Spray-Flame Synthesis of NASICON-Type Rhombohedral (α) Li1+xYxZr2−x(PO4)3 [x = 0–0.2] Solid Electrolytes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
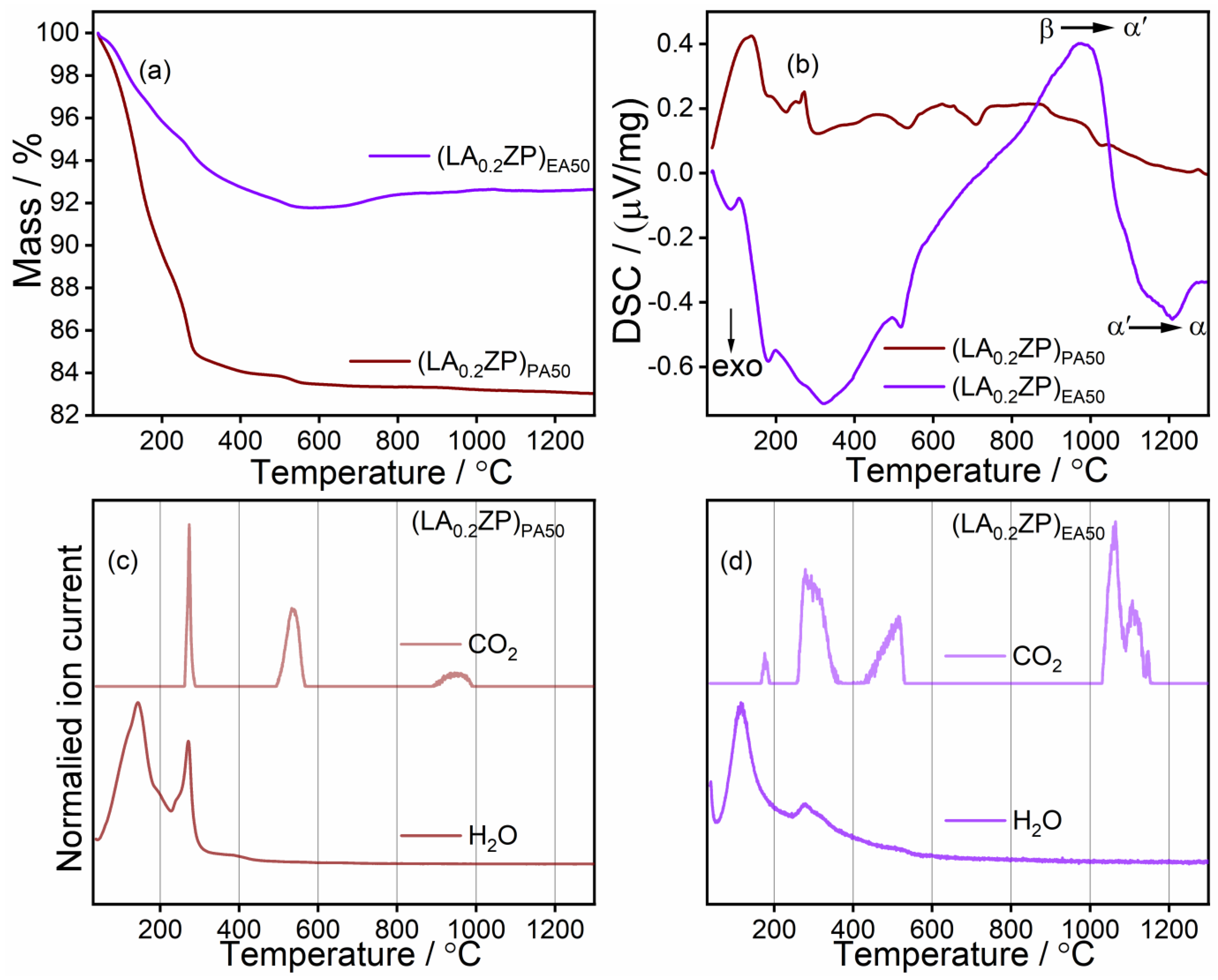
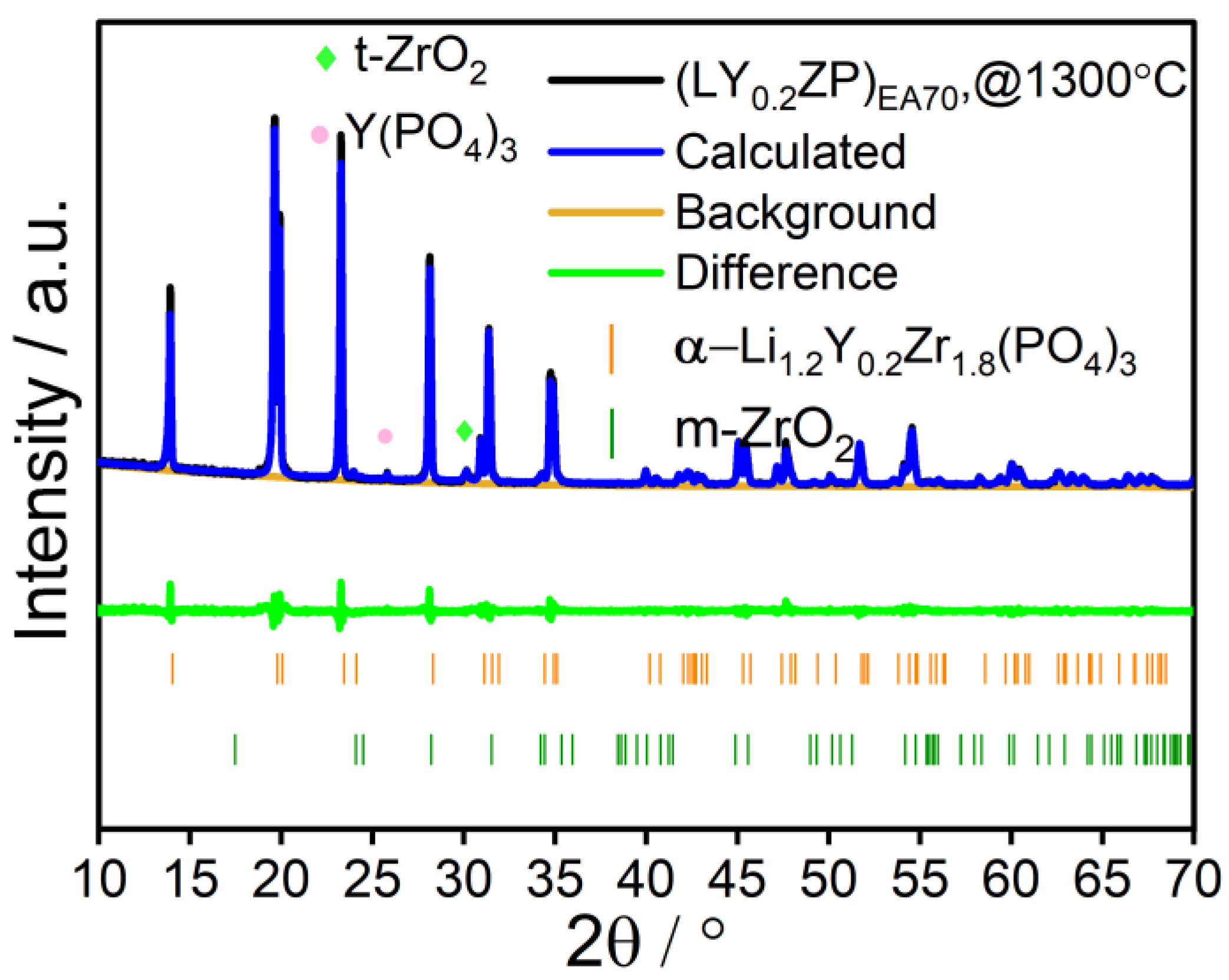
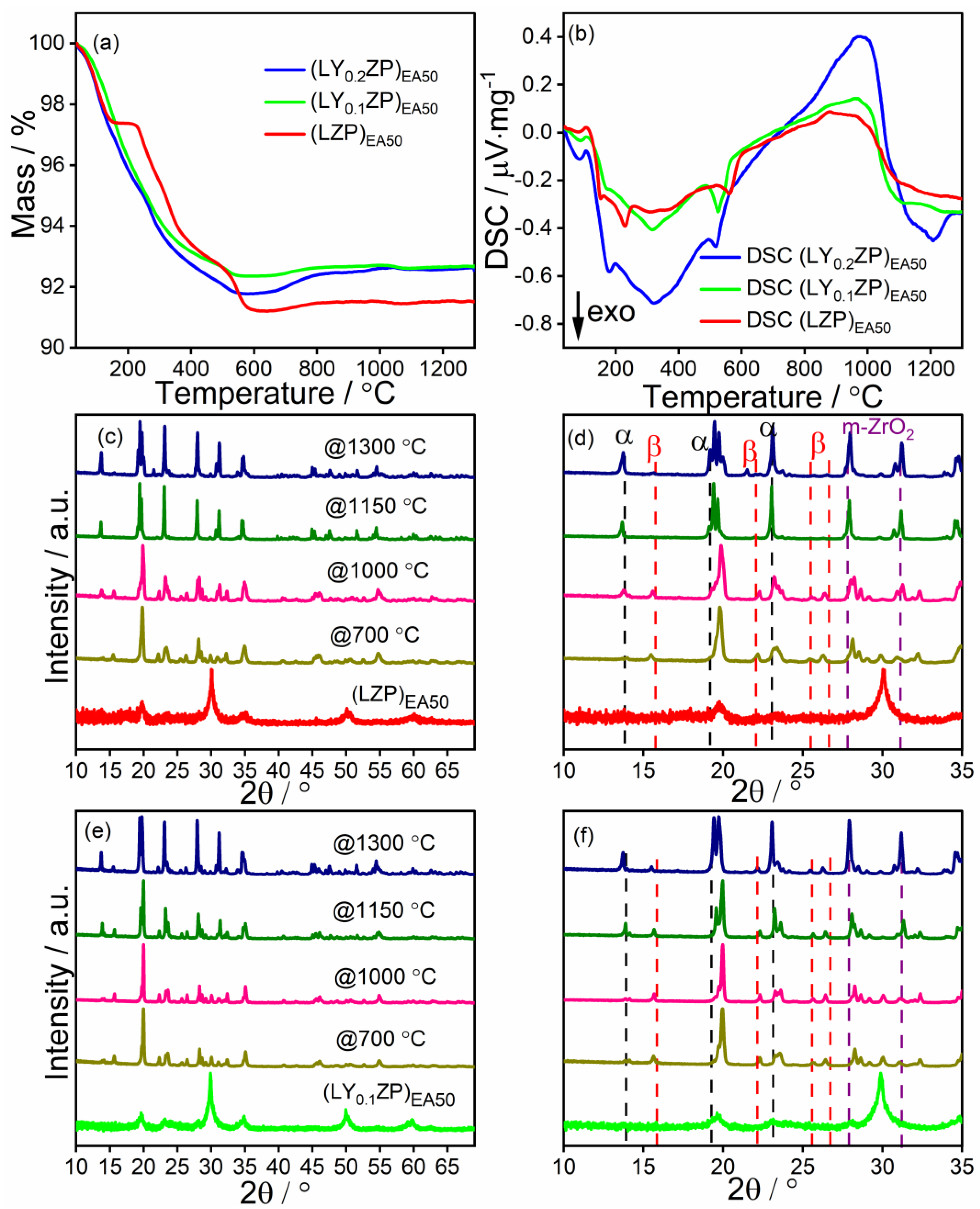
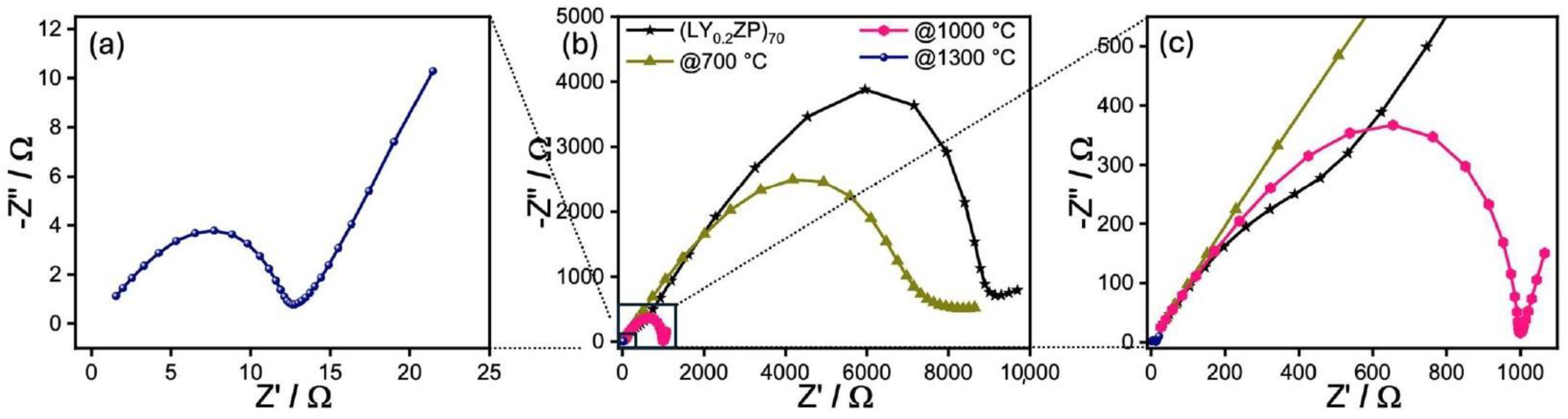
Characterization of (LY0.2ZP)PA50 and (LY0.2ZP)EA50 Nanoparticles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaies, B.; Guesmi, K.; Porcher, T.; Boroumand, R. Financial instability and oil price fluctuations: Evidence from oil exporting developing countries. Eur. J. Comp. Econ. 2020, 17, 55–71. [Google Scholar] [CrossRef]
- Brand, K. Umstrittene Ostsee-Pipeline: Grünes Licht für Nord Stream 2. 2021. Available online: https://www.tagesschau.de/ausland/amerika/nord-stream-2-einigung-103.html (accessed on 2 February 2022).
- Kougias, I. Hydropower—Technology Development Report 2020; Publications Office of the European Union: Luxembourg, 2021.
- Taylor, N.; Jager-Waldau, A. Photovoltaics: Technology Development Report 2020; Publications Office of the European Union: Luxembourg, 2021; p. 30504.
- Telsnig, T. Wind Energy Technology Development Report 2020; Publications Office of the European Union: Luxembourg, 2021.
