Towards Greener and More Sustainable Synthesis of MXenes: A Review
Abstract
1. Introduction
2. Synthesis of MXenes
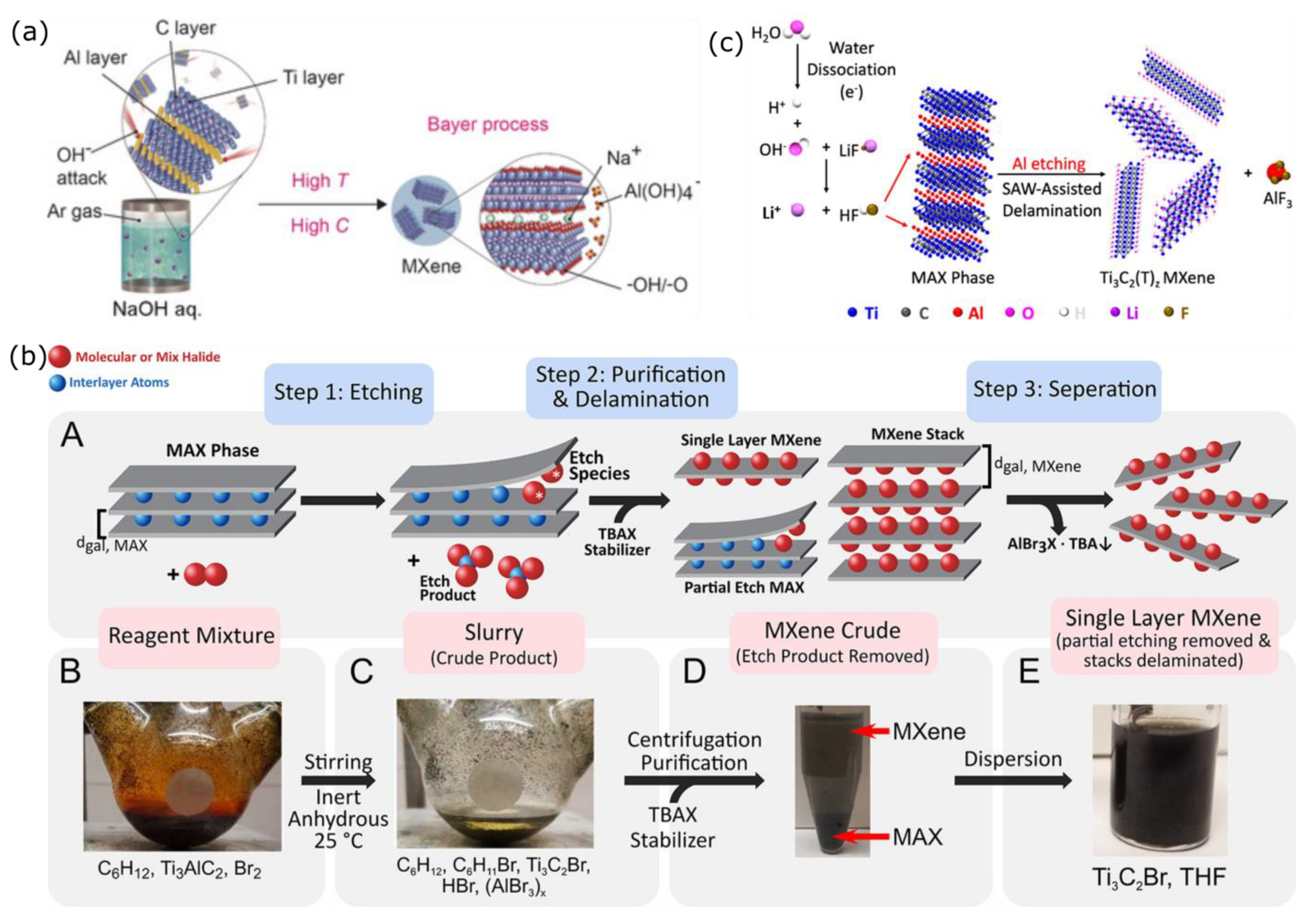
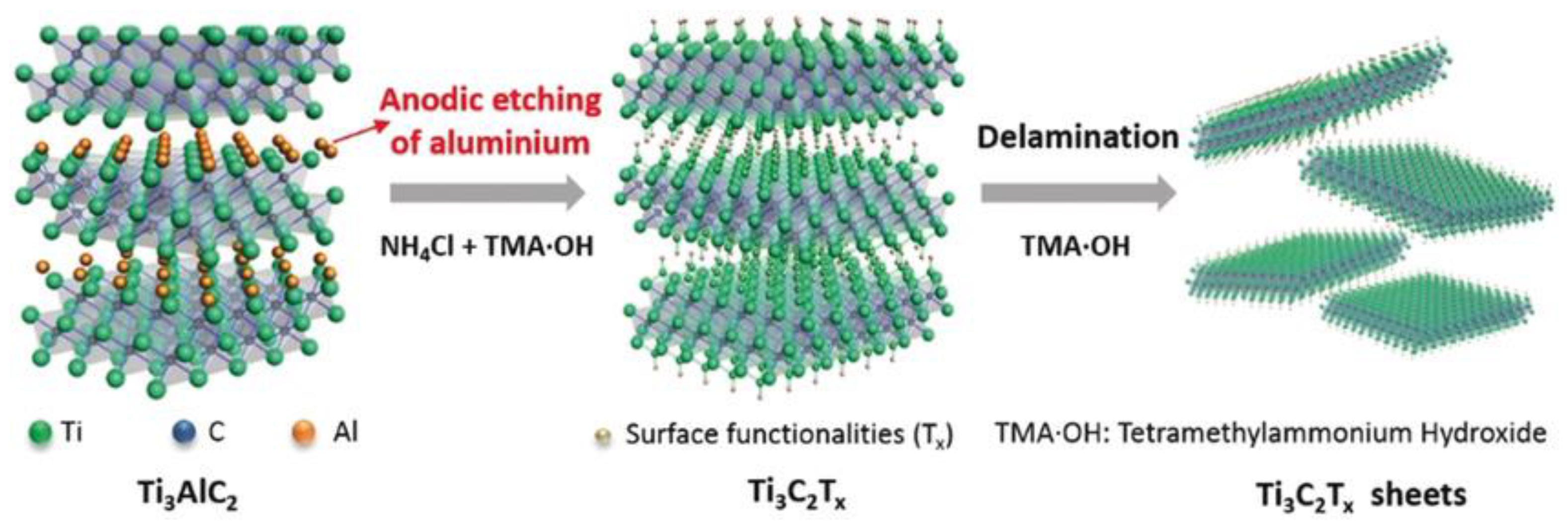
2.1. Conventional Synthesis of MXenes
2.2. Synthetic Strategies towards MXenes with Different Dimensionality
3. Recent Progress in Green Synthesis of MXenes
3.1. Low-Temperature MAX Synthesis
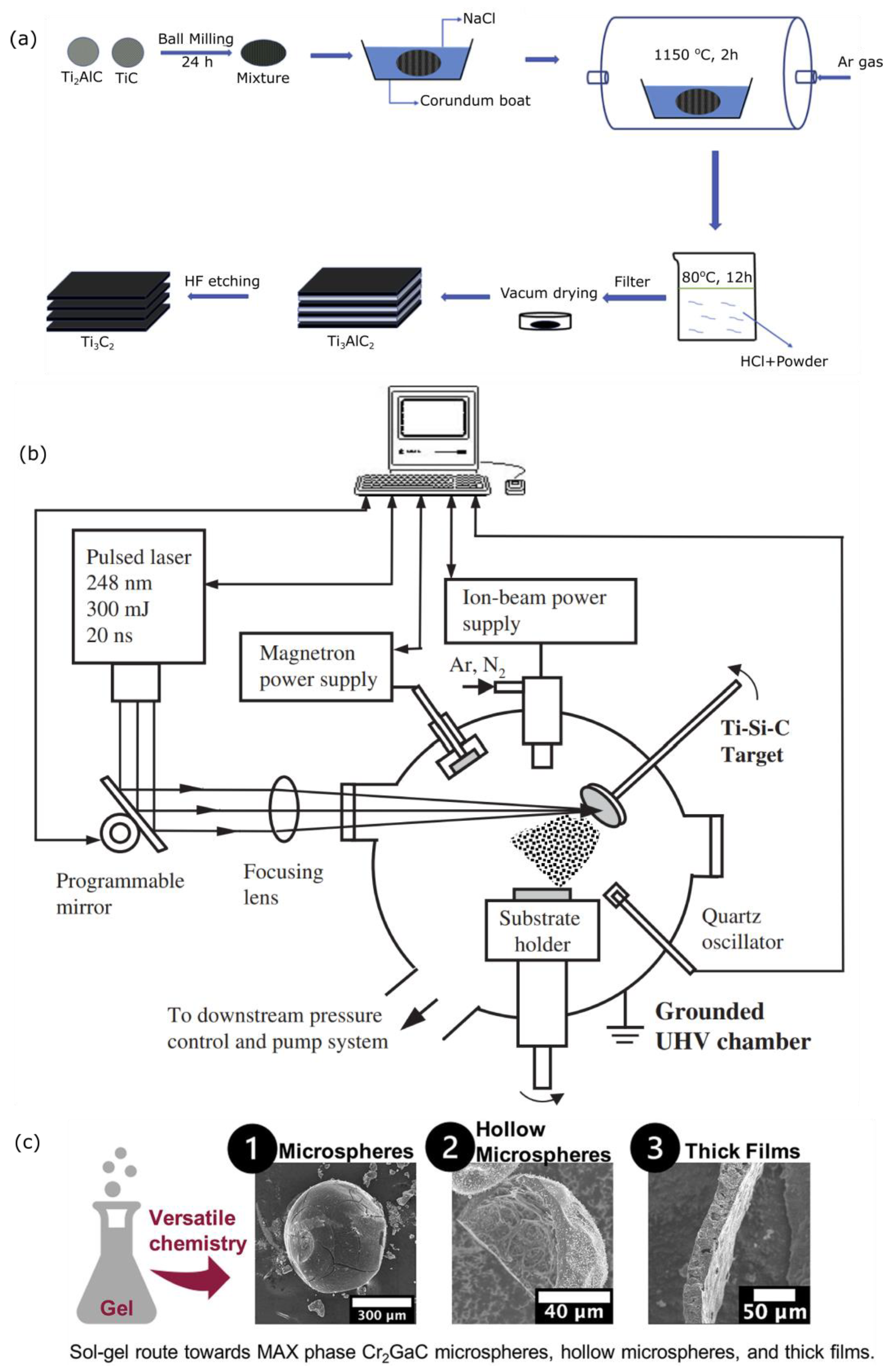
3.1.1. Molten Salt Method for Green Production of MAX Phases
3.1.2. Physical Method for Rapid Production of MAX Phases
3.1.3. Sol-Gel-Based Synthesis for Nanoparticulate MAX Phase
3.2. Replacing HF Etchant with Safer Chemicals
3.3. Electrochemical Exfoliation for HF-Free Etching Process of MXenes
3.4. Direct Synthesis of MXenes by Physical Methods
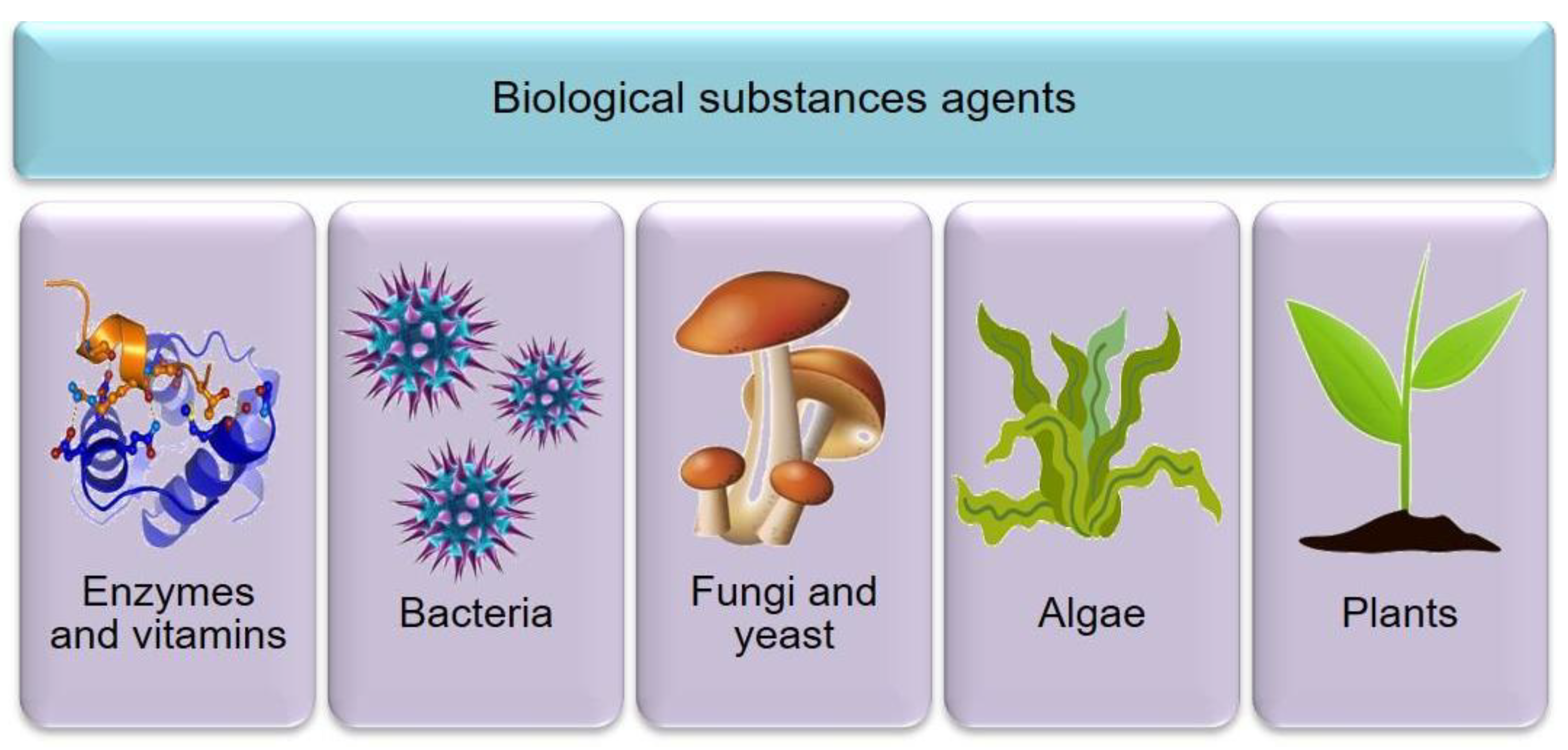
3.5. The Promise of the Bioagents as Exfoliation, Reduction, Capping, and Stabilizer Agents
4. Potential of Green MXenes for Environmental, Biomedical, Electronics, and Energy-Related Applications
4.1. Application of the Green MXenes for Environmental Remediation and Catalysis
4.2. Application of the Green MXenes for Biomedical, In Vitro, and In Vivo Studies
4.3. Application of Green MXenes in Biosensors, Chemical Sensors, and Gas Sensors
4.4. Application of the Green MXenes for Energy Harvesting
4.5. Application of the Green MXenes for Energy Storage
4.6. Application of the Green MXenes for Electromagnetic Interference Shielding
5. Future Perspective and Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef] [PubMed]
- Zamhuri, A.; Lim, G.P.; Ma, N.L.; Tee, K.S.; Soon, C.F. MXene in the lens of biomedical engineering: Synthesis, applications and future outlook. Biomed. Eng. Online 2021, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Amrillah, T. All Shapes and Phases of Nanometer-Sized Iron Oxides Made from Natural Sources and Waste Material via Green Synthesis Approach: A Review. Cryst. Growth Des. 2022, 22, 4640–4660. [Google Scholar] [CrossRef]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green Synthesis of Nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef]
- Jolly, S.; Paranthaman, M.P.; Naguib, M. Synthesis of Ti3C2Tz MXene from low-cost and environmentally friendly precursors. Mater. Today Adv. 2021, 10, 100139. [Google Scholar] [CrossRef]
- Luo, S.; Dong, S.; Lu, C.; Yu, C.; Ou, Y.; Luo, L.; Sun, J.; Sun, J. Rational and green synthesis of novel two-dimensional WS2/MoS2 heterojunction via direct exfoliation in ethanol-water targeting advanced visible-light-responsive photocatalytic performance. J. Colloid Interface Sci. 2018, 513, 389–399. [Google Scholar] [CrossRef]
- Gupta, A.; Badruddoza, A.Z.M.; Doyle, P.S. A General Route for Nanoemulsion Synthesis Using Low-Energy Methods at Constant Temperature. Langmuir 2017, 33, 7118–7123. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, J.-J.; Cheng, F.-F.; Zheng, T.-T.; Wang, C.; Zhu, J.-J. Green and facile synthesis of highly biocompatible graphene nanosheets and its application for cellular imaging and drug delivery. J. Mater. Chem. 2011, 21, 12034. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, B.; Liu, J.; Zhang, P.; Li, Z.; Luan, Y. Green fabricated reduced graphene oxide: Evaluation of its application as nano-carrier for pH-sensitive drug delivery. Int. J. Pharm. 2015, 496, 984–992. [Google Scholar] [CrossRef]
- Jeon, M.; Jun, B.-M.; Kim, S.; Jang, M.; Park, C.M.; Snyder, S.A.; Yoon, Y. A review on MXene-based nanomaterials as adsorbents in aqueous solution. Chemosphere 2020, 261, 127781. [Google Scholar] [CrossRef]
- Ronchi, R.M.; Arantes, J.T.; Santos, S.F. Synthesis, structure, properties and applications of MXenes: Current status and perspectives. Ceram. Int. 2019, 45, 18167–18188. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Y.; Li, Y.; Dai, J.; Song, Y. Hierarchical Ni2P/Cr2CTx (MXene) composites with oxidized surface groups as efficient bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2019, 7, 9324–9334. [Google Scholar] [CrossRef]
- Vasyukova, I.A.; Zakharova, O.V.; Kuznetsov, D.V.; Gusev, A.A. Synthesis, Toxicity Assessment, Environmental and Biomedical Applications of MXenes: A Review. Nanomaterials 2022, 12, 1797. [Google Scholar] [CrossRef]
- Yang, R.; Chen, X.; Ke, W.; Wu, X. Recent Research Progress in the Structure, Fabrication, and Application of MXene-Based Heterostructures. Nanomaterials 2022, 12, 1907. [Google Scholar] [CrossRef]
- Lim, K.R.G.; Shekhirev, M.; Wyatt, B.C.; Anasori, B.; Gogotsi, Y.; Seh, Z.W. Fundamentals of MXene synthesis. Nat. Synth. 2022, 1, 601–614. [Google Scholar] [CrossRef]
- Ding, M.; Han, C.; Yuan, Y.; Xu, J.; Yang, X. Advances and Promises of 2D MXenes as Cocatalysts for Artificial Photosynthesis. Sol. RRL 2021, 5, 2100603. [Google Scholar] [CrossRef]
- Jeitschko, W.; Nowotny, H.; Benesovsky, F. Carbides of formula T2MC. J. Common Met. 1964, 7, 133–138. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Guan, C.; Tian, Y.; Dang, P.; Wang, S.; Li, J.; Li, W.; Zhao, Z. Synthesis and tribological properties of ultrafine Cr2AlC MAX phase. J. Ceram. Soc. Jpn. 2019, 127, 754–760. [Google Scholar] [CrossRef]
- Shalini Reghunath, B.; Davis, D.; Sunaja Devi, K.R. Synthesis and characterization of Cr2AlC MAX phase for photocatalytic applications. Chemosphere 2021, 283, 131281. [Google Scholar] [CrossRef]
- Tunca, B.; Lapauw, T.; Karakulina, O.M.; Batuk, M.; Cabioc’h, T.; Hadermann, J.; Delville, R.; Lambrinou, K.; Vleugels, J. Synthesis of MAX Phases in the Zr-Ti-Al-C System. Inorg. Chem. 2017, 56, 3489–3498. [Google Scholar] [CrossRef]
- Du, Z.; Wu, C.; Chen, Y.; Zhu, Q.; Cui, Y.; Wang, H.; Zhang, Y.; Chen, X.; Shang, J.; Li, B.; et al. High-Entropy Carbonitride MAX Phases and Their Derivative MXenes. Adv. Energy Mater. 2022, 12, 2103228. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Sang, X.; Xie, Y.; Lin, M.-W.; Alhabeb, M.; Van Aken, K.L.; Gogotsi, Y.; Kent, P.R.C.; Xiao, K.; Unocic, R.R. Atomic Defects in Monolayer Titanium Carbide (Ti3C2Tx) MXene. ACS Nano 2016, 10, 9193–9200. [Google Scholar] [CrossRef]
- Tian, L.; Li, Z.; Wang, P.; Zhai, X.; Wang, X.; Li, T. Carbon quantum dots for advanced electrocatalysis. J. Energy Chem. 2021, 55, 279–294. [Google Scholar] [CrossRef]
- Cheng, H.; Ding, L.-X.; Chen, G.-F.; Zhang, L.; Xue, J.; Wang, H. Molybdenum Carbide Nanodots Enable Efficient Electrocatalytic Nitrogen Fixation under Ambient Conditions. Adv. Mater. 2018, 30, 1803694. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Han, X.; Liu, D.; Zhao, H.; Li, Z.; Xu, P.; Du, Y. Ultrasmall Mo2C Nanoparticle-Decorated Carbon Polyhedrons for Enhanced Microwave Absorption. ACS Appl. Nano Mater. 2018, 1, 5366–5376. [Google Scholar] [CrossRef]
- Xu, N.; Li, H.; Gan, Y.; Chen, H.; Li, W.; Zhang, F.; Jiang, X.; Shi, Y.; Liu, J.; Wen, Q.; et al. Zero-Dimensional MXene-Based Optical Devices for Ultrafast and Ultranarrow Photonics Applications. Adv. Sci. 2020, 7, 2002209. [Google Scholar] [CrossRef] [PubMed]
- Rafieerad, A.; Yan, W.; Amiri, A.; Dhingra, S. Bioactive and trackable MXene quantum dots for subcellular nanomedicine applications. Mater. Des. 2020, 196, 109091. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, J.; Khan, W.; Zeng, X.; Li, N.; Cao, Y.; Zhao, X.; Xu, M. Highly green fluorescent Nb2C MXene quantum dots. Chem. Commun. 2020, 56, 6648–6651. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhou, F.; Deng, Q.; Peng, C. Solvothermal synthesis of in situ nitrogen-doped Ti3C2 MXene fluorescent quantum dots for selective Cu2+ detection. Ceram. Int. 2020, 46, 8320–8327. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, Z.; Liu, N.; Sun, Y.; Han, D.; Liu, Y.; Niu, L.; Kang, Z. High-yield fabrication of Ti3C2Tx MXene quantum dots and their electrochemiluminescence behavior. Nanoscale 2018, 10, 14000–14004. [Google Scholar] [CrossRef]
- Yu, X.; Cai, X.; Cui, H.; Lee, S.-W.; Yu, X.-F.; Liu, B. Fluorine-free preparation of titanium carbide MXene quantum dots with high near-infrared photothermal performances for cancer therapy. Nanoscale 2017, 9, 17859–17864. [Google Scholar] [CrossRef]
- Pandey, P.; Sengupta, A.; Parmar, S.; Bansode, U.; Gosavi, S.; Swarnkar, A.; Muduli, S.; Mohite, A.D.; Ogale, S. CsPbBr3–Ti3C2Tx MXene QD/QD Heterojunction: Photoluminescence Quenching, Charge Transfer, and Cd Ion Sensing Application. ACS Appl. Nano Mater. 2020, 3, 3305–3314. [Google Scholar] [CrossRef]
