef]
- Abdullah, Q.; Yam, F.; Hassan, Z.; Bououdina, M. Hydrogen gas sensing performance of GaN nanowires-based sensor at low operating temperature. Sens. Actuators B Chem. 2014, 204, 497–506. [Google Scholar] [CrossRef]
- Pearton, S.; Ren, F.; Wang, Y.-L.; Chu, B.; Chen, K.; Chang, C.; Lim, W.; Lin, J.; Norton, D. Recent advances in wide bandgap semiconductor biological and gas sensors. Prog. Mater. Sci. 2010, 55, 1–59. [Google Scholar] [CrossRef]
- Espid, E.; Taghipour, F. UV-LED photo-activated chemical gas sensors: A review. Crit. Rev. Solid State Mater. Sci. 2017, 42, 416–432. [Google Scholar] [CrossRef]
- Shafa, M.; Priante, D.; ElAfandy, R.T.; Hedhili, M.N.; Mahmoud, S.T.; Ng, T.K.; Ooi, B.S.; Najar, A. Twofold Porosity and Surface Functionalization Effect on Pt–Porous GaN for High-Performance H2-Gas Sensors at Room Temperature. ACS Omega 2019, 4, 1678–1684. [Google Scholar] [CrossRef] [Green Version]
- Shafa, M.; Aravindh, S.A.; Hedhili, M.N.; Mahmoud, S.T.; Pan, Y.; Ng, T.K.; Ooi, B.S.; Najar, A. Improved H2 detection performance of GaN sensor with Pt/Sulfide treatment of porous active layer prepared by metal electroless etching. Int. J. Hydrog. Energy 2020. in Press. [Google Scholar] [CrossRef]
- Kishino, K.; Kamimura, J.; Kamiyama, K. Near-Infrared InGaN Nanocolumn Light-Emitting Diodes Operated at 1.46 µm. Appl. Phys. Express 2012, 5, 031001. [Google Scholar] [CrossRef]
- Nguyen, H.P.T.; Cui, K.; Zhang, S.; Fathololoumi, S.; Mi, Z. Full-color InGaN/GaN dot-in-a-wire light emitting diodes on silicon. Nanotechnology 2011, 22, 445202. [Google Scholar] [CrossRef]
- Philip, M.R.; Choudhary, D.D.; Djavid, M.; Bhuyian, M.N.; Piao, J.; Pham, T.T.; Misra, D.; Nguyen, H.P.T. Controlling color emission of InGaN/AlGaN nanowire light-emitting diodes grown by molecular beam epitaxy. J. Vac. Sci. Technol. B 2017, 35, 02B108. [Google Scholar] [CrossRef]
- Zhao, C.; Ng, T.K.; El Afandy, R.T.; Prabaswara, A.; Consiglio, G.B.; Ajia, I.A.; Roqan, I.S.; Janjua, B.; Shen, C.; Eid, J.; et al. Droop-Free, Reliable, and High-Power InGaN/GaN Nanowire Light-Emitting Diodes for Monolithic Metal-Optoelectronics. Nano Lett. 2016, 16, 4616–4623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frost, T.; Jahangir, S.; Stark, E.; Deshpande, S.; Hazari, A.; Zhao, C.; Ooi, B.S.; Bhattacharya, P. Monolithic Electrically Injected Nanowire Array Edge-Emitting Laser on (001) Silicon. Nano Lett. 2014, 14, 4535–4541. [Google Scholar] [CrossRef]
- Chen, F.; Ji, X.H.; Lau, S.P. Recent progress in group III-nitride nanostructures: From materials to applications. Mat. Sci. Eng. R 2020, 142. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lin, H.-W.; Shen, C.-H.; Gwo, S. Structure and photoluminescence properties of epitaxially oriented GaN nanorods grown on Si (111) by plasma-assisted molecular-beam epitaxy. Appl. Phys. Lett. 2006, 89, 243105. [Google Scholar] [CrossRef] [Green Version]
