Direct Polyphenol Attachment on the Surfaces of Magnetite Nanoparticles, Using Vitis vinifera, Vaccinium corymbosum, or Punica granatum
Abstract
:1. Introduction
Description and Properties of Polyphenols
2. Materials and Methods
2.1. Materials Used to Synthesize the MNPs@Polyphenols
2.2. Cell Viability Materials
2.3. Preparation of the Aqueous Extract
2.4. MNPs@Polyphenol Synthesis
3. Formation Mechanism of MNPs@Polyphenols
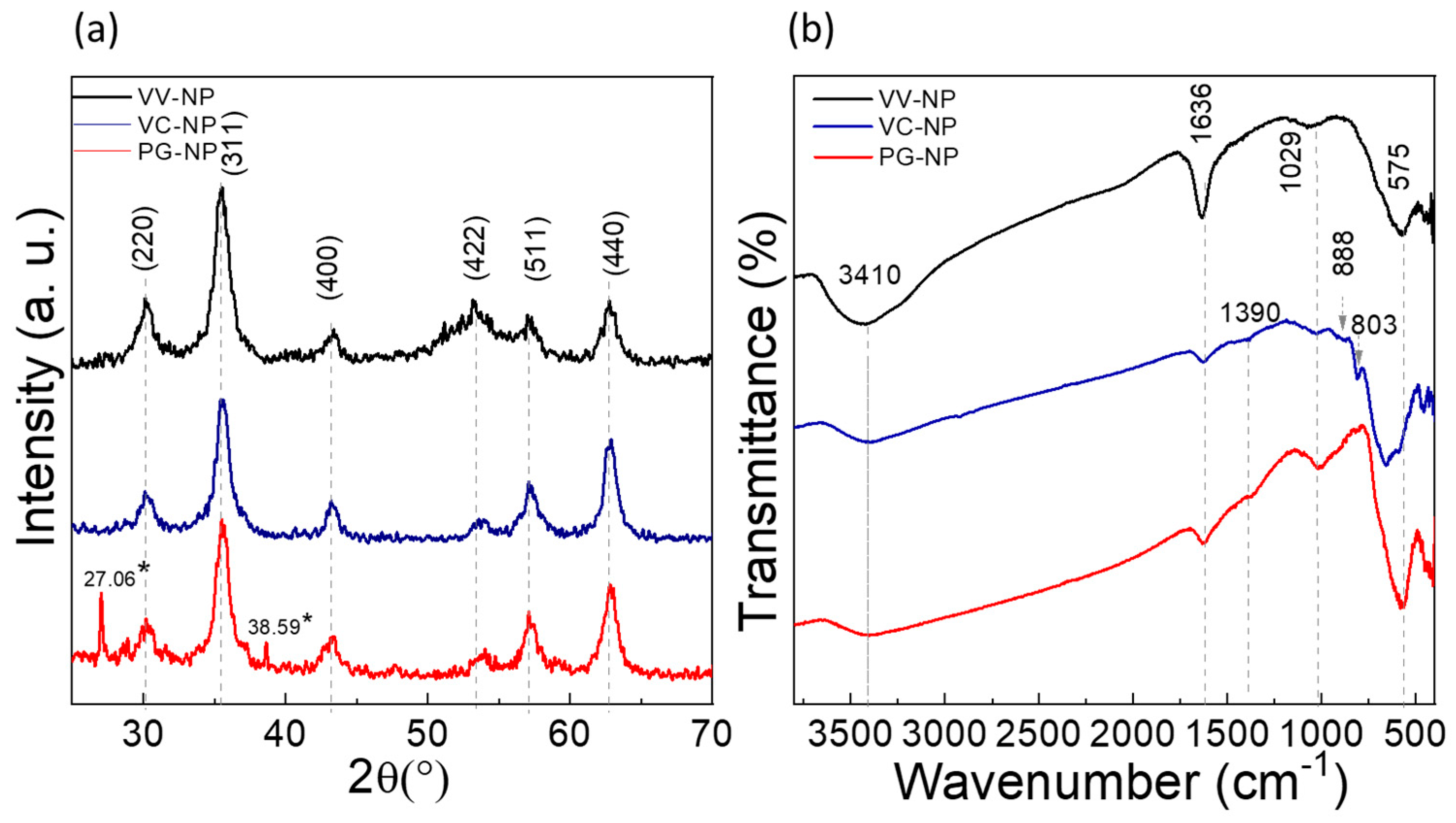
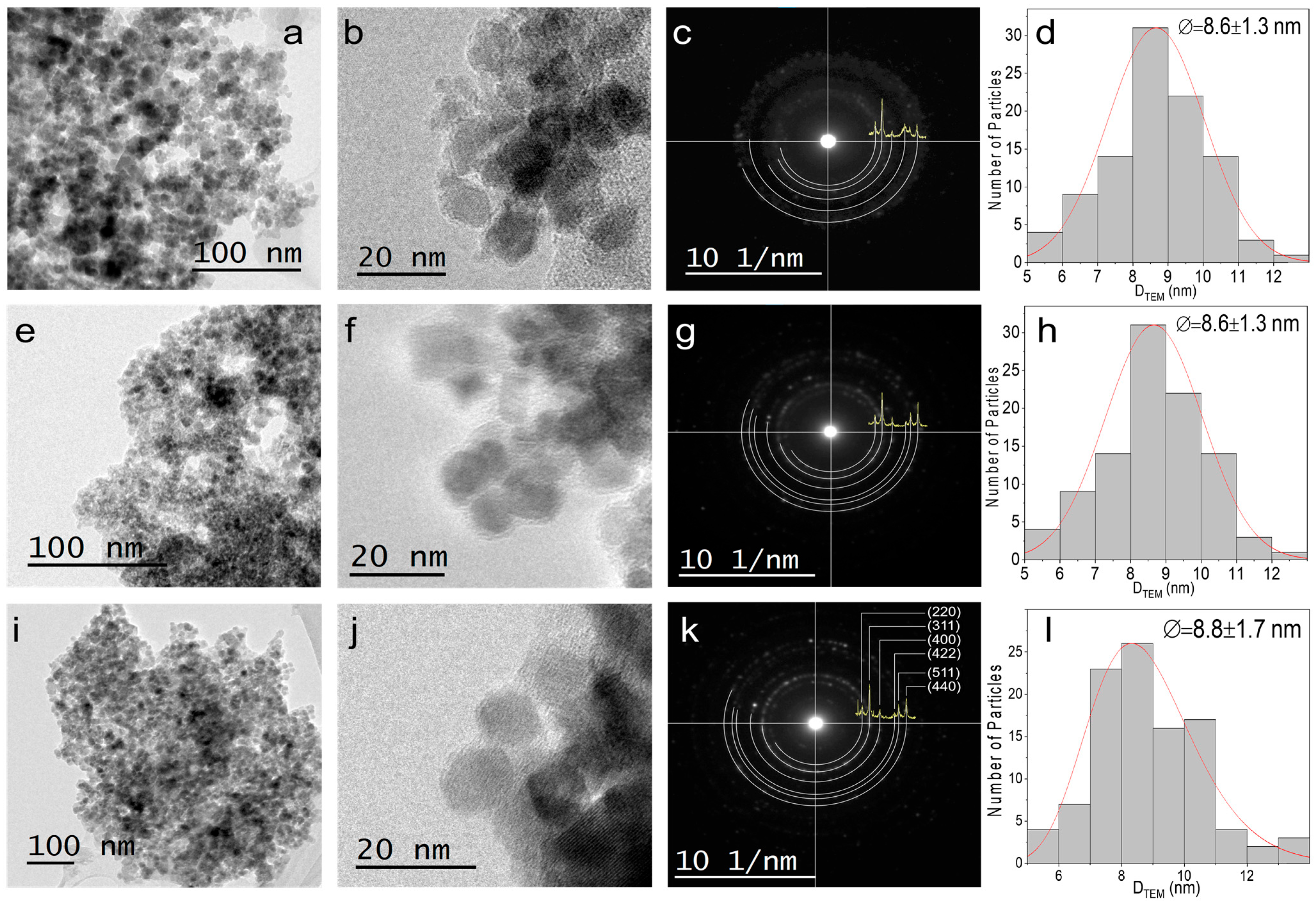
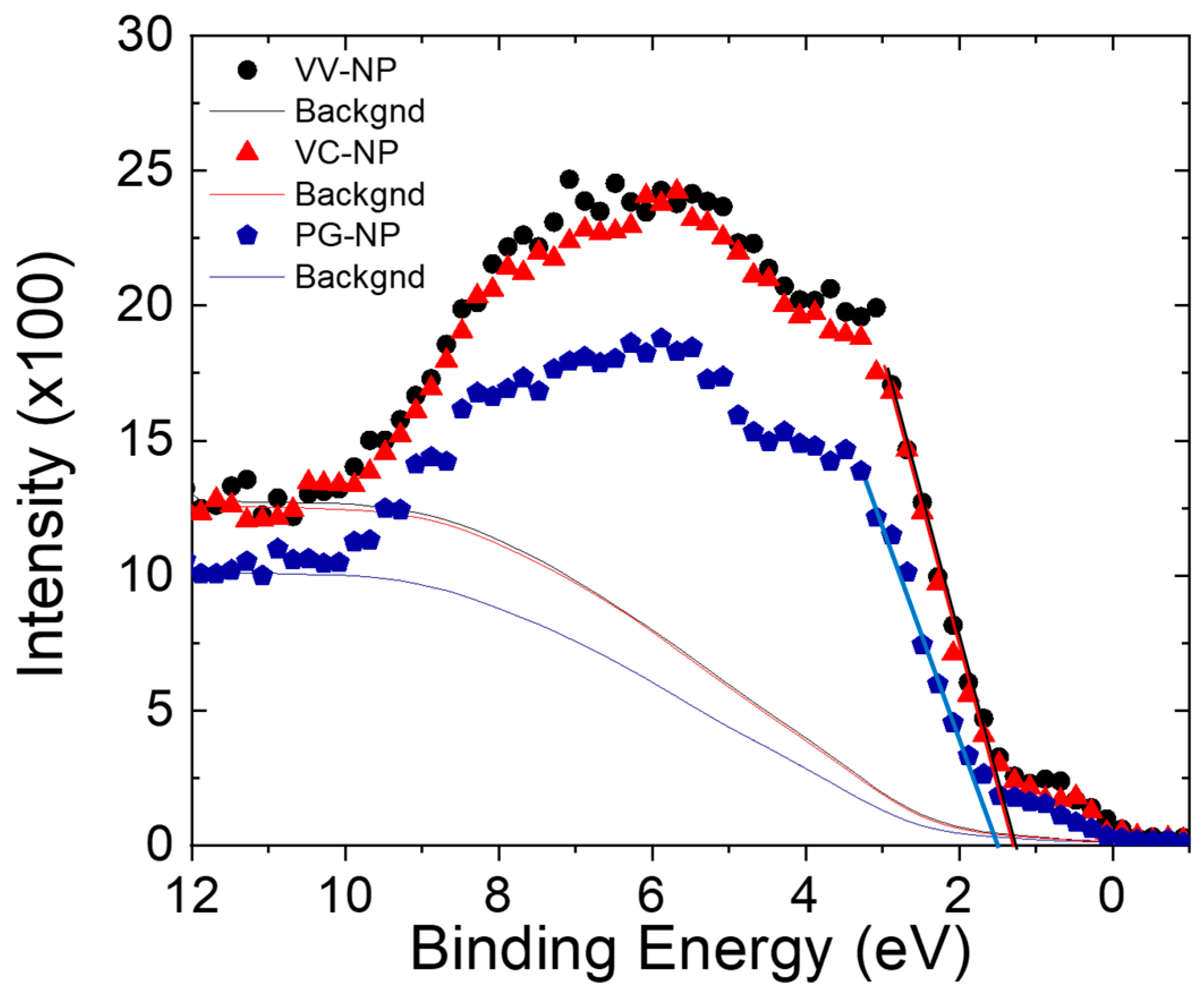
4. Physicochemical Characterization
5. Specific Loss Power (SLP) Measurements
6. Biological Evaluation
6.1. K562 Cell Line
6.2. Treatments
6.3. Cell Viability Determined via MTT and NR Uptake (NRu) Assays
7. Results
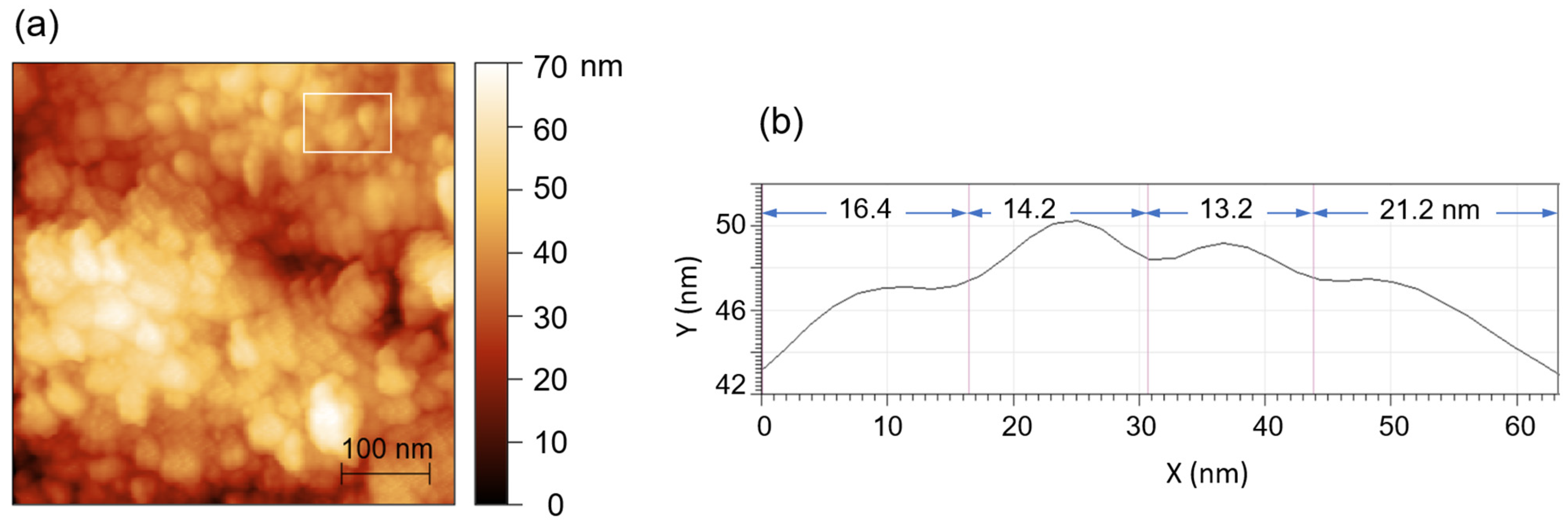
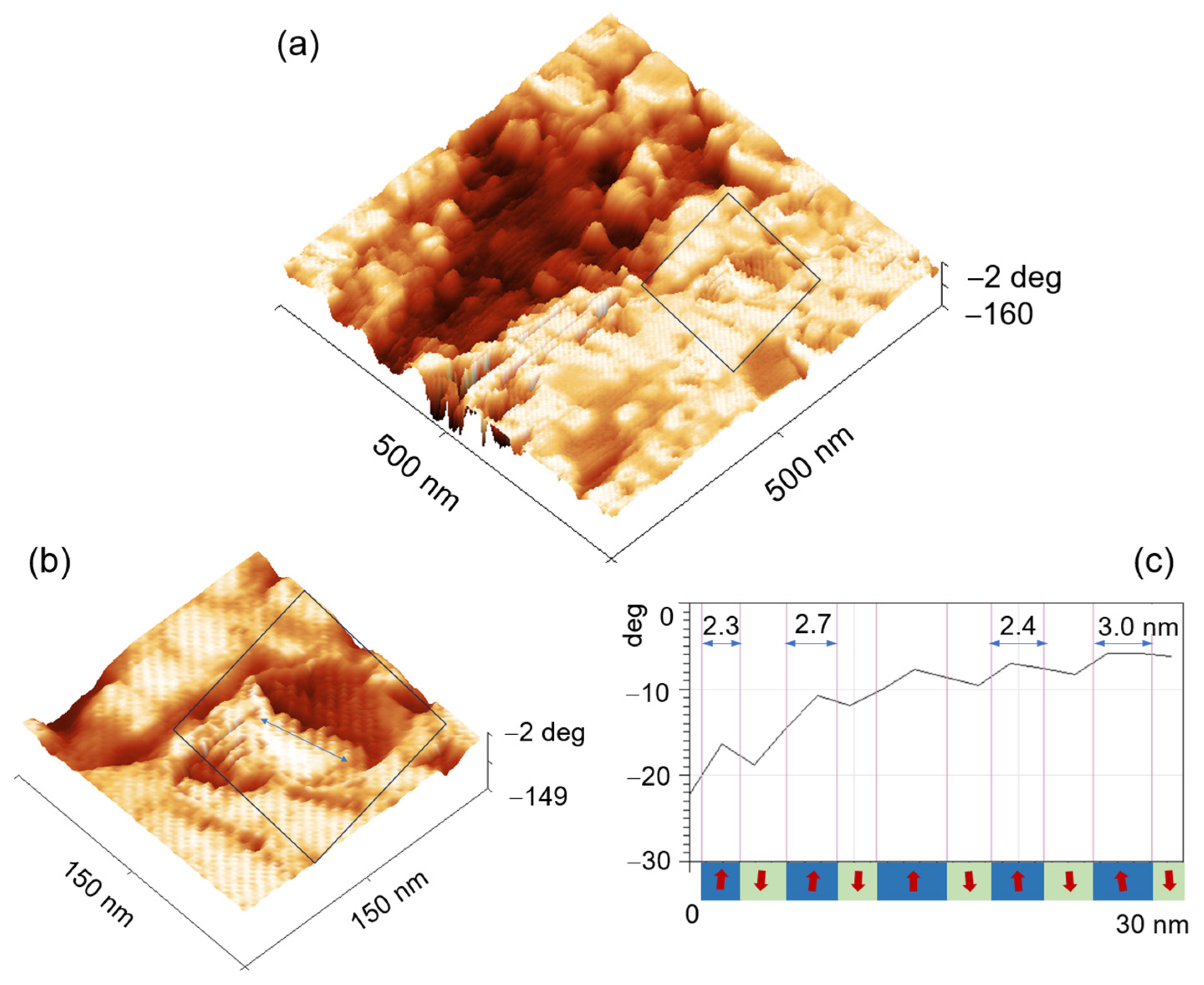
7.1. Structural Analysis
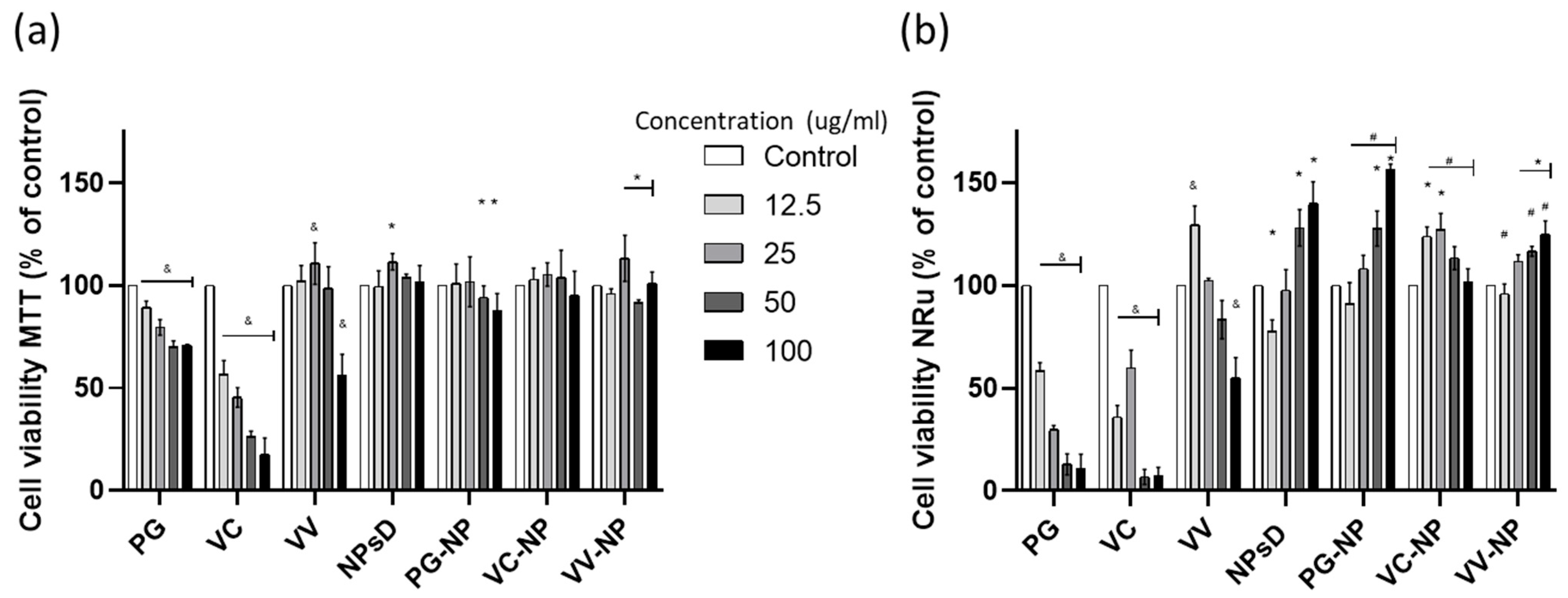
7.2. Cell Viability via the MTT and NRu Tests
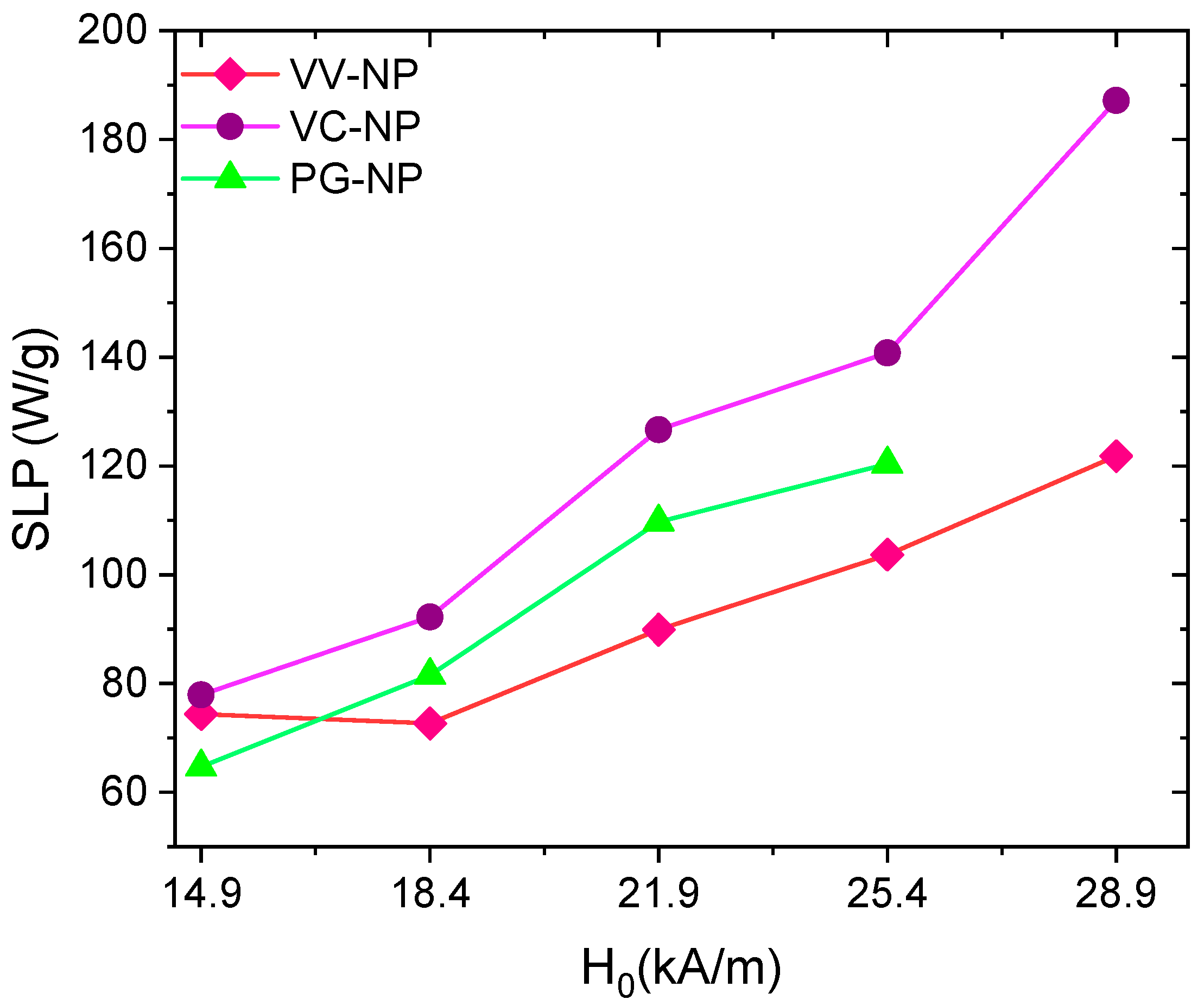
7.3. Power Loss under AC Magnetic Fields
8. Discussion
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Usov, N.A. Low Frequency Hysteresis Loops of Superparamagnetic Nanoparticles with Uniaxial Anisotropy. J. Appl. Phys. 2010, 107, 123909. [Google Scholar] [CrossRef]
- Niculaes, D.; Lak, A.; Anyfantis, G.C.; Marras, S.; Laslett, O.; Avugadda, S.K.; Cassani, M.; Serantes, D.; Hovorka, O.; Chantrell, R.; et al. Asymmetric Assembling of Iron Oxide Nanocubes for Improving Magnetic Hyperthermia Performance. ACS Nano 2017, 11, 12121–12133. [Google Scholar] [CrossRef]
- Kobylinska, N.; Klymchuk, D.; Khaynakova, O.; Duplij, V.; Matvieieva, N. Morphology-Controlled Green Synthesis of Magnetic Nanoparticles Using Extracts of ‘Hairy’ Roots: Environmental Application and Toxicity Evaluation. Nanomaterials 2022, 12, 4231. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-Cid, P.; Isasi, J.; Alcolea Palafox, M.; Martín-Hernández, F. Comparative Structural, Morphological and Magnetic Study of MFe2O4 Nanopowders Prepared by Different Synthesis Routes. Mater. Res. Bull. 2020, 123, 110726. [Google Scholar] [CrossRef]
- Knobel, M.; Nunes, W.C.; Socolovsky, L.M.; De Biasi, E.; Vargas, J.M.; Denardin, J.C. Superparamagnetism and Other Magnetic Features in Granular Materials: A Review on Ideal and Real Systems. J. Nanosci. Nanotechnol. 2008, 8, 2836–2857. [Google Scholar] [CrossRef]
- Bano, S.; Nazir, S.; Nazir, A.; Munir, S.; Mahmood, T.; Afzal, M.; Ansari, F.L.; Mazhar, K. Microwave-Assisted Green Synthesis of Superparamagnetic Nanoparticles Using Fruit Peel Extracts: Surface Engineering, T2 Relaxometry, and Photodynamic Treatment Potential. Int. J. Nanomed. 2016, 11, 3833–3848. [Google Scholar] [CrossRef]
- Barrow, M.; Taylor, A.; Murray, P.; Rosseinsky, M.J.; Adams, D.J. Design Considerations for the Synthesis of Polymer Coated Iron Oxide Nanoparticles for Stem Cell Labelling and Tracking Using MRI. Chem. Soc. Rev. 2015, 44, 6733–6748. [Google Scholar] [CrossRef]
- Nabil, G.; Bhise, K.; Sau, S.; Atef, M.; El-Banna, H.A.; Iyer, A.K. Nano-Engineered Delivery Systems for Cancer Imaging and Therapy: Recent Advances, Future Direction and Patent Evaluation. Drug Discov. Today 2019, 24, 462–491. [Google Scholar] [CrossRef]