- Xu, Q.; Ding, L.; Wen, Y.; Yang, W.; Zhou, H.; Chen, X.; Street, J.; Zhou, A.; Ong, W.-J.; Li, N. High photoluminescence quantum yield of 18.7% by using nitrogen-doped Ti3C2 MXene quantum dots. J. Mater. Chem. C 2018, 6, 6360–6369. [Google Scholar] [CrossRef]
- Lian, P.; Dong, Y.; Wu, Z.-S.; Zheng, S.; Wang, X.; Sen, W.; Sun, C.; Qin, J.; Shi, X.; Bao, X. Alkalized Ti3C2 MXene nanoribbons with expanded interlayer spacing for high-capacity sodium and potassium ion batteries. Nano Energy 2017, 40, 1–8. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, Z.-S.; Zheng, S.; Wang, X.; Qin, J.; Wang, S.; Shi, X.; Bao, X. Ti3C2 MXene-Derived Sodium/Potassium Titanate Nanoribbons for High-Performance Sodium/Potassium Ion Batteries with Enhanced Capacities. ACS Nano 2017, 11, 4792–4800. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Meng, R.; Zu, L.; Wang, Z.; Feng, N.; Yang, Z.; Yu, Y.; Yang, J. Sandwich-like Na0.23TiO2 nanobelt/Ti3C2 MXene composites from a scalable in situ transformation reaction for long-life high-rate lithium/sodium-ion batteries. Nano Energy 2018, 46, 20–28. [Google Scholar] [CrossRef]
- Li, N.; Jiang, Y.; Zhou, C.; Xiao, Y.; Meng, B.; Wang, Z.; Huang, D.; Xing, C.; Peng, Z. High-Performance Humidity Sensor Based on Urchin-Like Composite of Ti3C2 MXene-Derived TiO2 Nanowires. ACS Appl. Mater. Interfaces 2019, 11, 38116–38125. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Jin, S.; Miao, L.; Cai, Y.; Hou, Y.; Li, H.; Zhang, K.; Yan, Z.; Chen, J. A 3D Hydroxylated MXene/Carbon Nanotubes Composite as a Scaffold for Dendrite-Free Sodium-Metal Electrodes. Angew. Chem. Int. Ed. 2020, 59, 16705–16711. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chen, L.; Liu, Z.; Cheng, H.-M.; Ren, W. Bottom-Up Synthesis of 2D Transition Metal Carbides and Nitrides. In 2D Metal Carbides and Nitrides (MXenes); Anasori, B., Gogotsi, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 89–109. ISBN 978-3-030-19025-5. [Google Scholar]
- Druffel, D.L.; Lanetti, M.G.; Sundberg, J.D.; Pawlik, J.T.; Stark, M.S.; Donley, C.L.; McRae, L.M.; Scott, K.M.; Warren, S.C. Synthesis and Electronic Structure of a 3D Crystalline Stack of MXene-Like Sheets. Chem. Mater. 2019, 31, 9788–9796. [Google Scholar] [CrossRef]
- Shang, T.; Lin, Z.; Qi, C.; Liu, X.; Li, P.; Tao, Y.; Wu, Z.; Li, D.; Simon, P.; Yang, Q. 3D Macroscopic Architectures from Self-Assembled MXene Hydrogels. Adv. Funct. Mater. 2019, 29, 1903960. [Google Scholar] [CrossRef]
- Yuan, W.; Yang, K.; Peng, H.; Li, F.; Yin, F. A flexible VOCs sensor based on a 3D Mxene framework with a high sensing performance. J. Mater. Chem. A 2018, 6, 18116–18124. [Google Scholar] [CrossRef]
- Zhang, P.; Soomro, R.A.; Guan, Z.; Sun, N.; Xu, B. 3D carbon-coated MXene architectures with high and ultrafast lithium/sodium-ion storage. Energy Storage Mater. 2020, 29, 163–171. [Google Scholar] [CrossRef]
- Gonzalez-Julian, J. Processing of MAX phases: From synthesis to applications. J. Am. Ceram. Soc. 2021, 104, 659–690. [Google Scholar] [CrossRef]
- Liu, X.; Fechler, N.; Antonietti, M. Salt melt synthesis of ceramics, semiconductors and carbon nanostructures. Chem. Soc. Rev. 2013, 42, 8237. [Google Scholar] [CrossRef]
- Guan, K.; Lei, W.; Wang, H.; Liu, X.; Luo, J.; Liu, J.; Jia, Q.; Zhang, H.; Zhang, S. Efficient synthesis of Ti3AlC2 powders with high purity by microwave-assisted molten salt method. Ceram. Int. 2022, 48, 16357–16363. [Google Scholar] [CrossRef]
- Galvin, T.; Hyatt, N.C.; Rainforth, W.M.; Reaney, I.M.; Shepherd, D. Molten salt synthesis of MAX phases in the Ti-Al-C system. J. Eur. Ceram. Soc. 2018, 38, 4585–4589. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Yang, L.; Liu, R.; Zeng, C. Synthesis and characterization of nanosized Ti3AlC2 ceramic powder by elemental powders of Ti, Al and C in molten salt. J. Mater. Sci. Technol. 2020, 37, 77–84. [Google Scholar] [CrossRef]
- Liu, A.; Yang, Q.; Ren, X.; Meng, F.; Gao, L.; Gao, M.; Yang, Y.; Ma, T.; Wu, G. Energy- and cost-efficient NaCl-assisted synthesis of MAX-phase Ti3AlC2 at lower temperature. Ceram. Int. 2020, 46, 6934–6939. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J.; Yang, S.; Gao, L.; Qian, B. Preparation of Ti3SiC2 powders by the molten salt method. Mater. Lett. 2013, 111, 211–213. [Google Scholar] [CrossRef]
- Roy, C.; Banerjee, P.; Bhattacharyya, S. Molten salt shielded synthesis (MS3) of Ti2AlN and V2AlC MAX phase powders in open air. J. Eur. Ceram. Soc. 2020, 40, 923–929. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, A.; Hu, Q.; Wang, L. Synthesis and oxidation resistance of V2AlC powders by molten salt method. Int. J. Appl. Ceram. Technol. 2017, 14, 873–879. [Google Scholar] [CrossRef]
- Tian, W.-B.; Wang, P.-L.; Kan, Y.-M.; Zhang, G.-J. Cr2AlC powders prepared by molten salt method. J. Alloys Compd. 2008, 461, L5–L10. [Google Scholar] [CrossRef]
- Li, Y.; Ma, G.; Shao, H.; Xiao, P.; Lu, J.; Xu, J.; Hou, J.; Chen, K.; Zhang, X.; Li, M.; et al. Electrochemical Lithium Storage Performance of Molten Salt Derived V2SnC MAX Phase. Nano Micro Lett. 2021, 13, 158. [Google Scholar] [CrossRef]
- Wilhelmsson, O.; Eklund, P.; Högberg, H.; Hultman, L.; Jansson, U. Structural, electrical and mechanical characterization of magnetron-sputtered V–Ge–C thin films. Acta Mater. 2008, 56, 2563–2569. [Google Scholar] [CrossRef]
- Mertens, R.; Sun, Z.; Music, D.; Schneider, J.M. Effect of the Composition on the Structure of Cr-Al-C Investigated by Combinatorial Thin Film Synthesis and ab Initio Calculations. Adv. Eng. Mater. 2004, 6, 903–907. [Google Scholar] [CrossRef]
- Walter, C.; Sigumonrong, D.P.; El-Raghy, T.; Schneider, J.M. Towards large area deposition of Cr2AlC on steel. Thin Solid Films 2006, 515, 389–393. [Google Scholar] [CrossRef]
- Gao, L.; Han, T.; Guo, Z.; Zhang, X.; Pan, D.; Zhou, S.; Chen, W.; Li, S. Preparation and performance of MAX phase Ti3AlC2 by in-situ reaction of Ti-Al-C system. Adv. Powder Technol. 2020, 31, 3533–3539. [Google Scholar] [CrossRef]
- Zou, Y.; Sun, Z.; Hashimoto, H.; Tada, S. Low temperature synthesis of single-phase Ti3AlC2 through reactive sintering Ti/Al/C powders. Mater. Sci. Eng. A 2008, 473, 90–95. [Google Scholar] [CrossRef]
- Shu, R.; Ge, F.; Meng, F.; Li, P.; Wang, J.; Huang, Q.; Eklund, P.; Huang, F. One-step synthesis of polycrystalline V2AlC thin films on amorphous substrates by magnetron co-sputtering. Vacuum 2017, 146, 106–110. [Google Scholar] [CrossRef]
- Hu, J.J.; Bultman, J.E.; Patton, S.; Zabinski, J.S. Pulsed Laser Deposition and Properties of Mn+1AXn Phase Formulated Ti3SiC2 Thin Films. Tribol. Lett. 2004, 16, 113–122. [Google Scholar] [CrossRef]
- Nickl, J.J.; Schweitzer, K.K.; Luxenberg, P. Gasphasenabscheidung im system TiSiC. J. Common Met. 1972, 26, 335–353. [Google Scholar] [CrossRef]
- Pickering, E.; Lackey, W.J.; Crain, S. CVD of Ti3SiC2. Chem. Vap. Depos. 2000, 6, 289–295. [Google Scholar] [CrossRef]
- Goto, T.; Hirai, T. Chemically vapor deposited Ti3SiC2. Mater. Res. Bull. 1987, 22, 1195–1201. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Liu, Z.; Chen, L.; Guo, J.; Kang, N.; Ma, X.-L.; Cheng, H.-M.; Ren, W. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat. Mater. 2015, 14, 1135–1141. [Google Scholar] [CrossRef]
- Siebert, J.P.; Bischoff, L.; Lepple, M.; Zintler, A.; Molina-Luna, L.; Wiedwald, U.; Birkel, C.S. Sol–gel based synthesis and enhanced processability of MAX phase Cr2GaC. J. Mater. Chem. C 2019, 7, 6034–6040. [Google Scholar] [CrossRef]
- Siebert, J.P.; Mallett, S.; Juelsholt, M.; Pazniak, H.; Wiedwald, U.; Page, K.; Birkel, C.S. Structure determination and magnetic properties of the Mn-doped MAX phase Cr2GaC. Mater. Chem. Front. 2021, 5, 6082–6091. [Google Scholar] [CrossRef]
- Siebert, J.P.; Flores, M.; Birkel, C.S. Shape Control of MAX Phases by Biopolymer Sol–Gel Synthesis: Cr2GaC Thick Films, Microspheres, and Hollow Microspheres. ACS Org. Inorg. Au 2022, 2, 59–65. [Google Scholar] [CrossRef]
- Siebert, J.P.; Patarakun, K.; Birkel, C.S. Mechanistic Insights into the Nonconventional Sol–Gel Synthesis of MAX Phase M2GeC (M = V, Cr). Inorg. Chem. 2022, 61, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B.; et al. Fluorine-Free Synthesis of High-Purity Ti3C2Tx (T=OH, O) via Alkali Treatment. Angew. Chem. Int. Ed. 2018, 57, 6115–6119. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shah, S.A.; Chen, Y.; Tan, Z.; Gao, H.; Habib, T.; Radovic, M.; Green, M.J. Electrochemical etching of Ti2AlC to Ti2CTx (MXene) in low-concentration hydrochloric acid solution. J. Mater. Chem. A 2017, 5, 21663–21668. [Google Scholar] [CrossRef]