- Yoo, J.; Park, B.; An, K.; Al-Ammar, E.; Khan, Y.; Hur, K.; Kim, J. Look-Ahead Energy Management of a Grid-Connected Residential PV System with Energy Storage under Time-Based Rate Programs. Energies 2012, 5, 1116–1134. [Google Scholar] [CrossRef]
- Hesse, H.C.; Schimpe, M.; Kucevic, D.; Jossen, A. Lithium-Ion Battery Storage for the Grid—A Review of Stationary Battery Storage System Design Tailored for Applications in Modern Power Grids. Energies 2017, 10, 2107. [Google Scholar] [CrossRef]
- Meyers, W.F.; Simmons, J.W. Electric Current-Producing Cell with Anhydrous Organic Liquid Electrolyte. U.S. Patent 3,423,242, 21 January 1969. [Google Scholar]
- Brodd, R.J.; Tagawa, K. Lithium-Ion Cell Production Processes. In Advances in Lithium-Ion Batteries; van Schalkwijk, W.A., Scrosati, B., Eds.; Springer: Boston, MA, USA, 2002; pp. 267–288. [Google Scholar]
- Ravdel, B.; Abraham, K.M.; Gitzendanner, R.; DiCarlo, J.; Lucht, B.; Campion, C. Thermal stability of lithium-ion battery electrolytes. J. Power Sources 2003, 119–121, 805–810. [Google Scholar] [CrossRef]
- Abada, S.; Marlair, G.; Lecocq, A.; Petit, M.; Sauvant-Moynot, V.; Huet, F. Safety focused modeling of lithium-ion batteries: A review. J. Power Sources 2016, 306, 178–192. [Google Scholar] [CrossRef]
- Arbizzani, C.; Gabrielli, G.; Mastragostino, M. Thermal stability and flammability of electrolytes for lithium-ion batteries. J. Power Sources 2011, 196, 4801–4805. [Google Scholar] [CrossRef]
- Takada, K.; Nakano, S.; Inada, T.; Kajiyama, A.; Kouguchi, M.; Sasaki, H.; Kondo, S.; Watanabe, M.; Murayama, M.; Kanno, R. Solid-State Lithium Batteries with Sulfide-Based Solid Electrolytes. Solid State Ion. 2004, 172, 25–30. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Huang, S.; Komine, Y.; Notohara, H.; Urita, K.; Moriguchi, I.; Wei, M. Regulating the effects of SnS shrinkage in all-solid-state lithium-ion batteries with excellent electrochemical performance. Chem. Eng. J. 2022, 429, 132424. [Google Scholar] [CrossRef]
- Wang, J.; Okabe, J.; Komine, Y.; Notohara, H.; Urita, K.; Moriguchi, I.; Wei, M. The optimized interface engineering of VS2 as cathodes for high performance all-solid-state lithium-ion battery. Sci. China Technol. Sci. 2022, 65, 1859–1866. [Google Scholar] [CrossRef]
- Judez, X.; Zhang, H.; Li, C.; Eshetu, G.G.; González-Marcos, J.A.; Armand, M.; Rodriguez-Martinez, L.M. Review—Solid Electrolytes for Safe and High Energy Density Lithium-Sulfur Batteries: Promises and Challenges. J. Electrochem. Soc. 2018, 165, A6008. [Google Scholar] [CrossRef]
- Overhoff, G.M.; Ali, M.Y.; Brinkmann, J.-P.; Lennartz, P.; Orthner, H.; Hammad, M.; Wiggers, H.; Winter, M.; Brunklaus, G. Ceramic-in-Polymer Hybrid Electrolytes with Enhanced Electrochemical Performance. ACS Appl. Mater. Interfaces 2022, 14, 53636–53647. [Google Scholar] [CrossRef] [PubMed]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Monroe, C.; Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 2005, 152, A396–A404. [Google Scholar] [CrossRef]
- Brissot, C.; Rosso, M.; Chazalviel, J.N.; Lascaud, S. Dendritic growth mechanisms in lithium/polymer cells. J. Power Sources 1999, 81, 925–929. [Google Scholar] [CrossRef]
- Kerman, K.; Luntz, A.; Viswanathan, V.; Chiang, Y.M.; Chen, Z.B. Review-Practical Challenges Hindering the Development of Solid State Li Ion Batteries. J. Electrochem. Soc. 2017, 164, A1731–A1744. [Google Scholar] [CrossRef]
- Duan, H.; Fan, M.; Chen, W.-P.; Li, J.-Y.; Wang, P.-F.; Wang, W.-P.; Shi, J.-L.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Extended Electrochemical Window of Solid Electrolytes via Heterogeneous Multilayered Structure for High-Voltage Lithium Metal Batteries. Adv. Mater. 2019, 31, 1807789. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wang, S.; Lochala, J.; Desrochers, D.; Liu, B.; Zhang, W.; Yang, J.; Xiao, J. The role of the solid electrolyte interphase layer in preventing Li dendrite growth in solid-state batteries. Energy Environ. Sci. 2018, 11, 1803–1810. [Google Scholar] [CrossRef]
- Luo, W.; Gong, Y.; Zhu, Y.; Fu, K.K.; Dai, J.; Lacey, S.D.; Wang, C.; Liu, B.; Han, X.; Mo, Y.; et al. Transition from Superlithiophobicity to Superlithiophilicity of Garnet Solid-State Electrolyte. J. Am. Chem. Soc. 2016, 138, 12258–12262. [Google Scholar] [CrossRef]
- Lu, J.Y.; Li, Y. Perovskite-type Li-ion solid electrolytes: A review. J. Mater. Sci.-Mater. Electron. 2021, 32, 9736–9754. [Google Scholar] [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-Type Solid-State Electrolytes: Materials, Interfaces, and Batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef] [PubMed]