- Simeonidis, K.; Morales, M.P.; Marciello, M.; Angelakeris, M.; de la Presa, P.; Lazaro-Carrillo, A.; Tabero, A.; Villanueva, A.; Chubykalo-Fesenko, O.; Serantes, D. In-Situ Particles Reorientation during Magnetic Hyperthermia Application: Shape Matters Twice. Sci. Rep. 2016, 6, 38382. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Ahmad Khairudin, N.B.B.; Mohamad, S.E.B.; Naiki, T.; Lee, K.X. Green Biosynthesis of Superparamagnetic Magnetite Fe3O4 Nanoparticles and Biomedical Applications in Targeted Anticancer Drug Delivery System: A Review. Arab. J. Chem. 2020, 13, 2287–2308. [Google Scholar] [CrossRef]
- Mosayebi, J.; Kiyasatfar, M.; Laurent, S. Synthesis, Functionalization, and Design of Magnetic Nanoparticles for Theranostic Applications. Adv. Healthc. Mater. 2017, 6, 1700306. [Google Scholar] [CrossRef]
- Ghani, U. Polyphenols. In Alpha-Glucosidase Inhibitors; Elsevier: Amsterdam, The Netherlands, 2020; pp. 61–100. [Google Scholar]
- Alvarado-Noguez, M.L.; Matías-Reyes, A.E.; Pérez-González, M.; Tomás, S.A.; Hernández-Aguilar, C.; Domínguez-Pacheco, F.A.; Arenas-Alatorre, J.A.; Cruz-Orea, A.; Carbajal-Tinoco, M.D.; Galot-Linaldi, J.; et al. Processing and Physicochemical Properties of Magnetite Nanoparticles Coated with Curcuma longa L. Extract. Materials 2023, 16, 3020. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Salama, M.M.; Salem, M.A. Bioactive Lead Compounds and Molecular Targets for the Treatment of Heart Diseases. In Phytochemicals as Lead Compounds for New Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 67–94. [Google Scholar]
- Gupta, V.K.; Jaiswara, P.K.; Sonker, P.; Rawat, S.G.; Kumar, A. Adjunct Therapeutic Potential of Phytochemicals against Cancer. In Phytochemicals as Lead Compounds for New Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 117–126. [Google Scholar]
- Milinčić, D.D.; Popović, D.A.; Lević, S.M.; Kostić, A.Ž.; Tešić, Ž.L.; Nedović, V.A.; Pešić, M.B. Application of Polyphenol-Loaded Nanoparticles in Food Industry. Nanomater 2019, 9, 1629. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef]
- Conte, R.; Napoletano, A.; Valentino, A.; Margarucci, S.; Calarco, A. Polyphenols Nanoencapsulation for Therapeutic Applications. J. Biomol. Res. Ther. 2016, 5. [Google Scholar] [CrossRef]
- Ziaullah, H.P.V.R. Application of NMR Spectroscopy in Plant Polyphenols Associated with Human Health. In Application of NMR Spectroscopy; Bentham Science Publishers: Oak Par, IL, USA, 2015; Volume 2, pp. 3–92. [Google Scholar]
- Halake, K.; Cho, S.; Kim, J.; Lee, T.; Cho, Y.; Chi, S.; Park, M.; Kim, K.; Lee, D.; Ju, H.; et al. Applications Using the Metal Affinity of Polyphenols with Mussel-Inspired Chemistry. Macromol. Res. 2018, 26, 93–99. [Google Scholar] [CrossRef]
- Halake, K.; Birajdar, M.; Lee, J. Structural Implications of Polyphenolic Antioxidants. J. Ind. Eng. Chem. 2016, 35, 1–7. [Google Scholar] [CrossRef]
- Ramirez-Nuñez, A.L.; Jimenez-Garcia, L.F.; Goya, G.F.; Sanz, B.; Santoyo-Salazar, J. In Vitro Magnetic Hyperthermia Using Polyphenol-Coated Fe3O4@γFe2O3 Nanoparticles from Cinnamomun verum and Vanilla planifolia: The Concert of Green Synthesis and Therapeutic Possibilities. Nanotechnology 2018, 29, 074001. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Lampakis, D.; Skenderidis, P.; Leontopoulos, S. Technologies and Extraction Methods of Polyphenolic Compounds Derived from Pomegranate (Punica granatum) Peels. A Mini Review. Processes 2021, 9, 236. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Compounds as Beneficial Phytochemicals in Pomegranate (Punica granatum L.) Peel: A Review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef] [PubMed]