- Hazari, A.; Aiello, A.; Ng, T.-K.; Ooi, B.S.; Bhattacharya, P. III-nitride disk-in-nanowire 1.2 mu m monolithic diode laser on (001) silicon. Appl. Phys. Lett. 2015, 107. [Google Scholar] [CrossRef] [Green Version]
- Jahangir, S.; Frost, T.; Hazari, A.; Yan, L.; Stark, E.; LaMountain, T.; Millunchick, J.M.; Ooi, B.S.; Bhattacharya, P. Small signal modulation characteristics of red-emitting (lambda=610 nm) III-nitride nanowire array lasers on (001) silicon. Appl. Phys. Lett. 2015, 106. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Woo, S.Y.; Bugnet, M.; Liu, X.; Kang, J.; Botton, G.A.; Mi, Z. Three-Dimensional Quantum Confinement of Charge Carriers in Self-Organized AlGaN Nanowires: A Viable Route to Electrically Injected Deep Ultraviolet Lasers. Nano Lett. 2015, 15, 7801–7807. [Google Scholar] [CrossRef]
- Li, K.; Liu, X.; Wang, Q.; Zhao, S.; Mi, Z. Ultralow-threshold electrically injected AlGaN nanowire ultraviolet lasers on Si operating at low temperature. Nat. Nanotechnol. 2015, 10, 140–144. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, X.; Woo, S.Y.; Kang, J.; Botton, G.A.; Mi, Z. An electrically injected AlGaN nanowire laser operating in the ultraviolet-C band. Appl. Phys. Lett. 2015, 107, 043101. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, X.; Wu, Y.; Mi, Z. An electrically pumped 239 nm AlGaN nanowire laser operating at room temperature. Appl. Phys. Lett. 2016, 109, 5. [Google Scholar] [CrossRef]
- Mohseni, P.K.; Hyun Kim, S.; Zhao, X.; Balasundaram, K.; Dong Kim, J.; Pan, L.; Rogers, J.A.; Coleman, J.J.; Li, X. GaAs pillar array-based light emitting diodes fabricated by metal-assisted chemical etching. J. Appl. Phys. 2013, 114, 064909. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-L.; Tsujino, K.; Kanda, Y.; Ikeda, S.; Matsumura, M. Pore formation in silicon by wet etching using micrometre-sized metal particles as catalysts. J. Mater. Chem. 2008, 18, 1015–1020. [Google Scholar] [CrossRef]
- Huang, Z.; Geyer, N.; Liu, L.; Li, M.; Zhong, P. Metal-assisted electrochemical etching of silicon. Nanotechnology 2010, 21, 465301. [Google Scholar] [CrossRef] [PubMed]


















| GaN Substrate | Etching Solution | Etching Time (min) | Parameters of the Structures | Ref. | |
|---|---|---|---|---|---|
| Porous GaN | MOCVD-grown, Si-doped, carrier conc. 1.2 × 1018 cm−3 | CH3OH:HF:H2O2 (1:4:1) | 60–120 | D:55–60 nm | [67] |
| MBE-grown on Si, carrier conc. 4 × 1019 cm−3 | HF:CH3OH:H2O2 (2:1:1) | 10–25–35 | D:80–110 nm | [68] | |
| HVPE on sapphire, n = 3 × 1016 cm−3 | CH3OH:HF: H2O2 (1:2:1) | 60–120 | [69] | ||
| MBE-grown on Si, carrier conc. 4 × 1018 cm−3 | HF:CH3OH:H2O2 (4:1:1) | 10–25–35 | D:80–110 nm | [70] | |
| - | CH3OH:HF: H2O2 (1:2:2) | 60 | D:80–100 nm | [71] | |
| Si-doped grew on sapphire, electron conc. 1–3 × 1018 cm−3 | CH3OH:HF: H2O2 (1:4:1) | - | - | [72] | |
| Si-doped, HVPE on sapphire, conc. 1–3 × 1018 cm−3 | CH3OH:HF: H2O2 (1:2:2) | 60 | [73] | ||