- Peng, C.; Wei, P.; Chen, X.; Zhang, Y.; Zhu, F.; Cao, Y.; Wang, H.; Yu, H.; Peng, F. A hydrothermal etching route to synthesis of 2D MXene (Ti3C2, Nb2C): Enhanced exfoliation and improved adsorption performance. Ceram. Int. 2018, 44, 18886–18893. [Google Scholar] [CrossRef]
- Guo, Y.; Jin, S.; Wang, L.; He, P.; Hu, Q.; Fan, L.-Z.; Zhou, A. Synthesis of two-dimensional carbide Mo2CTx MXene by hydrothermal etching with fluorides and its thermal stability. Ceram. Int. 2020, 46, 19550–19556. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Yang, Q.; Liang, G.; Huang, Z.; Ma, L.; Wang, D.; Mo, F.; Dong, B.; Huang, Q.; et al. In Situ Electrochemical Synthesis of MXenes without Acid/Alkali Usage in/for an Aqueous Zinc Ion Battery. Adv. Energy Mater. 2020, 10, 2001791. [Google Scholar] [CrossRef]
- Pang, S.-Y.; Wong, Y.-T.; Yuan, S.; Liu, Y.; Tsang, M.-K.; Yang, Z.; Huang, H.; Wong, W.-T.; Hao, J. Universal Strategy for HF-Free Facile and Rapid Synthesis of Two-dimensional MXenes as Multifunctional Energy Materials. J. Am. Chem. Soc. 2019, 141, 9610–9616. [Google Scholar] [CrossRef]
- Jawaid, A.; Hassan, A.; Neher, G.; Nepal, D.; Pachter, R.; Kennedy, W.J.; Ramakrishnan, S.; Vaia, R.A. Halogen Etch of Ti3AlC2 MAX Phase for MXene Fabrication. ACS Nano 2021, 15, 2771–2777. [Google Scholar] [CrossRef]
- Ghazaly, A.E.; Ahmed, H.; Rezk, A.R.; Halim, J.; Persson, P.O.Å.; Yeo, L.Y.; Rosen, J. Ultrafast, One-Step, Salt-Solution-Based Acoustic Synthesis of Ti3C2 MXene. ACS Nano 2021, 15, 4287–4293. [Google Scholar] [CrossRef] [PubMed]
- Xiu, L.-Y.; Wang, Z.-Y.; Qiu, J.-S. General synthesis of MXene by green etching chemistry of fluoride-free Lewis acidic melts. Rare Met. 2020, 39, 1237–1238. [Google Scholar] [CrossRef]
- Arole, K.; Blivin, J.W.; Saha, S.; Zhao, X.; Holta, D.E.; Sarmah, A.; Cao, H.; Radovic, M.; Lutkenhaus, J.L.; Green, M.J. Water-Dispersible Ti3C2Tz MXene Nanosheets by Acid-Free, Molten Salt Etching. SSRN Electron. J. 2021. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, P.; Wang, F.; Ricciardulli, A.G.; Lohe, M.R.; Blom, P.W.M.; Feng, X. Fluoride-Free Synthesis of Two-Dimensional Titanium Carbide (MXene) Using A Binary Aqueous System. Angew. Chem. Int. Ed. 2018, 57, 15491–15495. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Li, Y.; Wang, R.; Al-Hartomy, O.A.; Al-Ghamdi, A.; Wageh, S.; Luo, X.; Tang, X.; Zhang, H. Synthesis of Ti3C2Fx MXene with controllable fluorination by electrochemical etching for lithium-ion batteries applications. Ceram. Int. 2021, 47, 28642–28649. [Google Scholar] [CrossRef]
- Song, M.; Pang, S.; Guo, F.; Wong, M.; Hao, J. Fluoride-Free 2D Niobium Carbide MXenes as Stable and Biocompatible Nanoplatforms for Electrochemical Biosensors with Ultrahigh Sensitivity. Adv. Sci. 2020, 7, 2001546. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Jiang, W.; Guo, L.; Zhao, S.; Tang, R.; Wang, J. One-pot green process to synthesize controllable surface terminations MXenes in molten salts. arXiv 2021, arXiv:2102.10262. [Google Scholar]
- Hermawan, A.; Amrillah, T.; Riapanitra, A.; Ong, W.; Yin, S. Prospects and Challenges of MXenes as Emerging Sensing Materials for Flexible and Wearable Breath-Based Biomarker Diagnosis. Adv. Healthc. Mater. 2021, 10, 2100970. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Wang, Y.; Wang, T.; Shang, S.; Ren, W. Magnetron-sputtering deposited molybdenum carbide MXene thin films as a saturable absorber for passively Q-switched lasers. J. Mater. Chem. C 2020, 8, 1608–1613. [Google Scholar] [CrossRef]
- Halim, J.; Lukatskaya, M.R.; Cook, K.M.; Lu, J.; Smith, C.R.; Näslund, L.-Å.; May, S.J.; Hultman, L.; Gogotsi, Y.; Eklund, P.; et al. Transparent Conductive Two-Dimensional Titanium Carbide Epitaxial Thin Films. Chem. Mater. 2014, 26, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Horak, P.; Vacik, J.; Bakardjieva, S.; Cannavo, A.; Ceccio, G.; Kupcik, J.; Klie, R. Ion Beam Sputtering for Controlled Synthesis of Thin MAX (MXene) Phases. Microsc. Microanal. 2019, 25, 1626–1627. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Z.; Wang, H.; Chan, C.H.; Chan, N.Y.; Chen, X.X.; Dai, J.-Y. Plasma-enhanced pulsed-laser deposition of single-crystalline Mo2C ultrathin superconducting films. Phys. Rev. Mater. 2017, 1, 034002. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, F.; Wang, H.; Ho Chan, C.; Lu, W.; Dai, J. Substrate orientation-induced epitaxial growth of face centered cubic Mo2C superconductive thin film. J. Mater. Chem. C 2017, 5, 10822–10827. [Google Scholar] [CrossRef]
- Sarr, M.; Bahlawane, N.; Arl, D.; Dossot, M.; McRae, E.; Lenoble, D. Atomic layer deposition of cobalt carbide films and their magnetic properties using propanol as a reducing agent. Appl. Surf. Sci. 2016, 379, 523–529. [Google Scholar] [CrossRef]
- Kim, J.B.; Jang, B.; Lee, H.-J.; Han, W.S.; Lee, D.-J.; Lee, H.-B.-R.; Hong, T.E.; Kim, S.-H. A controlled growth of WNx and WCx thin films prepared by atomic layer deposition. Mater. Lett. 2016, 168, 218–222. [Google Scholar] [CrossRef]
- Li, B.; Guo, H.; Wang, Y.; Zhang, W.; Zhang, Q.; Chen, L.; Fan, X.; Zhang, W.; Li, Y.; Lau, W.-M. Asymmetric MXene/monolayer transition metal dichalcogenide heterostructures for functional applications. NPJ Comput. Mater. 2019, 5, 16. [Google Scholar] [CrossRef]
- Choi, S.-K.; Kim, H.; Kim, J.; Cheon, T.; Seo, J.H.; Kim, S.-H. Properties of plasma-enhanced atomic layer deposited TiCx films as a diffusion barrier for Cu metallization. Thin Solid Films 2015, 590, 311–317. [Google Scholar] [CrossRef]
- Smuleac, V.; Varma, R.; Sikdar, S.; Bhattacharyya, D. Green synthesis of Fe and Fe/Pd bimetallic nanoparticles in membranes for reductive degradation of chlorinated organics. J. Membr. Sci. 2011, 379, 131–137. [Google Scholar] [CrossRef]
- Shao, Y.; Wu, C.; Wu, T.; Yuan, C.; Chen, S.; Ding, T.; Ye, X.; Hu, Y. Green synthesis of sodium alginate-silver nanoparticles and their antibacterial activity. Int. J. Biol. Macromol. 2018, 111, 1281–1292. [Google Scholar] [CrossRef]
- Uma Suganya, K.S.; Govindaraju, K.; Ganesh Kumar, V.; Stalin Dhas, T.; Karthick, V.; Singaravelu, G.; Elanchezhiyan, M. Blue green alga mediated synthesis of gold nanoparticles and its antibacterial efficacy against Gram positive organisms. Mater. Sci. Eng. C 2015, 47, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Meka Chufa, B.; Abdisa Gonfa, B.; Yohannes Anshebo, T.; Adam Workneh, G. A Novel and Simplest Green Synthesis Method of Reduced Graphene Oxide Using Methanol Extracted Vernonia Amygdalina: Large-Scale Production. Adv. Condens. Matter Phys. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Chen, I.-W.P.; Shie, M.-Y.; Liu, M.-H.; Huang, W.-M.; Chen, W.-T.; Chao, Y.-T. Scalable synthesis of two-dimensional nano-sheet materials with chlorophyll extracts: Enhancing the hydrogen evolution reaction. Green Chem. 2018, 20, 525–533. [Google Scholar] [CrossRef]
- Rasool, K.; Pandey, R.P.; Rasheed, P.A.; Berdiyorov, G.R.; Mahmoud, K.A. MXenes for Environmental and Water Treatment Applications. In 2D Metal Carbides and Nitrides (MXenes); Anasori, B., Gogotsi, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 417–444. ISBN 978-3-030-19025-5. [Google Scholar]
- Ihsanullah, I.; Ali, H. Technological challenges in the environmental applications of MXenes and future outlook. Case Stud. Chem. Environ. Eng. 2020, 2, 100034. [Google Scholar] [CrossRef]
- Ying, Y.; Liu, Y.; Wang, X.; Mao, Y.; Cao, W.; Hu, P.; Peng, X. Two-Dimensional Titanium Carbide for Efficiently Reductive Removal of Highly Toxic Chromium(VI) from Water. ACS Appl. Mater. Interfaces 2015, 7, 1795–1803. [Google Scholar] [CrossRef]
- Ren, C.E.; Hatzell, K.B.; Alhabeb, M.; Ling, Z.; Mahmoud, K.A.; Gogotsi, Y. Charge- and Size-Selective Ion Sieving Through Ti3C2Tx MXene Membranes. J. Phys. Chem. Lett. 2015, 6, 4026–4031. [Google Scholar] [CrossRef]
- Pandey, R.P.; Rasool, K.; Madhavan, V.E.; Aïssa, B.; Gogotsi, Y.; Mahmoud, K.A. Ultrahigh-flux and fouling-resistant membranes based on layered silver/MXene (Ti3C2Tx) nanosheets. J. Mater. Chem. A 2018, 6, 3522–3533. [Google Scholar] [CrossRef]
