Low-Temperature Fabrication of BiFeO3 Films on Aluminum Foils under a N2-Rich Atmosphere
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Performance of BiFeO3 Annealed at 440 ± 5 °C under Different Atmospheres
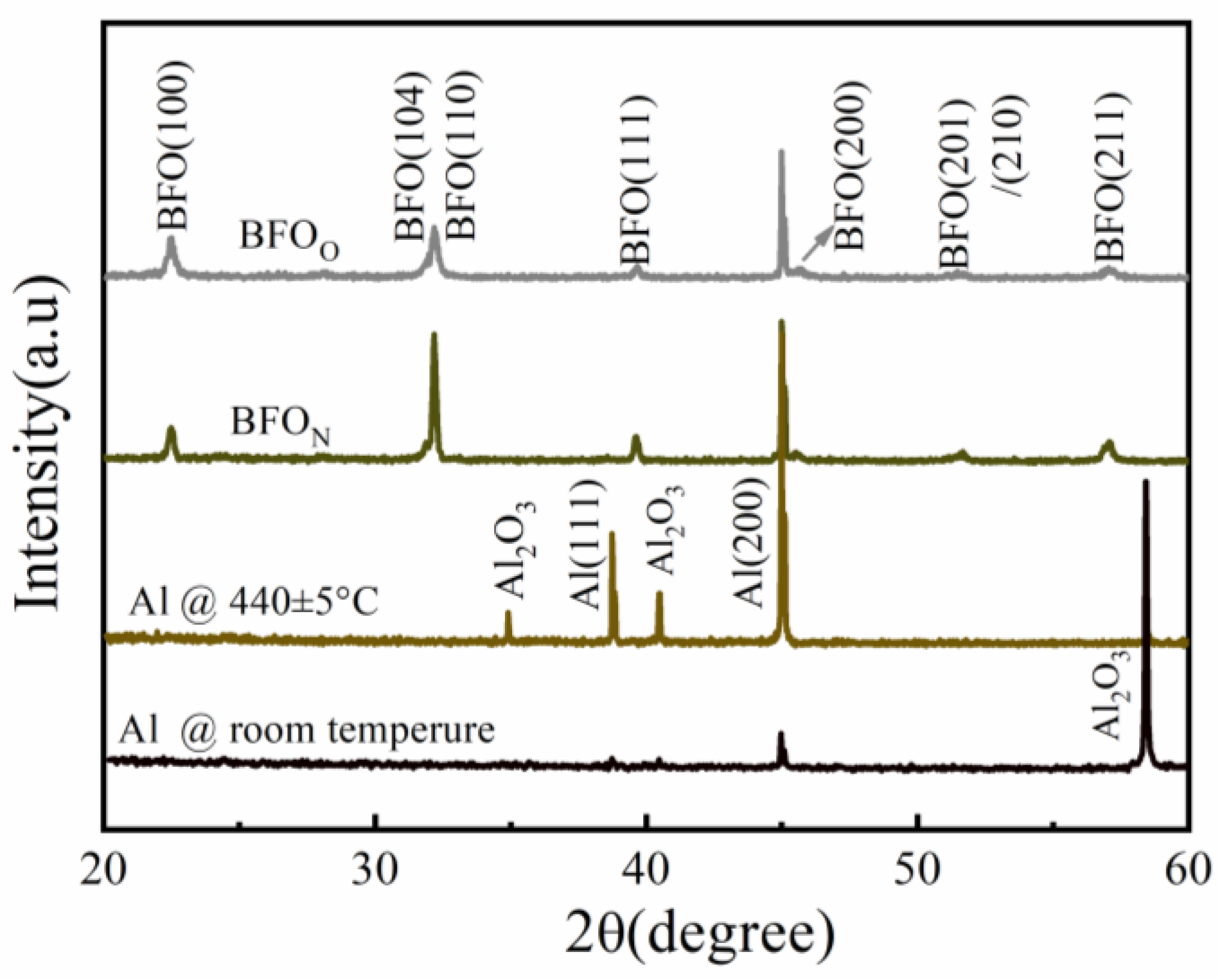
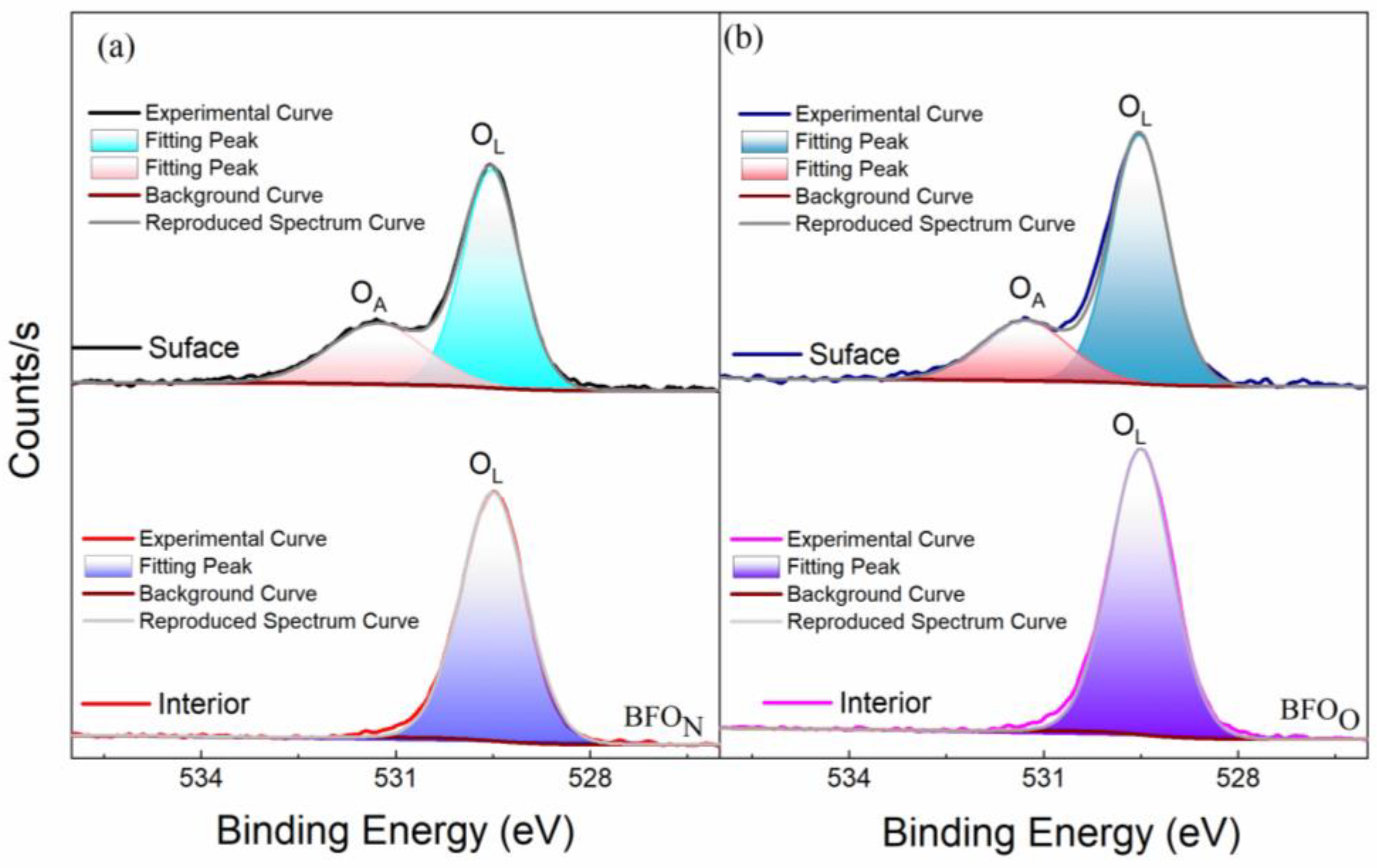
3.1.1. Microstructure Analysis
3.1.2. Room-Temperature Electrical Properties
3.2. Performance of BiFeO3 Films Annealed at 365 ± 5 °C under a N2-Rich Atmospheres
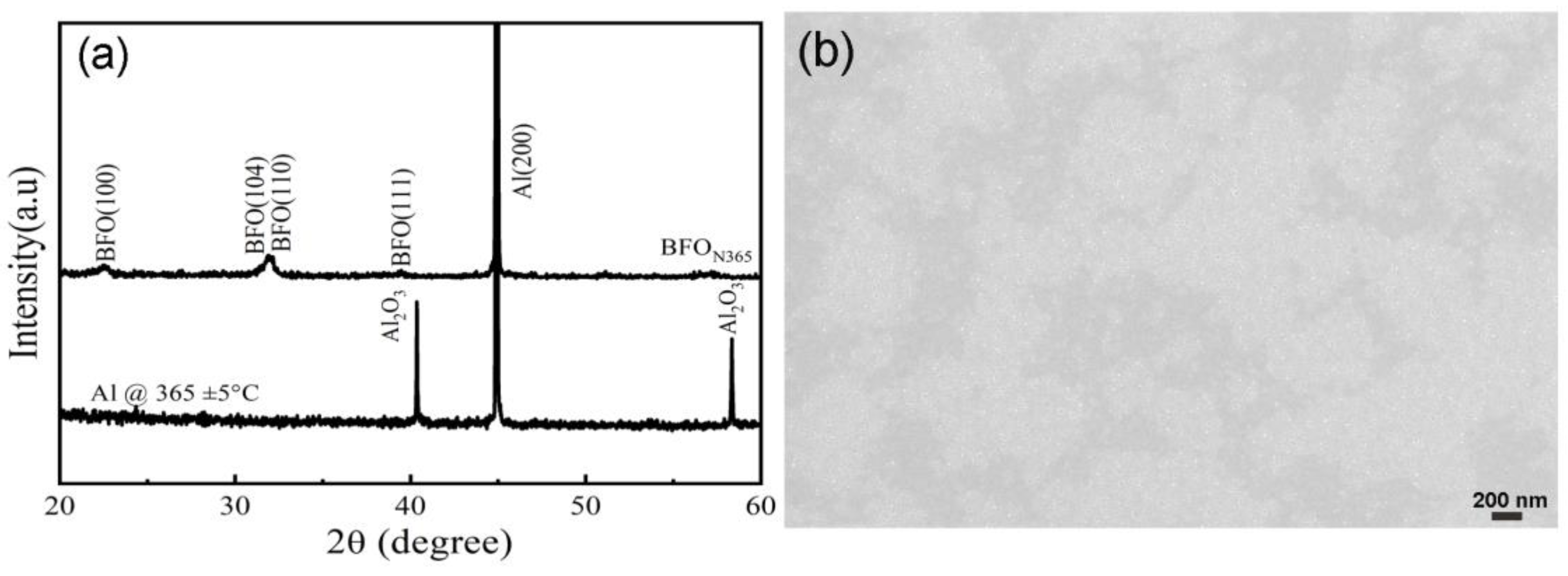
3.2.1. Microstructure Analysis
3.2.2. Room-Temperature Electrical Properties
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Wang, J. Orientation dependence of ferroelectric behavior of BiFeO3 thin films. J. Appl. Phys. 2009, 106, 104111. [Google Scholar] [CrossRef]
- Yan, J.; Ouyang, J.; Cheng, H.B.; Yan, P. Low temperature deposition of BiFeO3 films on Ti foils for piezoelectric applications. Scr. Mater. 2021, 204, 114152. [Google Scholar] [CrossRef]
- Wang, J.; Neaton, J.B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaithyanathan, V.; Schlom, D.G.; Waghmare, U.V.; et al. Epitaxial BiFeO3 multiferroic thin film heterostructures. Science 2003, 299, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.G.; Fan, Z.; Xiao, D.Q.; Zhu, J.G.; Wang, J. Multiferroic bismuth ferrite-based materials for multifunctional applications: Ceramic bulks, thin films and nanostructures. Prog. Mater. Sci. 2016, 84, 335–402. [Google Scholar] [CrossRef]
- Yang, B.B.; Jin, L.H.; Wei, R.H.; Tang, X.W.; Hu, L.; Tong, P.; Yang, J.; Song, W.H.; Dai, J.M.; Zhu, X.B.; et al. Chemical solution route for high-quality multiferroic BiFeO3 thin films. Small 2021, 17, 1903663. [Google Scholar] [CrossRef] [PubMed]