- David, H. Chapter 10 Pomegranate Ellagitannins, 2nd ed.; Iris, F.F., Benzie, S.W.-G., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2011; ISBN 9781439807132.
- Ko, K.; Dadmohammadi, Y.; Abbaspourrad, A. Nutritional and Bioactive Components of Pomegranate Waste Used in Food and Cosmetic Applications: A Review. Foods 2021, 10, 657. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.; Hegazi, N.; El-Shamy, S.; Farag, M.A. Pomegranate Juice as a Functional Food: A Comprehensive Review of Its Polyphenols, Therapeutic Merits, and Recent Patents. Food Funct. 2020, 11, 5768–5781. [Google Scholar] [CrossRef]
- Sun, S.; Huang, S.; Shi, Y.; Shao, Y.; Qiu, J.; Sedjoah, R.-C.A.-A.; Yan, Z.; Ding, L.; Zou, D.; Xin, Z. Extraction, Isolation, Characterization and Antimicrobial Activities of Non-Extractable Polyphenols from Pomegranate Peel. Food Chem. 2021, 351, 129232. [Google Scholar] [CrossRef]
- Li, R.; Chen, X.G.; Jia, K.; Liu, Z.P.; Peng, H.Y. A Systematic Determination of Polyphenols Constituents and Cytotoxic Ability in Fruit Parts of Pomegranates Derived from Five Chinese Cultivars. Springerplus 2016, 5, 914. [Google Scholar] [CrossRef]
- Pirzadeh, M.; Caporaso, N.; Rauf, A.; Shariati, M.A.; Yessimbekov, Z.; Khan, M.U.; Imran, M.; Mubarak, M.S. Pomegranate as a Source of Bioactive Constituents: A Review on Their Characterization, Properties and Applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 982–999. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Bălan, A.; Dima, L.; Toma, S.I.; Bîgiu, N.F.; Blidaru, A. Pharmacological and Therapeutic Properties of Punica granatum Phytochemicals: Possible Roles in Breast Cancer. Molecules 2021, 26, 1054. [Google Scholar] [CrossRef]
- Gato, E.; Perez, A.; Rosalowska, A.; Celeiro, M.; Bou, G.; Lores, M. Multicomponent Polyphenolic Extracts from Vaccinium Corymbosum at Lab and Pilot Scale. Characterization and Effectivity against Nosocomial Pathogens. Plants 2021, 10, 2801. [Google Scholar] [CrossRef]
- Pervin, M.; Hasnat, M.A.; Lim, J.-H.; Lee, Y.-M.; Kim, E.O.; Um, B.-H.; Lim, B.O. Preventive and Therapeutic Effects of Blueberry (Vaccinium corymbosum) Extract against DSS-Induced Ulcerative Colitis by Regulation of Antioxidant and Inflammatory Mediators. J. Nutr. Biochem. 2016, 28, 103–113. [Google Scholar] [CrossRef]
- Golovinskaia, O.; Wang, C.-K. Review of Functional and Pharmacological Activities of Berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Tenuta, M.C.; Loizzo, M.R.; Bonesi, M.; Finetti, F.; Trabalzini, L.; Deguin, B. Vaccinium Species (Ericaceae): From Chemical Composition to Bio-Functional Activities. Appl. Sci. 2021, 11, 5655. [Google Scholar] [CrossRef]