- Rasool, K.; Mahmoud, K.A.; Johnson, D.J.; Helal, M.; Berdiyorov, G.R.; Gogotsi, Y. Efficient Antibacterial Membrane based on Two-Dimensional Ti3C2Tx (MXene) Nanosheets. Sci. Rep. 2017, 7, 1598. [Google Scholar] [CrossRef]
- Han, X.; Huang, J.; Lin, H.; Wang, Z.; Li, P.; Chen, Y. 2D Ultrathin MXene-Based Drug-Delivery Nanoplatform for Synergistic Photothermal Ablation and Chemotherapy of Cancer. Adv. Healthc. Mater. 2018, 7, 1701394. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.; Mao, Y.; Li, Z. Progress and biomedical applications of MXenes. Nano Sel. 2021, 2, 1480–1508. [Google Scholar] [CrossRef]
- Shaw, Z.L.; Kuriakose, S.; Cheeseman, S.; Dickey, M.D.; Genzer, J.; Christofferson, A.J.; Crawford, R.J.; McConville, C.F.; Chapman, J.; Truong, V.K.; et al. Antipathogenic properties and applications of low-dimensional materials. Nat. Commun. 2021, 12, 3897. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. MXenes and MXene-based materials for tissue engineering and regenerative medicine: Recent advances. Mater. Adv. 2021, 2, 2906–2917. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Progress and Insights in the Application of MXenes as New 2D Nano-Materials Suitable for Biosensors and Biofuel Cell Design. Int. J. Mol. Sci. 2020, 21, 9224. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, X.; Ma, L.; Gao, J.; Jiang, Y. Acetylcholinesterase/chitosan-transition metal carbides nanocomposites-based biosensor for the organophosphate pesticides detection. Biochem. Eng. J. 2017, 128, 243–249. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, J.; Jiang, C.; Lin, J.; Huang, P. Biodegradable titanium nitride MXene quantum dots for cancer phototheranostics in NIR-I/II biowindows. Chem. Eng. J. 2020, 400, 126009. [Google Scholar] [CrossRef]
- Yang, Q.; Yin, H.; Xu, T.; Zhu, D.; Yin, J.; Chen, Y.; Yu, X.; Gao, J.; Zhang, C.; Chen, Y.; et al. Engineering 2D Mesoporous Silica@MXene-Integrated 3D-Printing Scaffolds for Combinatory Osteosarcoma Therapy and NO-Augmented Bone Regeneration. Small 2020, 16, 1906814. [Google Scholar] [CrossRef]
- Dai, C.; Chen, Y.; Jing, X.; Xiang, L.; Yang, D.; Lin, H.; Liu, Z.; Han, X.; Wu, R. Two-Dimensional Tantalum Carbide (MXenes) Composite Nanosheets for Multiple Imaging-Guided Photothermal Tumor Ablation. ACS Nano 2017, 11, 12696–12712. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.; Yu, L.; Chen, Y.; Shi, J. Two-Dimensional Ultrathin MXene Ceramic Nanosheets for Photothermal Conversion. Nano Lett. 2017, 17, 384–391. [Google Scholar] [CrossRef]
- Liu, G.; Zou, J.; Tang, Q.; Yang, X.; Zhang, Y.; Zhang, Q.; Huang, W.; Chen, P.; Shao, J.; Dong, X. Surface Modified Ti3C2 MXene Nanosheets for Tumor Targeting Photothermal/Photodynamic/Chemo Synergistic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 40077–40086. [Google Scholar] [CrossRef]
- Chen, K.; Qiu, N.; Deng, Q.; Kang, M.-H.; Yang, H.; Baek, J.-U.; Koh, Y.-H.; Du, S.; Huang, Q.; Kim, H.-E. Cytocompatibility of Ti3AlC2, Ti3SiC2, and Ti2AlN: In Vitro Tests and First-Principles Calculations. ACS Biomater. Sci. Eng. 2017, 3, 2293–2301. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Szuplewska, A.; Wojciechowski, T.; Chudy, M.; Ziemkowska, W.; Chlubny, L.; Rozmysłowska, A.; Olszyna, A. In vitro studies on cytotoxicity of delaminated Ti3C2 MXene. J. Hazard. Mater. 2017, 339, 1–8. [Google Scholar] [CrossRef]
- Xing, C.; Chen, S.; Liang, X.; Liu, Q.; Qu, M.; Zou, Q.; Li, J.; Tan, H.; Liu, L.; Fan, D.; et al. Two-Dimensional MXene (Ti3C2)-Integrated Cellulose Hydrogels: Toward Smart Three-Dimensional Network Nanoplatforms Exhibiting Light-Induced Swelling and Bimodal Photothermal/Chemotherapy Anticancer Activity. ACS Appl. Mater. Interfaces 2018, 10, 27631–27643. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, H.; Zhao, M.; Dai, C.; Zhang, S.; Peng, W.; Chen, Y. 2D Superparamagnetic Tantalum Carbide Composite MXenes for Efficient Breast-Cancer Theranostics. Theranostics 2018, 8, 1648–1664. [Google Scholar] [CrossRef]
- Scheibe, B.; Wychowaniec, J.K.; Scheibe, M.; Peplińska, B.; Jarek, M.; Nowaczyk, G.; Przysiecka, Ł. Cytotoxicity Assessment of Ti–Al–C Based MAX Phases and Ti3C2Tx MXenes on Human Fibroblasts and Cervical Cancer Cells. ACS Biomater. Sci. Eng. 2019, 5, 6557–6569. [Google Scholar] [CrossRef]
- Szuplewska, A.; Kulpińska, D.; Dybko, A.; Jastrzębska, A.M.; Wojciechowski, T.; Rozmysłowska, A.; Chudy, M.; Grabowska-Jadach, I.; Ziemkowska, W.; Brzózka, Z.; et al. 2D Ti2C (MXene) as a novel highly efficient and selective agent for photothermal therapy. Mater. Sci. Eng. C 2019, 98, 874–886. [Google Scholar] [CrossRef]
- Hussein, E.A.; Zagho, M.M.; Rizeq, B.R.; Younes, N.N.; Pintus, G.; Mahmoud, K.A.; Nasrallah, G.K.; Elzatahry, A.A. Plasmonic MXene-based nanocomposites exhibiting photothermal therapeutic effects with lower acute toxicity than pure MXene. Int. J. Nanomed. 2019, 14, 4529–4539. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, Y.; Mo, A. Multilayered Titanium Carbide MXene Film for Guided Bone Regeneration. Int. J. Nanomed. 2019, 14, 10091–10103. [Google Scholar] [CrossRef]
- Rozmysłowska-Wojciechowska, A.; Szuplewska, A.; Wojciechowski, T.; Poźniak, S.; Mitrzak, J.; Chudy, M.; Ziemkowska, W.; Chlubny, L.; Olszyna, A.; Jastrzębska, A.M. A simple, low-cost and green method for controlling the cytotoxicity of MXenes. Mater. Sci. Eng. C 2020, 111, 110790. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Szuplewska, A.; Rozmysłowska-Wojciechowska, A.; Chudy, M.; Olszyna, A.; Birowska, M.; Popielski, M.; Majewski, J.A.; Scheibe, B.; Natu, V.; et al. On tuning the cytotoxicity of Ti3C2 (MXene) flakes to cancerous and benign cells by post-delamination surface modifications. 2D Mater. 2020, 7, 025018. [Google Scholar] [CrossRef]
- Yan, X.; Ma, J.; Yu, K.; Li, J.; Yang, L.; Liu, J.; Wang, J.; Cai, L. Highly green fluorescent Nb2C MXene quantum dots for Cu2+ ion sensing and cell imaging. Chin. Chem. Lett. 2020, 31, 3173–3177. [Google Scholar] [CrossRef]
- Li, X.; Liu, F.; Huang, D.; Xue, N.; Dang, Y.; Zhang, M.; Zhang, L.; Li, B.; Liu, D.; Wang, L.; et al. Nonoxidized MXene Quantum Dots Prepared by Microexplosion Method for Cancer Catalytic Therapy. Adv. Funct. Mater. 2020, 30, 2000308. [Google Scholar] [CrossRef]
- Wu, W.; Ge, H.; Zhang, L.; Lei, X.; Yang, Y.; Fu, Y.; Feng, H. Evaluating the Cytotoxicity of Ti3C2 MXene to Neural Stem Cells. Chem. Res. Toxicol. 2020, 33, 2953–2962. [Google Scholar] [CrossRef]
- Gu, M.; Dai, Z.; Yan, X.; Ma, J.; Niu, Y.; Lan, W.; Wang, X.; Xu, Q. Comparison of toxicity of Ti3C2 and Nb2C Mxene quantum dots (QDs) to human umbilical vein endothelial cells. J. Appl. Toxicol. 2021, 41, 745–754. [Google Scholar] [CrossRef]
- Jang, J.-H.; Lee, E.-J. Influence of MXene Particles with a Stacked-Lamellar Structure on Osteogenic Differentiation of Human Mesenchymal Stem Cells. Materials 2021, 14, 4453. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Scheibe, B.; Szuplewska, A.; Rozmysłowska-Wojciechowska, A.; Chudy, M.; Aparicio, C.; Scheibe, M.; Janica, I.; Ciesielski, A.; Otyepka, M.; et al. On the rapid in situ oxidation of two-dimensional V2CTz MXene in culture cell media and their cytotoxicity. Mater. Sci. Eng. C 2021, 119, 111431. [Google Scholar] [CrossRef]
- Rashid, B.; Anwar, A.; Shahabuddin, S.; Mohan, G.; Saidur, R.; Aslfattahi, N.; Sridewi, N. A Comparative Study of Cytotoxicity of PPG and PEG Surface-Modified 2-D Ti3C2 MXene Flakes on Human Cancer Cells and Their Photothermal Response. Materials 2021, 14, 4370. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Zheng, Y.; Zhao, S.; Wei, W.; Zhang, D.; Fu, X.; Jiang, K.; Shen, G.; Han, W. Highly-stable polymer-crosslinked 2D MXene-based flexible biocompatible electronic skins for in vivo biomonitoring. Nano Energy 2021, 84, 105921. [Google Scholar] [CrossRef]
- Lim, G.P.; Soon, C.F.; Jastrzębska, A.M.; Ma, N.L.; Wojciechowska, A.R.; Szuplewska, A.; Wan Omar, W.I.; Morsin, M.; Nayan, N.; Tee, K.S. Synthesis, characterization and biophysical evaluation of the 2D Ti2CTx MXene using 3D spheroid-type cultures. Ceram. Int. 2021, 47, 22567–22577. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Lin, H.; Mo, A. 2D titanium carbide(MXene) nanosheets and 1D hydroxyapatite nanowires into free standing nanocomposite membrane: In vitro and in vivo evaluations for bone regeneration. Mater. Sci. Eng. C 2021, 118, 111367. [Google Scholar] [CrossRef]