- Catalan, G.; Scott, J.F. Physics and applications of bismuth ferrite. Adv. Mater. 2010, 21, 2463–2485. [Google Scholar] [CrossRef]
- Zhu, H.F.; Yang, Y.L.; Ren, W.; Niu, M.M.; Hu, W.; Ma, H.F.; Ouyang, J. Rhombohedral BiFeO3 thick films integrated on Si with a giant electric polarization and prominent piezoelectricity. Acta Mater. 2020, 200, 305–314. [Google Scholar] [CrossRef]
- Tomczyk, M.; Bretos, I.; Jiménez, R.; Mahajan, A.; Ramana, E.V.; Calzada, M.L.; Vilarinho, P.M. Direct fabrication of BiFeO3 thin films on polyimide substrates for flexible electronics. J. Mater. Chem. C 2017, 5, 12529–12537. [Google Scholar] [CrossRef]
- Wen, Z.; Shen, X.; Wu, J.X.; Wu, D.; Li, A.D.; Yang, B.; Wang, Z.; Chen, H.Z.; Wang, J.L. Temperature-dependent leakage current characteristics of Pr and Mn cosubstituted BiFeO3 thin films. Appl. Phys. Lett. 2010, 96, 202904. [Google Scholar] [CrossRef]
- Yan, J.; Hu, G.D.; Chen, X.M.; Wu, W.B.; Yang, C.H. Ferroelectric properties, morphologies, and leakage currents of Bi0.97La0.03FeO3 thin films deposited on indium tin oxide/glass substrates. J. Appl. Phys. 2008, 104, 076103. [Google Scholar] [CrossRef]
- Chen, D.Y.; Chen, Z.H.; He, Q.; Clarkson, J.D.; Serrao, C.R.; Yadav, A.K.; Nowakowski, M.E.; Fan, Z.; You, L.; Gao, X.S.; et al. Interface engineering of domain structures in BiFeO3 thin films. Nano Lett. 2017, 17, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.W.; Ortiz, D.; Baek, S.H.; Folkman, C.M.; Das, R.R.; Shafer, P.; Chen, Y.B.; Nelson, C.T.; Pan, X.Q.; Ramesh, R.; et al. Domain engineering for enhanced ferroelectric properties of epitaxial (001) BiFeO thin films. Adv. Mater. 2009, 21, 817–823. [Google Scholar] [CrossRef]