- DeWees, R.; Wang, H. Synthesis and Properties of NaSICON-type LATP and LAGP Solid Electrolytes. Chemsuschem 2019, 12, 3713–3725. [Google Scholar] [CrossRef]
- Hood, Z.D.; Wang, H.; Pandian, A.S.; Keum, J.K.; Liang, C.D. Li2OHCl Crystalline Electrolyte for Stable Metallic Lithium Anodes. J. Am. Chem. Soc. 2016, 138, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, J.; Vogel, S.; Wang, C.-A. The reaction of Li6.5La3Zr1.5Ta0.5O12 with water. Solid. State. Ion. 2014, 269, 57–61. [Google Scholar] [CrossRef]
- Shimonishi, Y.; Toda, A.; Zhang, T.; Hirano, A.; Imanishi, N.; Yamamoto, O.; Takeda, Y. Synthesis of Garnet-Type Li7−xLa3Zr2O12−1/2x and Its Stability in Aqueous Solutions. Solid State Ion. 2011, 183, 48–53. [Google Scholar] [CrossRef]
- Schroeder, D.J.; Hubaud, A.A.; Vaughey, J.T. Stability of the solid electrolyte Li3OBr to common battery solvents. Mater. Res. Bull. 2014, 49, 614–617. [Google Scholar] [CrossRef]
- Dashjav, E.; Ma, Q.; Xu, Q.; Tsai, C.-L.; Giarola, M.; Mariotto, G.; Tietz, F. The influence of water on the electrical conductivity of aluminum-substituted lithium titanium phosphates. Solid State Ion. 2018, 321, 83–90. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, Y.; Li, Y.; Peng, L.; Byon, H.R.; Goodenough, J.B.; Yu, G. A chemistry and material perspective on lithium redox flow batteries towards high-density electrical energy storage. Chem. Soc. Rev. 2015, 44, 7968–7996. [Google Scholar] [CrossRef]
- Bronowski, J. CHAPTER 6—D.C. CONDUCTIVITY. In Structural Chemistry of Glasses; Rao, K.J., Ed.; Elsevier Science Ltd.: Oxford, UK, 2002; pp. 203–261. [Google Scholar]
- Li, Y.; Liu, M.; Liu, K.; Wang, C.-A. High Li+ conduction in NASICON-type Li1+xYxZr2−x(PO4)3 at room temperature. J. Power Sources 2013, 240, 50–53. [Google Scholar] [CrossRef]
- Guo, Z.; Qin, X.; Xie, Y.; Lei, C.; Wei, T.; Zhang, Y. Advanced NASICON-type LiTi2(PO4)3 as electrode materials for lithium-ion batteries. Chem. Phys. Lett. 2022, 806, 140010. [Google Scholar] [CrossRef]
- Khatua, S.; Rao, Y.B.; Achary, K.R.; Patro, L.N. Li-ion transport studies of NASICON-type LiZr2(PO4)3 solid electrolyte crystallizing in rhombohedral structure at room temperature. Surf. Interfaces 2023, 41, 103212. [Google Scholar] [CrossRef]
- Hou, M.; Liang, F.; Chen, K.; Dai, Y.; Xue, D. Challenges and perspectives of NASICON-type solid electrolytes for all-solid-state lithium batteries. Nanotechnology 2020, 31, 132003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Shen, Y.; Lin, Y.; Nan, C.-W. Enhanced lithium-ion conductivity in a LiZr2(PO4)3 solid electrolyte by Al doping. Ceram. Int. 2017, 43, S598–S602. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, B.; Chien, P.-H.; Li, Y.; Huang, B.; Wu, N.; Xu, H.; Grundish, N.S.; Hu, Y.-Y.; Goodenough, J.B. NASICON Li1.2Mg0.1Zr1.9(PO4)3 Solid Electrolyte for an All-Solid-State Li-Metal Battery. Small Methods 2020, 4, 2000764. [Google Scholar] [CrossRef]
- Catti, M.; Comotti, A.; Di Blas, S. High-Temperature Lithium Mobility in α-LiZr2(PO4)3 NASICON by Neutron Diffraction. Chem. Mater. 2003, 15, 1628–1632. [Google Scholar] [CrossRef]
- Nomura, K.; Ikeda, S.; Ito, K.; Einaga, H. Ionic conduction behavior in zirconium phosphate framework. Solid State Ion. 1993, 61, 293–301. [Google Scholar] [CrossRef]
- Arbi, K.; Ayadi-Trabelsi, M.; Sanz, J. Li mobility in triclinic and rhombohedral phases of the Nasicon-type compound LiZr2(PO4)3 as deduced from NMR spectroscopy. J. Mater. Chem. 2002, 12, 2985–2990. [Google Scholar] [CrossRef]
- Li, Q.H.; Xu, C.; Huang, B.; Yin, X. Rhombohedral Li1+xYxZr2−x(PO4)3 Solid Electrolyte Prepared by Hot-Pressing for All-Solid-State Li-Metal Batteries. Materials 2020, 13, 035930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Wu, L.-R.; Ma, J.; Cui, G. Nanotechnology in solid state batteries, what’s next? Next Nanotechnol. 2023, 2, 100011. [Google Scholar] [CrossRef]
- Fuentes, R.O.; Figueiredo, F.; Marques, F.; Franco, J. Influence of microstructure on the electrical properties of NASICON materials. Solid State Ion. 2001, 140, 173–179. [Google Scholar] [CrossRef]
- Fuentes, R.; Figueiredo, F.; Marques, F.; Franco, J. Processing and Electrical Properties of NASICON Prepared from Yttria-Doped Zirconia Precursors. J. Eur. Ceram. Soc. 2001, 21, 737–743. [Google Scholar] [CrossRef]
- Buscaglia, M.T.; Bassoli, M.; Buscaglia, V. Solid-state synthesis of nanocrystalline BaTiO3: Reaction kinetics and powder properties. J. Am. Ceram. Soc. 2008, 91, 2862–2869. [Google Scholar] [CrossRef]
- Clabel, J.L.; Awan, I.T.; Pinto, A.H.; Nogueira, I.C.; Bezzon, V.D.N.; Leite, E.R.; Balogh, D.T.; Mastelaro, V.R.; Ferreira, S.O.; Marega, E. Insights on the mechanism of solid state reaction between TiO2 and BaCO3 to produce BaTiO3 powders: The role of calcination, milling, and mixing solvent. Ceram. Int. 2020, 46, 2987–3001. [Google Scholar] [CrossRef]