- Alhussain, H.; Augustine, R.; Hussein, E.A.; Gupta, I.; Hasan, A.; Al Moustafa, A.-E.; Elzatahry, A. MXene Nanosheets May Induce Toxic Effect on the Early Stage of Embryogenesis. J. Biomed. Nanotechnol. 2020, 16, 364–372. [Google Scholar] [CrossRef]
- Nasrallah, G.K.; Al-Asmakh, M.; Rasool, K.; Mahmoud, K.A. Ecotoxicological assessment of Ti3C2Tx (MXene) using a zebrafish embryo model. Environ. Sci. Nano 2018, 5, 1002–1011. [Google Scholar] [CrossRef]
- Rozmysłowska-Wojciechowska, A.; Karwowska, E.; Poźniak, S.; Wojciechowski, T.; Chlubny, L.; Olszyna, A.; Ziemkowska, W.; Jastrzębska, A.M. Influence of modification of Ti3C2 MXene with ceramic oxide and noble metal nanoparticles on its antimicrobial properties and ecotoxicity towards selected algae and higher plants. RSC Adv. 2019, 9, 4092–4105. [Google Scholar] [CrossRef]
- Joung, Y.-H. Development of Implantable Medical Devices: From an Engineering Perspective. Int. Neurourol. J. 2013, 17, 98. [Google Scholar] [CrossRef]
- Xu, B.; Zhi, C.; Shi, P. Latest advances in MXene biosensors. J. Phys. Mater. 2020, 3, 031001. [Google Scholar] [CrossRef]
- Kim, S.J.; Koh, H.-J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.-Y.; Anasori, B.; Kim, C.-K.; Choi, Y.-K.; Kim, J.; et al. Metallic Ti3C2 Tx MXene Gas Sensors with Ultrahigh Signal-to-Noise Ratio. ACS Nano 2018, 12, 986–993. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Hazra, A. MXene-based gas sensors. J. Mater. Chem. C 2021, 9, 15735–15754. [Google Scholar] [CrossRef]
- Xin, M.; Li, J.; Ma, Z.; Pan, L.; Shi, Y. MXenes and Their Applications in Wearable Sensors. Front. Chem. 2020, 8, 297. [Google Scholar] [CrossRef]
- Mehdi Aghaei, S.; Aasi, A.; Panchapakesan, B. Experimental and Theoretical Advances in MXene-Based Gas Sensors. ACS Omega 2021, 6, 2450–2461. [Google Scholar] [CrossRef]
- Hermawan, A.; Zhang, B.; Taufik, A.; Asakura, Y.; Hasegawa, T.; Zhu, J.; Shi, P.; Yin, S. CuO Nanoparticles/Ti3C2Tx MXene Hybrid Nanocomposites for Detection of Toluene Gas. ACS Appl. Nano Mater. 2020, 3, 4755–4766. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F.; Hermawan, A.; Asakura, Y.; Hasegawa, T.; Kumagai, H.; Kato, H.; Kakihana, M.; Zhu, J.; Yin, S. SnO-SnO2 modified two-dimensional MXene Ti3C2T for acetone gas sensor working at room temperature. J. Mater. Sci. Technol. 2021, 73, 128–138. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F.; Hermawan, A.; Zhu, J.; Yin, S. Surface engineering of Ti3C2Tx MXene by oxygen plasma irradiation as room temperature ethanol sensor. Funct. Mater. Lett. 2022, 15, 2251007. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F.; Hermawan, A.; Zhu, J.; Yin, S. A facile method for preparation of porous nitrogen-doped Ti3C2Tx MXene for highly responsive acetone detection at high temperature. Funct. Mater. Lett. 2021, 14, 2151043. [Google Scholar] [CrossRef]
- Wu, M.; An, Y.; Yang, R.; Tao, Z.; Xia, Q.; Hu, Q.; Li, M.; Chen, K.; Zhang, Z.; Huang, Q.; et al. V2CTx and Ti3C2Tx MXenes Nanosheets for Gas Sensing. ACS Appl. Nano Mater. 2021, 4, 6257–6268. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Xia, Y. Mesoporous MXene/ZnO nanorod hybrids of high surface area for UV-activated NO2 gas sensing in ppb-level. Sens. Actuators B Chem. 2022, 353, 131087. [Google Scholar] [CrossRef]
- Xu, Q.; Zong, B.; Li, Q.; Fang, X.; Mao, S.; Ostrikov, K. (Ken) H2S sensing under various humidity conditions with Ag nanoparticle functionalized Ti3C2Tx MXene field-effect transistors. J. Hazard. Mater. 2022, 424, 127492. [Google Scholar] [CrossRef]
- Sun, B.; Qin, F.; Jiang, L.; Gao, J.; Liu, Z.; Wang, J.; Zhang, Y.; Fan, J.; Kan, K.; Shi, K. Room-temperature gas sensors based on three-dimensional Co3O4/Al2O3@Ti3C2T MXene nanocomposite for highly sensitive NO detection. Sens. Actuators B Chem. 2022, 368, 132206. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Y.; Liu, B.; Duan, Z.; Pan, H.; Yuan, Z.; Xie, G.; Wang, J.; Fang, Z.; Tai, H. Ultrathin Nb2CT nanosheets-supported polyaniline nanocomposite: Enabling ultrasensitive NH3 detection. Sens. Actuators B Chem. 2021, 343, 130069. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, D.; Wang, S.; Duan, Z.; Yuan, Z.; Wei, G.; Xu, J.-L.; Tai, H.; Jiang, Y. Enhanced Blocking Effect: A New Strategy to Improve the NO2 Sensing Performance of Ti3C2Tx by γ-Poly(L-glutamic acid) Modification. ACS Sens. 2021, 6, 2858–2867. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, R.; Sa, B.; Zhou, J.; Sun, Z. MXenes: Promising donor and acceptor materials for high-efficiency heterostructure solar cells. Sustain. Energy Fuels 2021, 5, 135–143. [Google Scholar] [CrossRef]
- Bati, A.S.R.; Sutanto, A.A.; Hao, M.; Batmunkh, M.; Yamauchi, Y.; Wang, L.; Wang, Y.; Nazeeruddin, M.K.; Shapter, J.G. Cesium-doped Ti3C2Tx MXene for efficient and thermally stable perovskite solar cells. Cell Rep. Phys. Sci. 2021, 2, 100598. [Google Scholar] [CrossRef]
- Dong, Y.; Mallineni, S.S.K.; Maleski, K.; Behlow, H.; Mochalin, V.N.; Rao, A.M.; Gogotsi, Y.; Podila, R. Metallic MXenes: A new family of materials for flexible triboelectric nanogenerators. Nano Energy 2018, 44, 103–110. [Google Scholar] [CrossRef]
- Wu, T.; Pang, X.; Zhao, S.; Xu, S.; Liu, Z.; Li, Y.; Huang, F. One-Step Construction of Ordered Sulfur-Terminated Tantalum Carbide MXene for Efficient Overall Water Splitting. Small Struct. 2022, 3, 2100206. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, H.; Hu, T.; Fan, B.; Wang, X.; Li, Z. Emerging 2D MXenes for supercapacitors: Status, challenges and prospects. Chem. Soc. Rev. 2020, 49, 6666–6693. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Choi, C.; Ashby, D.S.; Butts, D.M.; DeBlock, R.H.; Wei, Q.; Lau, J.; Dunn, B. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 2020, 5, 5–19. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Peng, Y.-Y.; Akuzum, B.; Kurra, N.; Zhao, M.-Q.; Alhabeb, M.; Anasori, B.; Kumbur, E.C.; Alshareef, H.N.; Ger, M.-D.; Gogotsi, Y. All-MXene (2D titanium carbide) solid-state microsupercapacitors for on-chip energy storage. Energy Environ. Sci. 2016, 9, 2847–2854. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Z.; Fang, B.; Huang, T.; Cai, S.; Chen, H.; Liu, Y.; Gopalsamy, K.; Gao, W.; Gao, C. MXene/graphene hybrid fibers for high performance flexible supercapacitors. J. Mater. Chem. A 2017, 5, 22113–22119. [Google Scholar] [CrossRef]
- Fan, Q.; Zhao, R.; Yi, M.; Qi, P.; Chai, C.; Ying, H.; Hao, J. Ti3C2-MXene composite films functionalized with polypyrrole and ionic liquid-based microemulsion particles for supercapacitor applications. Chem. Eng. J. 2022, 428, 131107. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, J.; Wang, S.; Wang, B.; Shen, C.; Wang, L.; Hu, Q.; Huang, Q.; Zhou, A. Preparation of High-Purity V2C MXene and Electrochemical Properties as Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A709–A713. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; Lian, W.; Hu, Q.; Liu, X.; Zhou, A. The preparation of V2CTx by facile hydrothermal-assisted etching processing and its performance in lithium-ion battery. J. Mater. Res. Technol. 2020, 9, 984–993. [Google Scholar] [CrossRef]
- Du, F.; Tang, H.; Pan, L.; Zhang, T.; Lu, H.; Xiong, J.; Yang, J.; Zhang, C. (John) Environmental Friendly Scalable Production of Colloidal 2D Titanium Carbonitride MXene with Minimized Nanosheets Restacking for Excellent Cycle Life Lithium-Ion Batteries. Electrochim. Acta 2017, 235, 690–699. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, Q.; Miao, J.; Zhang, P.; Wan, P.; He, L.; Xu, B. Flexible 3D Porous MXene Foam for High-Performance Lithium-Ion Batteries. Small 2019, 15, 1904293. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Man Hong, S.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Feng, J. Flexible and Freestanding Silicon/MXene Composite Papers for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 10004–10011. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Zhu, Y.; Cao, B.; Zhu, Q.; Zhang, P.; Xu, B.; Wu, F.; Chen, R. Electrostatic Self-assembly of 0D–2D SnO2 Quantum Dots/Ti3C2Tx MXene Hybrids as Anode for Lithium-Ion Batteries. Nano Micro Lett. 2019, 11, 65. [Google Scholar] [CrossRef]