- Nunes, S.; Vieira, P.; Gomes, P.; Viana, S.D.; Reis, F. Blueberry as an Attractive Functional Fruit to Prevent (Pre)Diabetes Progression. Antioxidants 2021, 10, 1162. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Radha; Kumar, M.; Kumari, N.; Thakur, M.; Rathour, S.; Pundir, A.; Sharma, A.K.; Bangar, S.P.; Dhumal, S.; et al. Plant-Based Antioxidant Extracts and Compounds in the Management of Oral Cancer. Antioxidants 2021, 10, 1358. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Du, Y.; Li, X.; Xiong, X.; Cai, X.; Ren, X.; Kong, Q. An Investigation on Polyphenol Composition and Content in Skin of Grape (Vitis vinifera L. Cv. Hutai No.8) Fruit during Ripening by UHPLC-MS2 Technology Combined with Multivariate Statistical Analysis. Food Biosci. 2021, 43, 101276. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and Its Bioactive Constituents: An Update. Phyther. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef]
- Abu-Serie, M.M.; Habashy, N.H. Vitis vinifera Polyphenols from Seedless Black Fruit Act Synergistically to Suppress Hepatotoxicity by Targeting Necroptosis and Pro-Fibrotic Mediators. Sci. Rep. 2020, 10, 2452. [Google Scholar] [CrossRef]
- Jardim, F.R.; de Rossi, F.T.; Nascimento, M.X.; da Silva Barros, R.G.; Borges, P.A.; Prescilio, I.C.; de Oliveira, M.R. Resveratrol and Brain Mitochondria: A Review. Mol. Neurobiol. 2018, 55, 2085–2101. [Google Scholar] [CrossRef]
- Chan, S.; Kantham, S.; Rao, V.M.; Palanivelu, M.K.; Pham, H.L.; Shaw, P.N.; McGeary, R.P.; Ross, B.P. Metal Chelation, Radical Scavenging and Inhibition of Aβ42 Fibrillation by Food Constituents in Relation to Alzheimer’s Disease. Food Chem. 2016, 199, 185–194. [Google Scholar] [CrossRef]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In Vitro Antioxidant versus Metal Ion Chelating Properties of Flavonoids: A Structure-Activity Investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef]
- Prasad, C.; Gangadhara, S.; Venkateswarlu, P. Bio-Inspired Green Synthesis of Fe3O4 Magnetic Nanoparticles Using Watermelon Rinds and Their Catalytic Activity. Appl. Nanosci. 2016, 6, 797–802. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Su Yee, O.; Teow, S.-Y.; Hedayatnasab, Z.; Jahangirian, H.; Webster, T.J.; Kuča, K. Green Synthesis of Fe3O4 Nanoparticles Stabilized by a Garcinia Mangostana Fruit Peel Extract for Hyperthermia and Anticancer Activities. Int. J. Nanomed. 2021, 16, 2515–2532. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.K.; Saha, P.; Hasan, M.K.; Amin, M.K.; Haque, M.R. Green Synthesis of Magnetite Nanoparticles Using Lathyrus Sativus Peel Extract and Evaluation of Their Catalytic Activity. Clean. Eng. Technol. 2021, 3, 100117. [Google Scholar] [CrossRef]
- Vitta, Y.; Figueroa, M.; Calderon, M.; Ciangherotti, C. Synthesis of Iron Nanoparticles from Aqueous Extract of Eucalyptus Robusta Sm and Evaluation of Antioxidant and Antimicrobial Activity. Mater. Sci. Energy Technol. 2020, 3, 97–103. [Google Scholar] [CrossRef]
- Massart, R. Preparation of Aqueous Magnetic Liquids in Alkaline and Acidic Media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Sundaramurthy, A. 9—Phytosynthesized Nanoparticles for Orthopedic Applications. In Emerging Phytosynthesized Nanomaterials for Biomedical Applications; Dable-Tupas, G., Danquah, M.K., Jeevanandam, J., Sundaramurthy, A., Xian Tan, K., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2023; pp. 217–236. ISBN 978-0-12-824373-2. [Google Scholar]