- Kotobuki, M.; Koishi, M. Preparation of Li1.5Al0.5Ti1.5(PO4)(3) solid electrolyte via a sol-gel route using various Al sources. Ceram. Int. 2013, 39, 4645–4649. [Google Scholar] [CrossRef]
- Ugemuge, N.; Parauha, Y.R.; Dhoble, S.J. Chapter 15—Synthesis and luminescence study of silicate-based phosphors for energy-saving light-emitting diodes. In Energy Materials; Dhoble, S.J., Kalyani, N.T., Vengadaesvaran, B., Kariem Arof, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 445–480. [Google Scholar]
- Takada, K.; Fujimoto, K.; Inada, T.; Kajiyama, A.L.; Kouguchi, M.; Kondo, S.; Watanabe, M. Sol-gel preparation of Li+ ion conductive thin film. Appl. Surf. Sci. 2002, 189, 300–306. [Google Scholar] [CrossRef]
- Xu, X.X.; Wen, Z.Y.; Wu, J.G.; Yang, X.L. Preparation and electrical properties of NASICON-type structured Li1.4Al0.4Ti1.6(PO4)(3) glass-ceramics by the citric acid-assisted sol-gel method. Solid State Ion. 2007, 178, 29–34. [Google Scholar] [CrossRef]
- Ulrich, G.D. Flame Synthesis of Fine Particles. Chem. Eng. News 1984, 62, 22–29. [Google Scholar] [CrossRef]
- Guo, J.Z.; Goodings, J.M.; Hayhurst, A.N.; Taylor, S.G. A simple method for measuring positive ion concentrations in flames and the calibration of a nebulizer/atomizer. Combust. Flame 2003, 133, 335–343. [Google Scholar] [CrossRef]
- Madler, L.; Kammler, H.K.; Mueller, R.; Pratsinis, S.E. Controlled synthesis of nanostructured particles by flame spray pyrolysis. J. Aerosol Sci. 2002, 33, 369–389. [Google Scholar] [CrossRef]
- Dasgupta, M.; Fortugno, P.; Wiggers, H. Plasma-assisted gas-phase synthesis and in-line coating of silicon nanoparticles. Plasma Process Polym. 2020, 17, 1900245. [Google Scholar] [CrossRef]
- Chrystie, R.S.M.; Ebertz, F.L.; Dreier, T.; Schulz, C. Absolute SiO concentration imaging in low-pressure nanoparticle-synthesis flames via laser-induced fluorescence. Appl. Phys. B-Lasers Opt. 2019, 125, 29. [Google Scholar] [CrossRef]
- Abdali, A.; Moritz, B.; Gupta, A.; Wiggers, H.; Schulz, C. Hybrid microwave-plasma hot-wall reactor for synthesis of silica nanoparticles under well-controlled conditions. J. Optoelectron. Adv. Mater. 2010, 12, 440–444. [Google Scholar]
- Schneider, F.; Suleiman, S.; Menser, J.; Borukhovich, E.; Wlokas, I.; Kempf, A.; Wiggers, H.; Schulz, C. SpraySyn-A standardized burner configuration for nanoparticle synthesis in spray flames. Rev. Sci. Instrum. 2019, 90, 085108. [Google Scholar] [CrossRef] [PubMed]
- Angel, S.; Tapia, J.D.; Gallego, J.; Hagemann, U.; Wiggers, H. Spray-Flame Synthesis of LaMnO3+δ Nanoparticles for Selective CO Oxidation (SELOX). Energy Fuels 2021, 35, 4367–4376. [Google Scholar] [CrossRef]
- Alkan, B.; Cychy, S.; Varhade, S.; Muhler, M.; Schulz, C.; Schuhmann, W.; Wiggers, H.; Andronescu, C. Spray-Flame-Synthesized LaCo1−xFexO3 Perovskite Nanoparticles as Electrocatalysts for Water and Ethanol Oxidation. ChemElectroChem 2019, 6, 4266–4274. [Google Scholar] [CrossRef]
- Ali, M.Y.; Orthner, H.; Wiggers, H. Spray Flame Synthesis (SFS) of Lithium Lanthanum Zirconate (LLZO) Solid Electrolyte. Materials 2021, 14, 3472. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, H.; Kōmoto, K.; Miyayama, M.; Yamada, H. The Chemistry of Ceramics; Wiley: Chichester, UK, 1996. [Google Scholar]
- Hund, F. Anomale Mischkristalle im System ZrO2Y2O3 Kristallbau der Nernst-Stifte. Z. Elektrochem. Und Angew. Phys. Chem. 1951, 55, 363–366. [Google Scholar] [CrossRef]
- Yamada, T.; Kubota, Y.; Makinose, Y.; Suzuki, N.; Nakata, K.; Terashima, C.; Matsushita, N.; Okada, K.; Fujishima, A.; Katsumata, K.-i. Single Crystal ZrO2 Nanosheets Formed by Thermal Transformation for Solid Oxide Fuel Cells and Oxygen Sensors. ACS Appl. Nano Mater. 2019, 2, 6866–6873. [Google Scholar] [CrossRef]
- El-Shinawi, H.; Greaves, C.; Janek, J. Sol–gel synthesis and room-temperature properties of α-LiZr2(PO4)3. RSC Adv. 2015, 5, 17054–17059. [Google Scholar] [CrossRef]
- Lai, Y.; Sun, Z.; Jiang, L.; Hao, X.; Jia, M.; Wang, L.; Liu, F. Rapid sintering of ceramic solid electrolytes LiZr2(PO4)3 and Li1.2Ca0.1Zr1.9(PO4)3 using a microwave sintering process at low temperatures. Ceram. Int. 2019, 45, 11068–11072. [Google Scholar] [CrossRef]
- Lieber, C.M.; Wang, Z.L. Functional Nanowires. MRS Bull. 2007, 32, 99–108. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Madler, L. Flame spray pyrolysis: An enabling technology for nanoparticles design and fabrication. Nanoscale 2010, 2, 1324–1347. [Google Scholar] [CrossRef] [PubMed]
- PubChem Compound Summary for CID 26251, Zirconium Nitrate; National Library Medicine: Bethesda, MD, USA, 2021.