- Shen, C.; Wang, L.; Zhou, A.; Zhang, H.; Chen, Z.; Hu, Q.; Qin, G. MoS 2 -Decorated Ti3C2 MXene Nanosheet as Anode Material in Lithium-Ion Batteries. J. Electrochem. Soc. 2017, 164, A2654–A2659. [Google Scholar] [CrossRef]
- Chen, H.; Ke, G.; Wu, X.; Li, W.; Li, Y.; Mi, H.; Sun, L.; Zhang, Q.; He, C.; Ren, X. Amorphous MoS3 decoration on 2D functionalized MXene as a bifunctional electrode for stable and robust lithium storage. Chem. Eng. J. 2021, 406, 126775. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, H.; Zhang, X.; Xu, Y.; Fransaer, J. Cu2O Hybridized Titanium Carbide with Open Conductive Frameworks for Lithium-ion Batteries. Electrochim. Acta 2016, 202, 24–31. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, C.; Yi, R.; Li, Z.; Chen, Y.; Li, Y.; Mitrovic, I.; Taylor, S.; Chalker, P.; Yang, L.; et al. Facile preparation of Co3O4 nanoparticles incorporating with highly conductive MXene nanosheets as high-performance anodes for lithium-ion batteries. Electrochim. Acta 2020, 345, 136203. [Google Scholar] [CrossRef]
- Liang, X.; Garsuch, A.; Nazar, L.F. Sulfur Cathodes Based on Conductive MXene Nanosheets for High-Performance Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2015, 54, 3907–3911. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Su, D.; Xie, X.; Guo, X.; Bao, W.; Shao, G.; Wang, G. Immobilizing Polysulfides with MXene-Functionalized Separators for Stable Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2016, 8, 29427–29433. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Zhang, C.; Geng, C.; Wu, S.; Li, H.; Lv, W.; Tao, Y.; Chen, Z.; Zhou, G.; Li, J.; et al. Capture and Catalytic Conversion of Polysulfides by In Situ Built TiO2-MXene Heterostructures for Lithium–Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1900219. [Google Scholar] [CrossRef]
- Xie, X.; Kretschmer, K.; Anasori, B.; Sun, B.; Wang, G.; Gogotsi, Y. Porous Ti3C2Tx MXene for Ultrahigh-Rate Sodium-Ion Storage with Long Cycle Life. ACS Appl. Nano Mater. 2018, 1, 505–511. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, R.; Dong, S.; Miao, X.; Zhang, Z.; Wang, C.; Yin, L. Alkali-induced 3D crinkled porous Ti3C2 MXene architectures coupled with NiCoP bimetallic phosphide nanoparticles as anodes for high-performance sodium-ion batteries. Energy Environ. Sci. 2019, 12, 2422–2432. [Google Scholar] [CrossRef]
- Cao, J.; Wang, L.; Li, D.; Yuan, Z.; Xu, H.; Li, J.; Chen, R.; Shulga, V.; Shen, G.; Han, W. Ti3C2Tx MXene Conductive Layers Supported Bio-Derived Fex−1Sex/MXene/Carbonaceous Nanoribbons for High-Performance Half/Full Sodium-Ion and Potassium-Ion Batteries. Adv. Mater. 2021, 33, 2101535. [Google Scholar] [CrossRef]
- Zheng, J.; Ju, S.; Yao, L.; Xia, G.; Yu, X. Construction of solid solution sulfide embedded in MXene@N-doped carbon dual protection matrix for advanced aluminum ion batteries. J. Power Source 2021, 511, 230450. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Xiong, S.; Feng, J.; Qian, Y. A general method for constructing robust, flexible and freestanding MXene@metal anodes for high-performance potassium-ion batteries. J. Mater. Chem. A 2019, 7, 9716–9725. [Google Scholar] [CrossRef]
- Wang, T.; Shen, D.; Liu, H.; Chen, H.; Liu, Q.; Lu, B. A Sb2S3 Nanoflower/MXene Composite as an Anode for Potassium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 57907–57915. [Google Scholar] [CrossRef]
- Zhao, M.-Q.; Ren, C.E.; Alhabeb, M.; Anasori, B.; Barsoum, M.W.; Gogotsi, Y. Magnesium-Ion Storage Capability of MXenes. ACS Appl. Energy Mater. 2019, 2, 1572–1578. [Google Scholar] [CrossRef]
- Yun, T.; Kim, H.; Iqbal, A.; Cho, Y.S.; Lee, G.S.; Kim, M.; Kim, S.J.; Kim, D.; Gogotsi, Y.; Kim, S.O.; et al. Electromagnetic Shielding of Monolayer MXene Assemblies. Adv. Mater. 2020, 32, 1906769. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Sambyal, P.; Koo, C.M. 2D MXenes for Electromagnetic Shielding: A Review. Adv. Funct. Mater. 2020, 30, 2000883. [Google Scholar] [CrossRef]
- Han, M.; Shuck, C.E.; Rakhmanov, R.; Parchment, D.; Anasori, B.; Koo, C.M.; Friedman, G.; Gogotsi, Y. Beyond Ti3C2Tx: MXenes for Electromagnetic Interference Shielding. ACS Nano 2020, 14, 5008–5016. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Singha, K.; Pandit, P. Advanced applications of green materials in electromagnetic shielding. In Applications of Advanced Green Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 265–292. ISBN 978-0-12-820484-9. [Google Scholar]
- Li, Y.; Tian, X.; Gao, S.; Jing, L.; Li, K.; Yang, H.; Fu, F.; Lee, J.Y.; Guo, Y.; Ho, J.S.; et al. Reversible Crumpling of 2D Titanium Carbide (MXene) Nanocoatings for Stretchable Electromagnetic Shielding and Wearable Wireless Communication. Adv. Funct. Mater. 2020, 30, 1907451. [Google Scholar] [CrossRef]
- Ma, C.; Cao, W.-T.; Zhang, W.; Ma, M.-G.; Sun, W.-M.; Zhang, J.; Chen, F. Wearable, ultrathin and transparent bacterial celluloses/MXene film with Janus structure and excellent mechanical property for electromagnetic interference shielding. Chem. Eng. J. 2021, 403, 126438. [Google Scholar] [CrossRef]
- He, P.; Wang, X.-X.; Cai, Y.-Z.; Shu, J.-C.; Zhao, Q.-L.; Yuan, J.; Cao, M.-S. Tailoring Ti 3 C 2 T x nanosheets to tune local conductive network as an environmentally friendly material for highly efficient electromagnetic interference shielding. Nanoscale 2019, 11, 6080–6088. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.-T.; Chen, F.-F.; Zhu, Y.-J.; Zhang, Y.-G.; Jiang, Y.-Y.; Ma, M.-G.; Chen, F. Binary Strengthening and Toughening of MXene/Cellulose Nanofiber Composite Paper with Nacre-Inspired Structure and Superior Electromagnetic Interference Shielding Properties. ACS Nano 2018, 12, 4583–4593. [Google Scholar] [CrossRef] [PubMed]
- Sambyal, P.; Iqbal, A.; Hong, J.; Kim, H.; Kim, M.-K.; Hong, S.M.; Han, M.; Gogotsi, Y.; Koo, C.M. Ultralight and Mechanically Robust Ti3C2Tx Hybrid Aerogel Reinforced by Carbon Nanotubes for Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2019, 11, 38046–38054. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-W.; Zhang, H.-B.; Liu, J.; Zhao, S.; Xie, X.; Liu, L.; Yang, R.; Koratkar, N.; Yu, Z.-Z. Multifunctional and Water-Resistant MXene-Decorated Polyester Textiles with Outstanding Electromagnetic Interference Shielding and Joule Heating Performances. Adv. Funct. Mater. 2019, 29, 1806819. [Google Scholar] [CrossRef]
- Murali, G.; Reddy Modigunta, J.K.; Park, Y.H.; Lee, J.-H.; Rawal, J.; Lee, S.-Y.; In, I.; Park, S.-J. A Review on MXene Synthesis, Stability, and Photocatalytic Applications. ACS Nano 2022, 16, 13370–13429. [Google Scholar] [CrossRef]
- Barsoum, M.W. On the Synthesis of Low-Cost, Titanium-Based MXenes. J. Ceram. Sci. Technol. 2016, 7, 301–306. [Google Scholar] [CrossRef]
- Dash, A.; Vaßen, R.; Guillon, O.; Gonzalez-Julian, J. Molten salt shielded synthesis of oxidation prone materials in air. Nat. Mater. 2019, 18, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Bärmann, P.; Haneke, L.; Wrogemann, J.M.; Winter, M.; Guillon, O.; Placke, T.; Gonzalez-Julian, J. Scalable Synthesis of MAX Phase Precursors toward Titanium-Based MXenes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 26074–26083. [Google Scholar] [CrossRef] [PubMed]




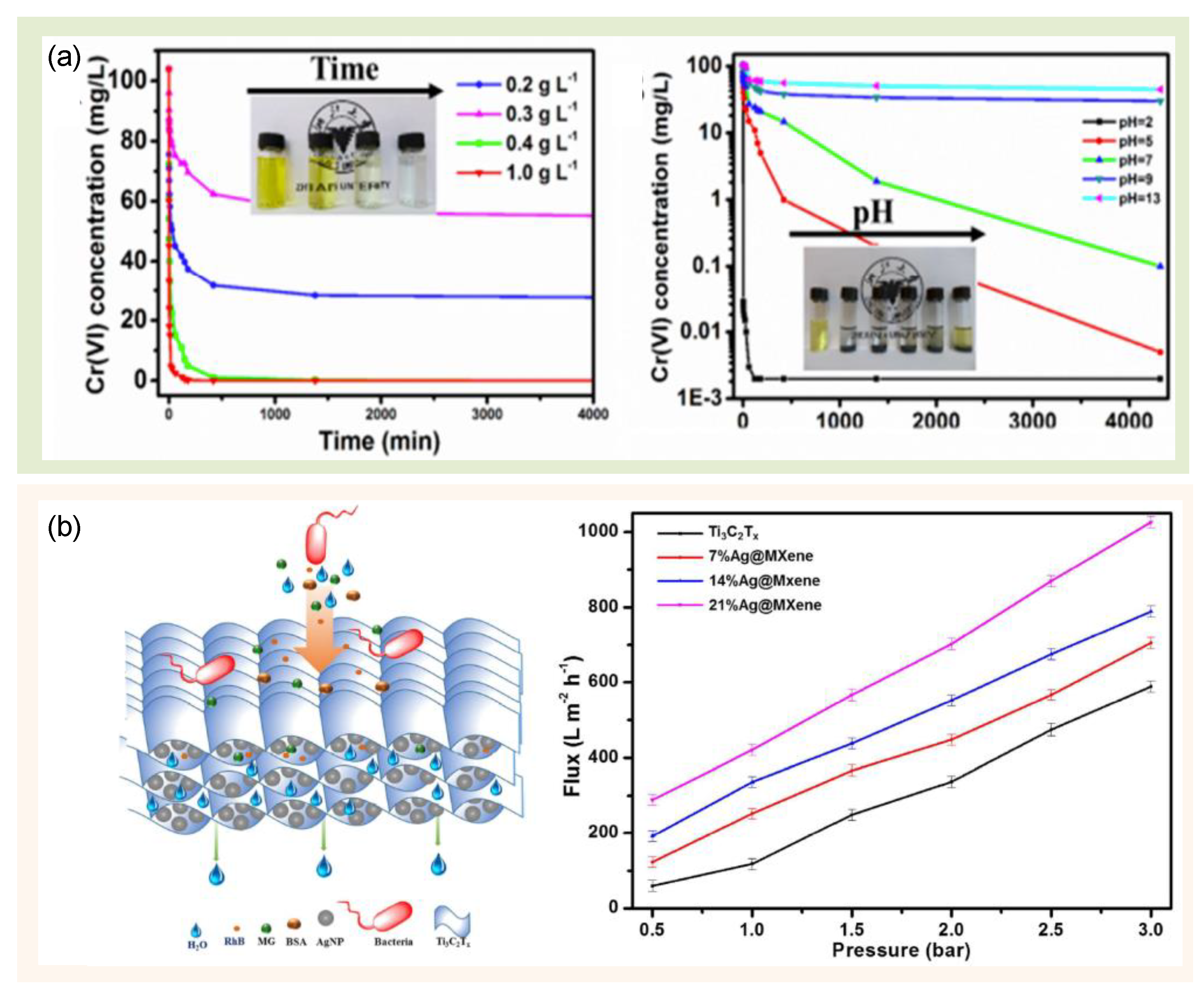


| Type of MXene/MXene-Based Materials | Fabrication Method | Toxicity Test Approach | Toxicity Test Assay | Findings | References |
|---|---|---|---|---|---|
| MnOx/ Ta4C3-SP composite nanosheets | Two-step exfoliation | In vitro 4T1 (mouse mammary tumor) cell line | CCK-8 | MnOx/Ta4C3-SP does not affect 4T1 cell survival even at high concentrations for 24 and 28 h, indicating excellent cytocompatibility. | [119] |
| Ti3C2, Ti3C2-SP nanosheets | Chemical exfoliation and intercalation | In vitro 4T1 (mouse mammary tumor) cell line | CCK-8 | The toxicity of Ti3C2-SP to 4T1 breast cancer cells is assessed for 24 and 48 h and revealed no effect on the survival of 4T1 cells. | [120] |
| Ti3C2 | Etching | In vitro HCT-116 (human colorectal carcinoma cell line) and A2780 (ovarian cancer cell line) | MTT assay | The cytotoxicity of Ti3C2 nanosheets is assessed using an MTT assay, indicating that cytotoxicity is dose-dependent and cell-type-dependent. | [121] |