- Yan, J.; Zhu, H.F.; Ouyang, J.; Kanno, I.; Yan, P.; Wang, Y.Y.; Onishi, K.; Nishikado, T. Highly (00l)-textured BiFeO3 thick films integrated on stainless steel foils with an optimized piezoelectric performance. J. Eur. Ceram. Soc. 2022, 42, 3454–3462. [Google Scholar] [CrossRef]
- Chen, X.M.; Hu, G.D.; Wang, J.C.; Cheng, L.; Yang, C.H.; Wu, W.B. Thickness effects of Bi0.89Ti0.11FeO3 thin films deposited on PbZr0.2Ti0.79Nb0.01O3 buffer layers. J. Alloys Compd. 2011, 509, 431–434. [Google Scholar] [CrossRef]
- Li, H.; Ding, Y.W.; Ren, K.J.; Zeng, Z.X.; Chen, C.; Deng, X.L.; Gao, R.L.; Cai, W.; Wang, Z.H.; Fu, C.L.; et al. Effect of rapid/slow annealing routes on the magnetic and photoelectric properties of BiFeO3/CoFe2O4 multilayer thin films. J. Alloys Compd. 2024, 989, 174203. [Google Scholar] [CrossRef]
- Pan, T.M.; Chen, Z.Y.; Weng, W.C.; Her, J.L. Electrical and structural properties of BiFeO3 thin films with four distinct buffer layers: La2O3, Pr2O3, Sm2O3, and Tm2O3. Phys. B Condens. Matter. 2024, 689, 416219. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, J.Q.; Zhang, G.C.; Zhu, X.J. Significantly improved ferroelectric properties of (Zn,Co) co-doped BiFeO3 thin films. prepared by sol-gel method. Ceram. Int. 2024, 50, 28449–28457. [Google Scholar] [CrossRef]
- Wang, Y.P.; Zhou, L.; Zhang, M.F.; Chen, X.Y.; Liu, J.M.; Liu, Z.G. Room-Temperature Saturated Ferroelectric Polarization in BiFeO3 Ceramics Synthesized by Rapid Liquid Phase Sintering. Appl. Phys. Lett. 2004, 84, 1731–1733. [Google Scholar] [CrossRef]