- Thakur, M.; Poojary, S.; Swain, N. Green Synthesis of Iron Oxide Nanoparticles and Its Biomedical Applications. In Nanotechnology Applications in Health and Environmental Sciences; Springer: Berlin/Heidelberg, Germany, 2021; pp. 83–109. [Google Scholar]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Mills, S.C.; Starr, N.E.; Bohannon, N.J.; Andrew, J.S. Chelating Agent Functionalized Substrates for the Formation of Thick Films via Electrophoretic Deposition. Front. Chem. 2021, 9, 703528. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, C.; Mallavarapu, M. Characterization of Iron–Polyphenol Complex Nanoparticles Synthesized by Sage (Salvia officinalis) Leaves. Environ. Technol. Innov. 2015, 4, 92–97. [Google Scholar] [CrossRef]
- Calatayud, M.P.; Soler, E.; Torres, T.E.; Campos-Gonzalez, E.; Junquera, C.; Ibarra, M.R.; Goya, G.F. Cell Damage Produced by Magnetic Fluid Hyperthermia on Microglial BV2 Cells. Sci. Rep. 2017, 7, 8627. [Google Scholar] [CrossRef]
- Natividad, E.; Castro, M.; Mediano, A. Adiabatic vs. Non-Adiabatic Determination of Specific Absorption Rate of Ferrofluids. J. Magn. Magn. Mater. 2009, 321, 1497–1500. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Noqta, O.A.; Khaniabadi, P.M.; Mehrdel, B. Simple Rapid Stabilization Method through Citric Acid Modification for Magnetite Nanoparticles. Sci. Rep. 2020, 10, 10793. [Google Scholar] [CrossRef]
- Karade, V.C.; Waifalkar, P.P.; Dongle, T.D.; Sahoo, S.C.; Kollu, P.; Patil, P.S.; Patil, P.B. Greener Synthesis of Magnetite Nanoparticles Using Green Tea Extract and Their Magnetic Properties. Mater. Res. Express 2017, 4, 096102. [Google Scholar] [CrossRef]
- Hwang, S.J.; Jun, S.H.; Park, Y.; Cha, S.-H.; Yoon, M.; Cho, S.; Lee, H.-J.; Park, Y. Green Synthesis of Gold Nanoparticles Using Chlorogenic Acid and Their Enhanced Performance for Inflammation. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1677–1688. [Google Scholar] [CrossRef]
- Sahu, N.; Soni, D.; Chandrashekhar, B.; Satpute, D.B.; Saravanadevi, S.; Sarangi, B.K.; Pandey, R.A. Synthesis of Silver Nanoparticles Using Flavonoids: Hesperidin, Naringin and Diosmin, and Their Antibacterial Effects and Cytotoxicity. Int. Nano Lett. 2016, 6, 173–181. [Google Scholar] [CrossRef]
- Rahmani, R.; Gharanfoli, M.; Gholamin, M.; Darroudi, M.; Chamani, J.; Sadri, K.; Hashemzadeh, A. Plant-Mediated Synthesis of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) Using Aloe Vera and Flaxseed Extracts and Evaluation of Their Cellular Toxicities. Ceram. Int. 2020, 46, 3051–3058. [Google Scholar] [CrossRef]
- Santhoshkumar, J.; Rajeshkumar, S.; Venkat Kumar, S. Phyto-Assisted Synthesis, Characterization and Applications of Gold Nanoparticles—A Review. Biochem. Biophys. Rep. 2017, 11, 46–57. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Ali, R.R.; Pang, S.-W.; Teow, S.-Y. Evaluating Anticancer Activity of Plant-Mediated Synthesized Iron Oxide Nanoparticles Using Punica granatum Fruit Peel Extract. J. Mol. Struct. 2020, 1204, 127539. [Google Scholar] [CrossRef]
- Oh, B.-T.; Jeong, S.-Y.; Velmurugan, P.; Park, J.-H.; Jeong, D.-Y. Probiotic-Mediated Blueberry (Vaccinium corymbosum L.) Fruit Fermentation to Yield Functionalized Products for Augmented Antibacterial and Antioxidant Activity. J. Biosci. Bioeng. 2017, 124, 542–550. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of Multiplet Splitting of Fe 2p XPS Spectra and Bonding in Iron Compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Pérez-González, M.; Tomás, S.A. Surface Chemistry of TiO2-ZnO Thin Films Doped with Ag. Its Role on the Photocatalytic Degradation of Methylene Blue. Catal. Today 2021, 360, 129–137. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS Spectra of Fe2+ and Fe3+ Ions in Oxide Materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Santoyo Salazar, J.; Perez, L.; de Abril, O.; Truong Phuoc, L.; Ihiawakrim, D.; Vazquez, M.; Greneche, J.-M.; Begin-Colin, S.; Pourroy, G. Magnetic Iron Oxide Nanoparticles in 10−40 Nm Range: Composition in Terms of Magnetite/Maghemite Ratio and Effect on the Magnetic Properties. Chem. Mater. 2011, 23, 1379–1386. [Google Scholar] [CrossRef]