| MOCVD grown n-GaN on SiC, | CH3OH:HF: H2O2 (1:1:1) | 15–40 | [58] | ||
| MOCVD grown n-GaN on Al2O3, n = 1 × 1017 cm−3 | HF:CH3OH:H2O2 (1:4:4) | 15–60 | - | [74] | |
| n-GaN grown on sapphire, conc. 3.4 × 1017 cm−3 | HF:CH3OH:H2O2 (2:1:2) | 45–90 | ≈65 nm | [48] | |
| GaN grew by HVPE, conc. 1 × 1017 cm−3 | CH3OH:HF: H2O2 (1:2:2) | 15–75 | 0.5 μm depth | [75] | |
| GaN Nanowires | Si-doped, HVPE on sapphire, con: 4 × 1018 cm−3 | HF: H2O2 (4M:1M) | 50 | 8–24 nm | [53] |
| MOCVD on sapphire, conc. 1 × 1017 cm−3 | HF:CH3OH | 10–50 | 200–300 nm | [55] | |
| MOCVD on sapphire, conc. 1 × 1017 cm−3 | HF:H2O (1:1) | 30–90 | [76] | ||
| HVPE on sapphire, conc. ≈1018 cm−3 | AgNO3:HF: H2O/CH3OH | 5–30 | D:15–25 nm | [40] | |
| HVPE on sapphire, con: 4.8 × 1018 cm−3 | K2S2O8:KOH (0.1M:1M) | 30 | D:13–52, 8–50 nm | [77] | |
| MOCVD grown GaN, conc. 3 × 1018 cm−3 | AgNO3:HF:DI&CuSO4:HF:DI | 10–50 | 1.8 μm depth | [54] | |
| Si doped, n-type GaN, resistivity 0.05 Ω.cm | H2O2:HF: CH3OH (2:1:2) | 180 | D:35 nm | [78] | |
| Si doped, n-type GaN, resistivity 0.05 Ω.cm | H2O2:HF: CH3OH (2:1:2) | 90–300 | D:35 nm | [46] | |
| HVPE grown n-type GaN, conc. ≈1018 cm−3 | CH3OH: H2O2:HF (1:2:2) | 30 | D:50–100 nm | [39] |
| InGaN Substrate | Etching Solution | Etching Time (min) | Parameters of the Structures | Ref. |
|---|---|---|---|---|
| Undoped InGaN grew on doped GaN, conc. 5 × 1017 cm−3 | CH3OH:H2O2:HF(2:1:2) | 10–30 | 280 nm length Nanowires | [61] |
| Undoped InGaN grown on Si-doped n-type GaN, resistivity 0.05 Ω·cm | CH3OH:H2O2:HF (2:1:2) | 30 | 280 nm length Nanowires | [78] |
| Material | Structure | Lattice Constant (Å) | Thermal Expansion (10−6 K−1) | Thermal Conductivity (W/cm·K) | Ref. | ||
|---|---|---|---|---|---|---|---|
| a | c | a | c | ||||
| GaN | Wurtzite | 3.189 | 5.186 | 5.59 | 3.17 | 1.3 | [101] |
| AlN | Wurtzite | 3.11 | 4.979 | 5.27 | 4.15 | 2.85 | [102] |
| InN | Wurtzite | 3.533 | 5.693 | 3.8 | 2.9 | 0.45 | [103] |
| Sapphire | Wurtzite | 4.765 | 12.982 | 5.0 | 9.03 | 0.23 | [104] |
| SiC (6H) | Wurtzite | 3.0730 | 15.118 | 4.3 | 4.7 | 4.9 | [105] |
| Si | Diamond | 5.431 | 2.6 | 1.3 | [106] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soopy, A.K.K.; Li, Z.; Tang, T.; Sun, J.; Xu, B.; Zhao, C.; Najar, A. In(Ga)N Nanostructures and Devices Grown by Molecular Beam Epitaxy and Metal-Assisted Photochemical Etching. Nanomaterials 2021, 11, 126. https://doi.org/10.3390/nano11010126
Soopy AKK, Li Z, Tang T, Sun J, Xu B, Zhao C, Najar A. In(Ga)N Nanostructures and Devices Grown by Molecular Beam Epitaxy and Metal-Assisted Photochemical Etching. Nanomaterials. 2021; 11(1):126. https://doi.org/10.3390/nano11010126
Chicago/Turabian StyleSoopy, Abdul Kareem K., Zhaonan Li, Tianyi Tang, Jiaqian Sun, Bo Xu, Chao Zhao, and Adel Najar. 2021. "In(Ga)N Nanostructures and Devices Grown by Molecular Beam Epitaxy and Metal-Assisted Photochemical Etching" Nanomaterials 11, no. 1: 126. https://doi.org/10.3390/nano11010126