- Angel, S.; Neises, J.; Dreyer, M.; Friedel Ortega, K.; Behrens, M.; Wang, Y.; Arandiyan, H.; Schulz, C.; Wiggers, H. Spray-flame synthesis of La(Fe, Co)O3 nano-perovskites from metal nitrates. AIChE J. 2020, 66, e16748. [Google Scholar] [CrossRef]
- Wang, D.; Kou, R.; Ren, Y.; Sun, C.-J.; Zhao, H.; Zhang, M.-J.; Li, Y.; Huq, A.; Ko, J.Y.P.; Pan, F.; et al. Synthetic Control of Kinetic Reaction Pathway and Cationic Ordering in High-Ni Layered Oxide Cathodes. Adv. Mater. 2017, 29, 1606715. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, S.; Wilson, H.; Zhao, F.; Manthiram, A. Y-Doped NASICON-type LiZr2(PO4)3 Solid Electrolytes for Lithium-Metal Batteries. Chem. Mater. 2017, 29, 7206–7212. [Google Scholar] [CrossRef]
- Chraska, T.; King, A.H.; Berndt, C.C.; Karthikeyan, J. Phase Transformation as a Function of Particle Size in Nanocrystalline Zirconia. MRS Online Proc. Libr. 1997, 481, 613–617. [Google Scholar] [CrossRef]
- Ramirez, L.; Mecartney, M.L.; Krumdieck, S.P. Nanocrystalline ZrO2 thin films on silicon fabricated by pulsed-pressure metalorganic chemical vapor deposition (PP-MOCVD). J. Mater. Res. 2008, 23, 2202–2211. [Google Scholar] [CrossRef]
- Srinivasan, R.; Rice, L.; Davis, B. Critical Particle Size and Phase Transformation in Zirconia: Transmission Electron Microscopy and X-Ray Diffraction Studies. J. Am. Ceram. Soc. 2005, 73, 3528–3530. [Google Scholar] [CrossRef]
- Lackner, P.; Zou, Z.; Mayr, S.; Diebold, U.; Schmid, M. Using photoelectron spectroscopy to observe oxygen spillover to zirconia. Phys. Chem. Chem. Phys. 2019, 21, 17613–17620. [Google Scholar] [CrossRef] [PubMed]
- Egger, P.; Dirè, S.; Ischia, M.; Campostrini, R. Pyrolysis study of sol-gel derived zirconia by TG-GC-MS. J. Therm. Anal. Calorim. 2005, 81, 407–415. [Google Scholar] [CrossRef]
- Shi, L.; Qu, T.; Liu, D.; Deng, Y.; Yang, B.; Dai, Y. Process of Thermal Decomposition of Lithium Carbonate. In Proceedings of the Materials Processing Fundamentals 2020, Cham, Switzerland, 9 January 2020; pp. 107–116. [Google Scholar]
- Geiculescu, A.C.; Spencer, H.G. Thermal Decomposition and Crystallization of Aqueous Sol-Gel Derived Zirconium Acetate Gels: Effects of the Additive Anions. J. Sol-Gel Sci. Technol. 2000, 17, 25–35. [Google Scholar] [CrossRef]
- Efaw, C.M.; Vandegrift, J.L.; Reynolds, M.; McMurdie, S.; Jaques, B.J.; Hu, H.; Xiong, H.; Hurley, M.F. Characterization of zirconium oxides part I: Raman mapping and spectral feature analysis. Nucl. Mater. Energy 2019, 21, 100707. [Google Scholar] [CrossRef]
- Kim, D.-J.; Jung, H.-J.; Yang, I.-S. Raman Spectroscopy of Tetragonal Zirconia Solid Solutions. J. Am. Ceram. Soc. 1993, 76, 2106–2108. [Google Scholar] [CrossRef]
- Colbea, C.; Avram, D.; Cojocaru, B.; Negrea, R.; Ghica, C.; Kessler, V.G.; Seisenbaeva, G.A.; Parvulescu, V.; Tiseanu, C. Full Tetragonal Phase Stabilization in ZrO2 Nanoparticles Using Wet Impregnation: Interplay of Host Structure, Dopant Concentration and Sensitivity of Characterization Technique. Nanomaterials 2018, 8, 988. [Google Scholar] [CrossRef] [PubMed]