- Palkar, V.R.; Pinto, R. BiFeO3 thin films: Novel effects. Pramana J. Phys. 2002, 58, 1003–1008. [Google Scholar] [CrossRef]
- Kingon, A.I.; Srinivasan, S. Lead zirconate titanate thin films directly on copper electrodes for ferroelectric, dielectric and piezoelectric applications. Nat. Mater. 2005, 4, 233–237. [Google Scholar] [CrossRef]
- Hidnert, P.; Krider, H.S. Thermal Expansion of Aluminum and Some Aluminum Alloys. J. Res. Natl. Bur. Stand. 1952, 48, 209–220. [Google Scholar] [CrossRef]
- Yim, W.M.; Paff, R.J. Thermal expansion of AlN, sapphire, and silicon. J. Appl. Phys. 1974, 45, 1456–1457. [Google Scholar] [CrossRef]
- Niu, M.M.; Zhu, H.F.; Wang, Y.Y.; Yan, J.; Chen, N.; Yan, P.; Ouyang, J. Integration-friendly, chemically stoichiometric BiFeO3 films with a piezoelectric performance challenging that of PZT. ACS Appl. Mater. Interfaces 2020, 12, 33899–33907. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Pu, Y.P.; Shi, X.; Yuan, Q.B. Dielectric and ferroelectric properties of BiFeO3 ceramics sintered in different atmospheres. Ceram. Int. 2013, 39, S217–S220. [Google Scholar] [CrossRef]
- Lee, J.S.; Joo, S.K. Analysis of grain-boundary effects on the electrical properties of Pb(Zr,Ti)O3 thin films. Appl. Phys. Lett. 2002, 81, 2602–2604. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, X.M.; Hu, G.D. Leakage mechanisms of partially self-polarized BiFeO3 film. Ceram. Int. 2018, 44, 13765–13772. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, Z.; Luo, J.; Wang, K.; Li, J.F. Leakage current characteristics and Sm/Ti doping effect in BiFeO3 thin films on silicon wafers. J. Appl. Phys. 2017, 121, 64101–64107. [Google Scholar] [CrossRef]