- Fujii, T.; de Groot, F.M.F.; Sawatzky, G.A.; Voogt, F.C.; Hibma, T.; Okada, K. In Situ XPS Analysis of Various Iron Oxide Films Grown by NO2-Assisted Molecular-Beam Epitaxy. Phys. Rev. B 1999, 59, 3195–3202. [Google Scholar] [CrossRef]
- Granada-Ramirez, D.A.; Cardona-Bedoya, J.A.; Hernandez-Rojas, U.; Pulzara-Mora, A.; Delgado-Rosero, M.I.; Durán-Ledezma, A.A.; Pérez-González, M.; Panecatl Bernal, Y.; Tomás, S.A.; Alvarado-Pulido, J.J.; et al. Assessment of Cr Doping on TiO2 Thin Films Deposited by a Wet Chemical Method. Ceram. Int. 2023, 43, 30347–30354. [Google Scholar] [CrossRef]
- Minati, L.; Micheli, V.; Rossi, B.; Migliaresi, C.; Dalbosco, L.; Bao, G.; Hou, S.; Speranza, G. Application of Factor Analysis to XPS Valence Band of Superparamagnetic Iron Oxide Nanoparticles. Appl. Surf. Sci. 2011, 257, 10863–10868. [Google Scholar] [CrossRef]
- García-Zepeda, S.P.; Santoyo-Salazar, J. Functional Addressable Magnetic Domains and Their Potential Applications in Theranostics. In Magnetic Nanoparticles in Human Health and Medicine; Wiley: Hoboken, NJ, USA, 2021; pp. 164–180. [Google Scholar]
- Stan, M.; Lung, I.; Soran, M.-L.; Leostean, C.; Popa, A.; Stefan, M.; Lazar, M.D.; Opris, O.; Silipas, T.-D.; Porav, A.S. Removal of Antibiotics from Aqueous Solutions by Green Synthesized Magnetite Nanoparticles with Selected Agro-Waste Extracts. Process Saf. Environ. Prot. 2017, 107, 357–372. [Google Scholar] [CrossRef]
- Rajan, A.; Sharma, M.; Sahu, N.K. Assessing Magnetic and Inductive Thermal Properties of Various Surfactants Functionalised Fe3O4 Nanoparticles for Hyperthermia. Sci. Rep. 2020, 10, 15045. [Google Scholar] [CrossRef]
- Ribeiro, V.G.P.; Barreto, A.C.H.; Denardin, J.C.; Mele, G.; Carbone, L.; Mazzetto, S.E.; Sousa, E.M.B.; Fechine, P.B.A. Magnetic Nanoparticles Coated with Anacardic Acid Derived from Cashew Nut Shell Liquid. J. Mater. Sci. 2013, 48, 7875–7882. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In Vitro Cytotoxicity Assays: Comparison of LDH, Neutral Red, MTT and Protein Assay in Hepatoma Cell Lines following Exposure to Cadmium Chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
- de Arruda Nascimento, E.; de Lima Coutinho, L.; da Silva, C.J.; de Lima, V.L.A.G.; dos Santos Aguiar, J. In Vitro Anticancer Properties of Anthocyanins: A Systematic Review. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188748. [Google Scholar] [CrossRef]