- Long, D.A. Infrared and Raman characteristic group frequencies. Tables and charts George Socrates John Wiley and Sons, Ltd., Chichester, Third Edition, 2001. Price £135. J. Raman Spectrosc. 2004, 35, 905. [Google Scholar] [CrossRef]
- Strobel, R.; Pratsinis, S.E. Effect of solvent composition on oxide morphology during flame spray pyrolysis of metal nitrates. Phys. Chem. Chem. Phys. 2011, 13, 92469252. [Google Scholar] [CrossRef] [PubMed]
- Stodt, M.F.B.; Groeneveld Jan, D.; Mädler, L.; Kiefer, J.; Fritsching, U. Microexplosions of multicomponent drops in spray flames. Combust. Flame 2022, 240, 112043. [Google Scholar] [CrossRef]
- Wang, J.; He, T.; Yang, X.; Cai, Z.; Wang, Y.; Lacivita, V.; Kim, H.; Ouyang, B.; Ceder, G. Design principles for NASICON super-ionic conductors. Nat. Commun. 2023, 14, 5210. [Google Scholar] [CrossRef] [PubMed]



| Nomenclature | Precursors | Solvents | ||||
|---|---|---|---|---|---|---|
| Li | Y | Zr | P | (A) | (B) | |
| LiNO3 (50% excess Li) | Y(NO3)3 ·6H2O | ZP | TBP | Propanol/Propionic Acid (PrOH/PA) V/V | Ethanol/2-Ethylhexanoic Acid (EtOH/2-EHA) V/V | |
| (LZP)EA50 | ✓ | 0 | ✓ | ✓ | × | 1:1 |
| (LY0.1ZP)EA50 | ✓ | 0.1 | ✓ | ✓ | × | 1:1 |
| (LY0.2ZP)PA50 | ✓ | 0.2 | ✓ | ✓ | 1:1 | × |
| (LY0.2ZP)EA50 | ✓ | 0.2 | ✓ | ✓ | × | 1:1 |
| (LY0.2ZP)EA70 | ✓ | 0.2 | ✓ | ✓ | × | 3:7 |
| Operating Parameters | ||||||
|---|---|---|---|---|---|---|
| Dispersion CH4 [slm] | Dispersion O2 [slm] | Pilot Flame CH4 [slm] | Pilot Flame O2 [slm] | Quench Gas Air [slm] | Coaxial Sheath Air [slm] | Reactor Pressure [mbar] |
| 1 | 9 | 2 | 16 | 240 | 140 | 800–820 |
| Status | Content of Phase [%] | ||||
|---|---|---|---|---|---|
| t-ZrO2 | m-ZrO2 | α-LYZP | β-LYZP | ||
| (LY0.2ZP)PA50 | As-synthesized | 34.0 | 39.9 | / | / |
| @1300 °C | 0.6 | 27.6 | 31.5 | 40.3 | |
| (LY0.2ZP)EA50 | As-synthesized | 21.8 | 63.5 | 14.7 | / |
| @ 1300 °C | 0.5 | 14.2 | 49.6 | 35.6 | |
| Nomenclature | Solvent Mixture | Composition [wt%] | ||
|---|---|---|---|---|
| Propanol/Propionic Acid (1:1 by Volume) | α-LYZP | β-LYZP | m-ZrO2 | |
| (LY0.2ZP)PA50 | Ethanol/2-EHA (1:1 by volume) | 31.5 | 40.3 | 27.6 |
| (LY0.2ZP)EA50 | Ethanol/2-EHA (3:7 by volume) | 49.6 | 35.6 | 14.2 |
| (LY0.2ZP)EA70 | Propanol/propionic acid (1:1 by volume) | 94.7 | / | 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.Y.; Chen, T.; Orthner, H.; Wiggers, H. Spray-Flame Synthesis of NASICON-Type Rhombohedral (α) Li1+xYxZr2−x(PO4)3 [x = 0–0.2] Solid Electrolytes. Nanomaterials 2024, 14, 1278. https://doi.org/10.3390/nano14151278
Ali MY, Chen T, Orthner H, Wiggers H. Spray-Flame Synthesis of NASICON-Type Rhombohedral (α) Li1+xYxZr2−x(PO4)3 [x = 0–0.2] Solid Electrolytes. Nanomaterials. 2024; 14(15):1278. https://doi.org/10.3390/nano14151278
Chicago/Turabian StyleAli, Md Yusuf, Tianyu Chen, Hans Orthner, and Hartmut Wiggers. 2024. "Spray-Flame Synthesis of NASICON-Type Rhombohedral (α) Li1+xYxZr2−x(PO4)3 [x = 0–0.2] Solid Electrolytes" Nanomaterials 14, no. 15: 1278. https://doi.org/10.3390/nano14151278