| Ti3AlC2, Ti3SiC2, and Ti2AlN | Hot pressing and in situ sintering | In vitro MC3T3-E1 (mouse pre-osteoblast) and L929 (mouse fibroblast) cell lines | MTS assay | Compared to commercial Ti–6Al–4V alloy and pure Ti, all phases are not toxic to pre-osteoblasts and fibroblasts cell lines. Ti2AlN performed best in the MAX phases for cell proliferation and differentiation. | [122] |
| Ti3C2 | Self-propagation high-temperature synthesis (SHS) | In vitro A549 (human alveolar basal epithelial cells), MRC-5 (human normal lung cells), A375 (human skin malignant melanoma cells), and HaCaT (human immortalized keratinocytes) | MTT assay | Toxicity increases with the concentration of MXene. The results reveal that the toxic effects are higher against cancerous (A549 and A375) cells than they are against normal (MRC-5 and HaCaT) cells. | [123] |
| Ti3C2 QDs | Etching-assisted exfoliation method, mechanical force-assisted liquid exfoliation | In vitro HeLa (human cervical cancer), MCF-7 (human breast cancer), U251 (human malignant glioblastoma), and HEK 293 (human embryonic kidney) cells | MTT assay | MXene QDs show no cytotoxicity to all tested cell lines (HeLa, MCF-7, U251, and HEK 293), even at the highest concentration of 100 ppm. Data obtained show excellent biocompatibility and indicate high clinical potential application. | [37] |
| Ti3C2-based MXene Integrated Cellulose Hydrogels | Etching and exfoliation | In vitro HepA1-6 (mouse hepatoma cells), SMMC-7721 and HepG2 (human hepatocellular carcinoma cells), U-118MG (human glioblastoma cells), and U-251MG (human astroglioma cells) | CCK-8 assay | The Ti3C2-based MXene integrated cellulose hydrogel exhibits excellent cellular biocompatibility as the addition of Ti3C2 MXene nanosheets to hydrogels do not affect cell viability regardless of MXene concentration. MXene integration into the hydrogel also reduces in vitro toxicity compared to dispersed MXenes. | [124] |
| Ti3C2-SP ultrathin nanosheets | Two-step exfoliation | In vitro 4T1 (mouse mammary tumor) cell line | CCK-8 assay | In vitro cytotoxicity of Ti3C2-SP using CCK-8 assay indicates that 4T1 cells treated with Ti3C2-SP at various concentrations for 12, 24, and 48 h have no obvious cytotoxicity, even at the highest concentration of 600 µg mL−1. | [111] |
| Ta4C3-IONP-SP | Liquid exfoliation | In vitro 4T1 (mouse mammary tumor) cell line | CCK-8 assay | In breast cancer cell lines, 4T1 cells exposed to various concentrations of Ta4C3-IONP-SP indicate no detectable cytotoxicity, even at the highest concentration of 200 ppm for 24 h. | [125] |
| TiC, Ti2AlC, and Ti3AlC2 | Etching, etching coupled with intercalation | In vitro HeLa (cervical cancer cells) and MSU1.1 (normal fibroblasts) | WST-1 assay, Live/Dead assay | TiC, Ti2AlC, and Ti3AlC2 at concentrations (≥400 μg/mL) induce a significant cytotoxic effect in HeLa cells, while MSU1.1 cells demonstrate slight cytotoxic behavior for all Ti3C2Tx forms. The cytotoxicity is also cell-type-dependent, with cancer cells exhibiting greater toxicity than normal cells do. | [126] |
| Ti2C-PEG | Etching | In vitro A375 (human skin malignant melanoma cells), HaCaT (human immortalized keratinocytes), MCF-7 (human breast cancer cells), and MCF-10A (normal human mammary epithelial cells) | MTT assay | Normal (nonmalignant) cells retain 70% viability after 24 h of exposure to Ti2C-PEG MXene flakes, indicating that delaminated Ti2C-PEG is biocompatible. Ti2C-PEG is cytotoxic to a malignant breast cancer cell line. In comparison, at all concentrations tested, MXenes have a negligible effect on the viability of skin cancer cells. After 48 h of exposure, the viability of each cell line decreases significantly, indicating that Ti2C flakes are toxic in a time-dependent manner. | [127] |
| Ti3C2Tx-based nanocomposites (Au/MXene and Au/Fe3O4/MXene) | In situ reduction of tetrachloroauric acid using NaBH4 | In vitro MCF7 (human breast cancer/adenocarcinoma cell line) | Alamar Blue assay | Both new composites inhibit human breast cancer cells MCF7 in vitro in a dose-dependent manner. Even at high concentrations, no cytotoxicity is observed, indicating the composites’ high biocompatibility. | [128] |
| Multilayered Ti3C2Tx | Etching | In vitro MC3T3-E1 (pre-osteoblast cell line) | EdU-488 assay, Live/dead double staining | Cells cultured on MXene films show no evidence of cytotoxicity as assessed using the EdU assay. Ti3C2Tx MXene demonstrates favorable cytocompatibility, cell spreading, and proliferation, proving that Ti3C2Tx MXene is extremely biocompatible in vitro. | [129] |
| Ti3C2 and Ti2C | Self-propagation high-temperature synthesis (SHS) | In vitro A375 (Human skin malignant melanoma cells), HaCaT (human immortalized keratinocytes), MCF-7 (human breast cancer cells), and MCF-10A (Mammary epithelial cells) | MTT assay | Biocompatibility of 2D Ti3C2 and Ti2C MXenes enhances post-modification with collagen compared to cultures exposed to pure 2D MXenes. Additionally, the reduction in cell viability is negligible across a broad tested concentration range. | [130] |
| Ti3C2 | LiF/HCl delamination method followed by post-treatment using probe sonication and thermal oxidation | In vitro MCF-10A (human epithelial breast), MCF-7 (breast cancer), HaCaT (human immortalized keratinocytes), A375 (human malignant melanoma) | MTT assay | Selective cytotoxicity is against tumor cells compared to normal cells, even at high concentrations of tested materials. The most cytotoxic effect is seen in samples that are thermally oxidized. The thermally oxidized samples are also cytotoxic to all cancer cell lines. | [131] |
| Nb2C quantum dots | Hydrothermal method and nitrogen and sulfur co-doping | In vitro Caco-2 (human colorectal adenocarcinoma) cells | CCK-8 assay | Cellular viability is completely lost at the highest concentration of 20 mg/mL. None of the concentrations tested at lower concentrations of less than 10 mg/mL reduce cellular viability. | [132] |
| Non-oxidized MXene-Ti3C2Tx quantum dots (NMQDs-Ti3C2Tx) | Micro-explosion method | In vitro HeLa (cervical cancer cell), MCF-7 (breast cancer cell), and normal ADSCs (Adipose-derived stem cells) | CCK-8 assay | NMQDs-Ti3C2Tx selectively kills cancer cells. | [133] |
| Ti3C2 | Etching | In vitro Primary mouse and derived NSCs (neural stem cells) | CCK-8 assay | MXene biocompatibility in the nervous system and NSC-Ti3C2 nanosheet interactions are evaluated. Ti3C2 shows dose-dependent toxicity towards primary NSCs and differentiated NSCs. Ti3C2 nanosheets cause apoptosis, membrane disruption, stress, and inflammation at higher concentrations. | [134] |
| Ti3C2 and Nb2C quantum dots | Acid reflux followed by hydrothermal method | In vitro HUVECs (human umbilical vein endothelial cells) | CCK-8 assay | Ti3C2 QDs are more toxic than Nb2C QDs are, and HUVEC toxicity is expected owing to the metal ions. The morphology of QDs-based also contributes to cytotoxicity. | [135] |
| Ti3C2 | Intercalation | In vitro hMSCs, (human mesenchymal stem cells) | MTS assay | MXene concentrations >50 µg/mL are cytotoxic over 7 days of the exposure period. | [136] |
| V2AlC, m-V2CTz, pristine and oxidized s-V2CTz | Etching | In vitro A375 (human skin malignant melanoma cells), HaCaT (human immortalized keratinocytes) | MTT assay | Immortalized keratinocyte (HaCaT) and malignant melanomas (A375) human cell lines are exposed to s-V2CTz-ox24 and s- V2CTz -ox48 flakes. At concentrations above 50 µg/mL, only 50% of cells are viable, suggesting oxidized V2CTz increases cytotoxicity toward human cells. | [137] |
| Ti3C2, Ti3C2-PEG, Ti3C2-PPG | Etching | In vitro MCF-7 (human breast cancer cells), MCF-10A (normal human mammary epithelial cells), A375 (human skin malignant melanoma cells), and HaCaT (human immortalized keratinocytes) | MTT assay | MXene cytotoxicity is directly related to cell line type, with HaCaT being the least toxic and A375 being the most. PEGylated and PPGylated MXenes show increased toxicity to both normal and cancerous cell lines. | [138] |
| Ti3C2Tx -PVA Free-standing Ti3C2Tx and PVA-Ti3C2Tx films | Etching and vacuum-assisted filtration | In vitro HUVECs (human umbilical vein endothelial cells) | Live/dead assay | There is no evidence of cytotoxicity based on the assay. More live cells (green) than dead cells (red) are observed, indicating that most HUVECs are healthy for 7 days with viability of 99.8%. PVA-MXene’s in vitro biocompatibility is critical for biomedical applications. | [139] |