- Asmaa, M.S.; Ali, A.-J.; Farid, J.; Azman, S. Growth Inhibitory Effects of Crude Pomegranate Peel Extract on Chronic Myeloid Leukemia, K562 Cells. Int. J. Appl. Basic Med. Res. 2015, 5, 100. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, W.; Fan, Q.; Ma, H.; Yin, Y.; Long, Y.; Guan, J. Polyphenol-Mediated Synthesis of Superparamagnetic Magnetite Nanoclusters for Highly Stable Magnetically Responsive Photonic Crystals. In Advanced Functional Materials; Wiley: Hoboken, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Chen, Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- Etemadifar, R.; Kianvash, A.; Arsalani, N.; Abouzari-Lotf, E.; Hajalilou, A. Green Synthesis of Superparamagnetic Magnetite Nanoparticles: Effect of Natural Surfactant and Heat Treatment on the Magnetic Properties. J. Mater. Sci. Mater. Electron. 2018, 29, 17144–17153. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.; Akram, M.; Udego, I.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [PubMed]
- Tombácz, E.; Turcu, R.; Socoliuc, V.; Vékás, L. Magnetic Iron Oxide Nanoparticles: Recent Trends in Design and Synthesis of Magnetoresponsive Nanosystems. Biochem. Biophys. Res. Commun. 2015, 468, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qiu, C.; Li, X.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z. Advances in Research on Interactions between Polyphenols and Biology-Based Nano-Delivery Systems and Their Applications in Improving the Bioavailability of Polyphenols. Trends Food Sci. Technol. 2021, 116, 492–500. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface Modification of Inorganic Nanoparticles for Development of Organic–Inorganic Nanocomposites—A Review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic Biochemical Mechanisms behind the Health Benefits of Polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Szekeres, M.; Tóth, I.; Illés, E.; Hajdú, A.; Zupkó, I.; Farkas, K.; Oszlánczi, G.; Tiszlavicz, L.; Tombácz, E. Chemical and Colloidal Stability of Carboxylated Core-Shell Magnetite Nanoparticles Designed for Biomedical Applications. Int. J. Mol. Sci. 2013, 14, 14550–14574. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Gupta, R.P.; Sen, S.K. Calculation of Multiplet Structure of Core p-Vacancy Levels. II. Phys. Rev. B 1975, 12, 15–19. [Google Scholar] [CrossRef]
- Pratt, A.; Muir, I.; Nesbitt, H. X-Ray Photoelectron and Auger Electron Spectroscopic Studies of Pyrrhotite and Mechanism of Air Oxidation. Geochim. Cosmochim. Acta 1994, 58, 827–841. [Google Scholar] [CrossRef]
- Lin, T.-C.; Seshadri, G.; Kelber, J.A. A Consistent Method for Quantitative XPS Peak Analysis of Thin Oxide Films on Clean Polycrystalline Iron Surfaces. Appl. Surf. Sci. 1997, 119, 83–92. [Google Scholar] [CrossRef]
- Thomas, G.; Demoisson, F.; Boudon, J.; Millot, N. Efficient Functionalization of Magnetite Nanoparticles with Phosphonate Using a One-Step Continuous Hydrothermal Process. Dalt. Trans. 2016, 45, 10821–10829. [Google Scholar] [CrossRef]
- Nene, A.G.; Takahashi, M.; Somani, P.R. Fe3O4 and Fe Nanoparticles by Chemical Reduction of Fe(acac)3 by Ascorbic Acid: Role of Water. World J. Nano Sci. Eng. 2016, 6, 20–28. [Google Scholar] [CrossRef]
- Zeiri, Y.; Elia, P.; Zach, R.; Hazan, S.; Kolusheva, S.; Porat, Z. Green Synthesis of Gold Nanoparticles Using Plant Extracts as Reducing Agents. Int. J. Nanomed. 2014, 9, 4007–4021. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, D.; Lee, C.S. Synthesis and Characterization of CoFe2O4 Magnetic Nanoparticles Prepared by Temperature-Controlled Coprecipitation Method. Phys. B Condens. Matter 2003, 337, 42–51. [Google Scholar] [CrossRef]
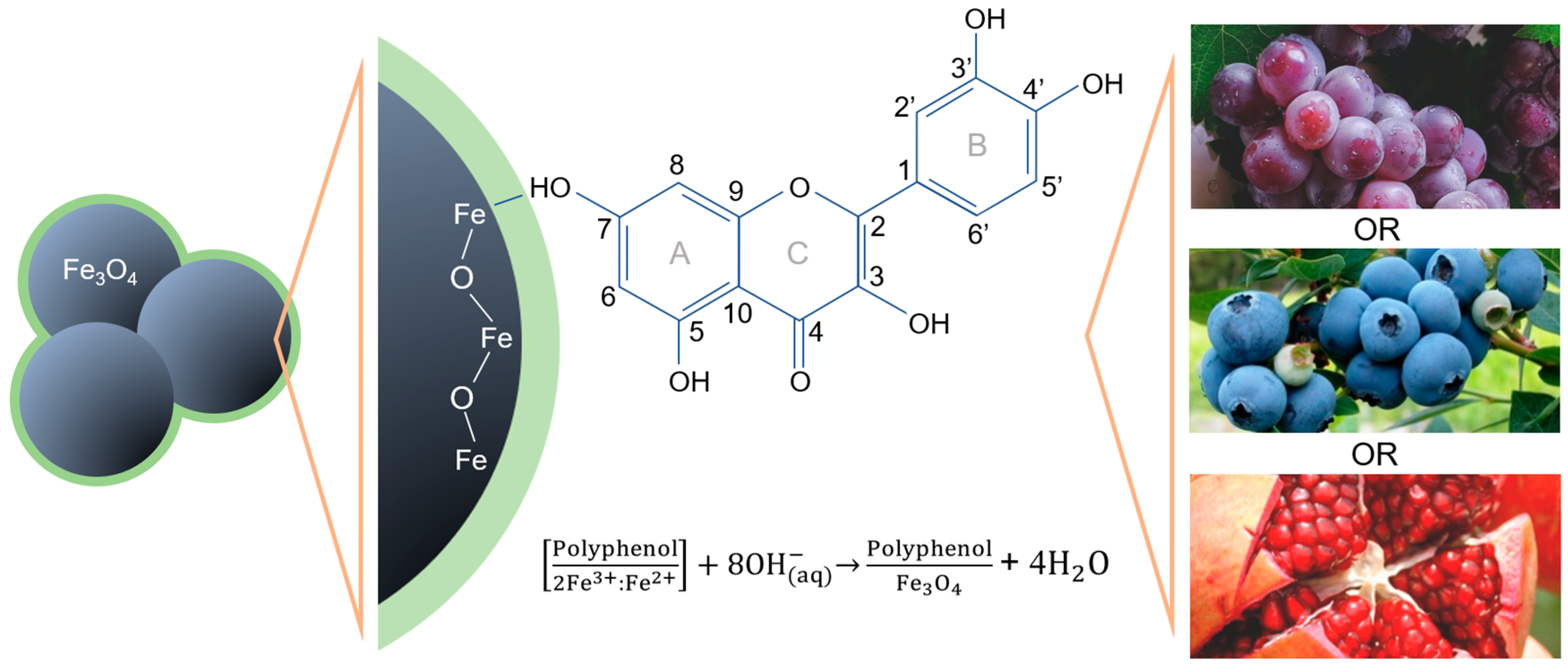


| Polyphenolic Compounds | VC (3.1–4.5 pH) | PG (5.5 pH) | VV (3.5–4.5) |
|---|---|---|---|
| Cyanidin | * | * | * |