| Ti2CTX | Etching | In vitro HeLa (cervical cancer cell) | MTT assay, calcein-AM staining | Two-dimensional and three-dimensional model HeLa cells are used to assess the cytotoxicity of Ti2CTx MXenes. Two-dimensional culture system shows that cells’ viability decreased to half with increasing Ti2CTx MXenes concentration. Three-dimensional spheroid better mimics real physiological conditions, so cells are highly viable after 15 days of culture at the lowest concentration of Ti2CTx MXenes, and some cells are found non-viable when concentration increases to 500 μg/mL. | [140] |
| Ti3C2Tx-UHAPNWs | Etching | In vitro MC3T3-E1 (pre-osteoblast cell line) | Live/dead double staining | Staining for live/dead cells demonstrates no discernible difference between cells seeded on glass and UHAPNWs/MXene, indicating that the films are non-toxic. MC3T3-E1 cells are tightly attached to the surface and appear flattened, spread more rapidly, and even confluence. Osteogenic differentiation in MC3T3-E1 cultures grown on Ti3C2Tx nanosheet also increases. | [141] |
| Type of MXene/MXene-Based Materials | Fabrication Method | Model of the Living Organism Used | Findings | References |
|---|---|---|---|---|
| Ti3C2 QDs | Etching-assisted exfoliation method, mechanical force-assisted liquid exfoliation | Balb/c mice | There are no obvious signs of abnormal mouse weight, diet, or activity. H and E staining reveals no obvious tissue or organ damage. MXene QDs are not toxic to mice at the dosages examined, which could be attributed to the synthesis using ultrasonication rather than to the toxic organic solvents and chemicals, which ensures the safe use of MXene QDs in medicine. | [37] |
| MnOx/^breakTa4C3-SP composite nanosheets | Two-step exfoliation | Balb/c mice | When they are used as intended, the MnOx/ Ta4C3-SP composite nanosheets are highly biocompatible and biosafe. The authors use a rational chemical composition (Ta4C3) and surface engineering for functionalization (MnOx integration). After 60 days, mice treated with MnOx/ Ta4C3-SP remains healthy with no evidence of tumor recurrence, confirming the high therapeutic efficacy of MnOx/Ta4C3-SP composite nanosheets in vivo. | [119] |
| Ti3C2 | Etching | Athymic nude mice | Histopathologically, H and E staining reveals the formation of small nuclei in the tumors of DOX and Ti3C2-DOX-treated mice, indicating cell apoptosis. The low dose and the stimuli-responsive drug release prevented significant organ damage. There are no significant morphology and pathology changes in the treated mice. | [121] |
| Ti3C2-SP | Two-step exfoliation | Balb/c mice | No significant morphology and pathology changes in major organs of treated mice suggest that Ti3C2-SP nanosheets have no obvious acute toxicity and side effects. Intravenous Ti3C2-SP injection can be easily excreted from the body via urine and feces. | [111] |
| Ta4C3-IONP-SP | Liquid exfoliation | BALB/c mice | The in vivo biocompatibility of Ta4C3-IONP-SP composite nanosheets are assessed in four groups of healthy Kunming mice (5, 10, and 20 mg kg−1). Ta4C3-IONP-SP is given intravenously for a month. None of the mice lose weight or die. The experimental mice’s main organ H and E staining sections show no significant damage or acute inflammation compared to the control group. Based on preliminary biocompatibility data, Ta4C3-IONP-SP composite nanosheets are safe to use in clinical settings, especially when used to guide hyperthermia and ablation of breast tumors in vivo. | [125] |
| Ti3C2Tx-based nanocomposites (Au/MXene and Au/Fe3O4/MXene) | In situ reduction of tetrachloroauric acid using NaBH4 | Zebrafish embryo | Au/MXene and Au/Fe3O4/MXene-treated embryo groups normally develop. Data obtained indicate that Au/MXene and Au/MXene have almost no acute toxic or teratogenic effect on zebrafish embryos. Au/MXene and Au/Fe3O4/MXene are biocompatible and safe when compared to pure MXene. | [128] |
| Multilayered Ti3C2Tx | Etching | Sprague Dawley rats | Inflammatory reactions are not seen in nearly all defect spaces under MXene films. The regenerated bone is flat and uniform with an osteoid collagen fiber separated MXene films from the osteoid tissue. The MXene group’s new bone volume is much larger than the control group’s one is. The host tissue response to Ti3C2Tx MXene films confirms their safety and high biocompatibility in vivo. Morphologically, MXene films promote early osteogenesis, mineralization, and bone regeneration in rats. These findings show Ti3C2Tx MXene is highly biocompatible. | [129] |
| Non-oxidized MXene- Ti3C2Tx quantum dots (NMQDs-Ti3C2Tx) | Micro-explosion method | BALB/c mice | The NMQDs- Ti3C2Tx killing tumor spreads from within. The H and E staining reveals significant destruction of tumor cells, and TUNEL images show increased cell apoptosis. NMQDs- Ti3C2Tx does not cause obvious pathological changes in the tissues of the heart, liver, spleen, lung, and kidney, demonstrating its high biocompatibility in vivo. | [133] |
| Ti₃C₂Tx | Etching | Avian embryos | The toxicity of MXene nanosheets on early embryonic development and angiogenesis is evaluated using 3- and 5-day-old avian embryos as a model. Forty-six percent of MXene-exposed embryos die 1–5 days after exposure, indicating MXene may cause early embryonic death. After 5 days of exposure to MXene, the inhibition of embryonic chorioallantoic membrane angiogenesis is detected. | [142] |
| Ti3C2Tx-UHAPNWs | Etching | Sprague Dawley rats | MXene films can promote osteogenic differentiation and bone formation, which accelerate bone regeneration in a calvarial bone defect in rats. The fabricated nanocomposite films’ novel structure and surface morphology result in excellent mechanical properties, biocompatibility, and osteoinductivity. MXene and MXene-based nanocomposites are critical in biomedical applications, particularly bone regeneration. | [141] |
| Ti3C2Tx | Delaminating and ultrasonication | Zebrafish embryo | No teratogenic effects on zebrafish embryos are observed at 100 µM, where Ti3C2Tx is in a homogeneous solution. High concentrations of Ti3C2Tx (>100 µM) have a minimal teratogenic effect on embryos. The mortality effect may be due to the Ti3C2Tx aggregation, and the embryos cannot tolerate it. Ti3C2Tx is completely non-toxic to aquatic life. It may be necessary to assess the toxicity of Ti3C2Tx (MXene) nanosheets in aquatic ecosystems other than zebrafishes. | [143] |
| Ti3C2 MXene with ceramic oxide and noble metal | SHS technique with a local ignition system | Green algae Desmodesmus quadricauda | Pristine Ti3C2 MXene stimulates algal growth at low concentrations. The effect of nano component concentration on stimulation decreases. Ecotoxicity against algae depends not only on concentration but also on modification type (ceramic oxide and noble metal used). | [144] |
| Ti3C2 MXene with ceramic oxide and noble metal | SHS technique with a local ignition system | Seed of sorghum (Sorghum saccharatum) and charlock (Sinapis alba) | Modification of Ti3C2 MXene is used to study its phytotoxicity. It becomes more phytotoxic when it is modified with SiO2/Ag or SiO2/Pd is added, but not when Ti3C2/Al2O3/Ag is added. The modified nanocomposites have a lower inhibitory effect on germination than Ti3C2 MXene. When different nanoparticles are added to pure Ti3C2 MXene, its phytotoxic properties can change. | [144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amrillah, T.; Abdullah, C.A.C.; Hermawan, A.; Sari, F.N.I.; Alviani, V.N. Towards Greener and More Sustainable Synthesis of MXenes: A Review. Nanomaterials 2022, 12, 4280. https://doi.org/10.3390/nano12234280
Amrillah T, Abdullah CAC, Hermawan A, Sari FNI, Alviani VN. Towards Greener and More Sustainable Synthesis of MXenes: A Review. Nanomaterials. 2022; 12(23):4280. https://doi.org/10.3390/nano12234280
Chicago/Turabian StyleAmrillah, Tahta, Che Azurahanim Che Abdullah, Angga Hermawan, Fitri Nur Indah Sari, and Vani Novita Alviani. 2022. "Towards Greener and More Sustainable Synthesis of MXenes: A Review" Nanomaterials 12, no. 23: 4280. https://doi.org/10.3390/nano12234280
APA StyleAmrillah, T., Abdullah, C. A. C., Hermawan, A., Sari, F. N. I., & Alviani, V. N. (2022). Towards Greener and More Sustainable Synthesis of MXenes: A Review. Nanomaterials, 12(23), 4280. https://doi.org/10.3390/nano12234280