| Delphinidin | * | * | * |
| Malvidin | * | * | |
| Peonidin | * | * | |
| Petunidin | * | * | |
| Pelargonidin 3-O-glucoside | * | ||
| Phloridzin | * | ||
| (-)-Epicatechin | * | * | * |
| (+)-Catechin | * | * | |
| (+)-Gallocatechin | * | ||
| (−)-Epigallocatechin | * | * | |
| Procyanidin dimer | * | * | |
| Kaempferol | * | ||
| Myricetin | * | ||
| Quercetin | * | * | * |
| 4-Hydroxybenzoic acid 4-O-glucoside | * | ||
| Gallic acid | * | * | |
| Protocatechuic acid 4-O-glucoside | * | * | |
| Ellagic acid | * | * | |
| Epigallocatechin gallate | * | ||
| Galloyl glucose | * | ||
| Punicalagin | * | ||
| Caffeoylquinic acid | * | * | |
| 5-p-Coumaroylquinic acid | * | ||
| Caffeic acid 4-O-glucoside | * | * | |
| Ferulic acid 4-O-glucoside | * | * | |
| p-Coumaric acid 4-O-glucoside | * | * | |
| Cis-Caffeoyl tartaric acid | * | ||
| Trans-Caffeoyl tartaric acid | * | ||
| Cis-p-Coumaroyl tartaric acid | * | ||
| Caffeoyl tartaric acid | * | ||
| p-Coumaroyl tartaric acid | * | ||
| Trans-p-Coumaroyl tartaric acid | * | ||
| -Piceatannol | * | ||
| Resveratrol | * | ||
| Resveratrol 3-O-glucoside | * | ||
| Trans-Resveratrol | * | ||
| Trans-Resveratrol 3-O-glucoside | * |
| MNPs | a (Å) | Error (%) | ϵ (a. u.) | ||
|---|---|---|---|---|---|
| VV–NPs | 8.368 | 0.003227727 | 0.046835967 | 7.34 ± 0.06 | 7.79 ± 1.63 |
| VC–NPs | 8.362 | 0.004085279 | 0.042566305 | 8.09 ± 0.10 | 8.66 ± 1.37 |
| PG–NPs | 8.357 | 0.004561696 | 0.042210834 | 8.15 ± 0.09 | 8.8 ± 2.61 |
| Sample | Fe (%) | O (%) | C (%) |
|---|---|---|---|
| VV–NPs | 29.81 | 47.16 | 23.03 |
| VC–NPs | 30.21 | 49.48 | 20.31 |
| PG–NPs | 23.72 | 40.74 | 35.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matías-Reyes, A.E.; Alvarado-Noguez, M.L.; Pérez-González, M.; Carbajal-Tinoco, M.D.; Estrada-Muñiz, E.; Fuentes-García, J.A.; Vega-Loyo, L.; Tomás, S.A.; Goya, G.F.; Santoyo-Salazar, J. Direct Polyphenol Attachment on the Surfaces of Magnetite Nanoparticles, Using Vitis vinifera, Vaccinium corymbosum, or Punica granatum. Nanomaterials 2023, 13, 2450. https://doi.org/10.3390/nano13172450
Matías-Reyes AE, Alvarado-Noguez ML, Pérez-González M, Carbajal-Tinoco MD, Estrada-Muñiz E, Fuentes-García JA, Vega-Loyo L, Tomás SA, Goya GF, Santoyo-Salazar J. Direct Polyphenol Attachment on the Surfaces of Magnetite Nanoparticles, Using Vitis vinifera, Vaccinium corymbosum, or Punica granatum. Nanomaterials. 2023; 13(17):2450. https://doi.org/10.3390/nano13172450
Chicago/Turabian StyleMatías-Reyes, Ana E., Margarita L. Alvarado-Noguez, Mario Pérez-González, Mauricio D. Carbajal-Tinoco, Elizabeth Estrada-Muñiz, Jesús A. Fuentes-García, Libia Vega-Loyo, Sergio A. Tomás, Gerardo F. Goya, and Jaime Santoyo-Salazar. 2023. "Direct Polyphenol Attachment on the Surfaces of Magnetite Nanoparticles, Using Vitis vinifera, Vaccinium corymbosum, or Punica granatum" Nanomaterials 13, no. 17: 2450. https://doi.org/10.3390/nano13172450
APA StyleMatías-Reyes, A. E., Alvarado-Noguez, M. L., Pérez-González, M., Carbajal-Tinoco, M. D., Estrada-Muñiz, E., Fuentes-García, J. A., Vega-Loyo, L., Tomás, S. A., Goya, G. F., & Santoyo-Salazar, J. (2023). Direct Polyphenol Attachment on the Surfaces of Magnetite Nanoparticles, Using Vitis vinifera, Vaccinium corymbosum, or Punica granatum. Nanomaterials, 13(17), 2450. https://doi.org/10.3390/nano13172450