- Luo, J.M.; Lin, S.P.; Zheng, Y.; Wang, B. Nonpolar resistive switching in Mn-doped BiFeO3 thin films by chemical solution deposition. Appl. Phys. Lett. 2012, 101, 62902–62903. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.Q.; Wang, H.; Jia, Q.X. Oxygen concentration and its effect on the leakage current in BiFeO3 thin films. Appl. Phys. Lett. 2010, 96, 12909–12913. [Google Scholar] [CrossRef]
- Hu, G.D.; Fan, S.H.; Yang, C.H.; Wu, W.B. Low leakage current and enhanced ferroelectric properties of Ti and Zn codoped BiFeO3 thin film. Appl. Phys. Lett. 2008, 92, 192905–192913. [Google Scholar] [CrossRef]




| Film | BFON | BFOO | |
|---|---|---|---|
| Global average grain size via the Scherrer formula | (100)-oriented grains | 180 nm | 120 nm |
| (111)-oriented grains | 75 nm | 50 nm | |
| (211)-oriented grains | 30 nm | 23 nm | |
| Local average grain size via SEM analysis (using 100 grains) | 105 nm | 55 nm | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J. Low-Temperature Fabrication of BiFeO3 Films on Aluminum Foils under a N2-Rich Atmosphere. Nanomaterials 2024, 14, 1343. https://doi.org/10.3390/nano14161343
Yan J. Low-Temperature Fabrication of BiFeO3 Films on Aluminum Foils under a N2-Rich Atmosphere. Nanomaterials. 2024; 14(16):1343. https://doi.org/10.3390/nano14161343
Chicago/Turabian StyleYan, Jing. 2024. "Low-Temperature Fabrication of BiFeO3 Films on Aluminum Foils under a N2-Rich Atmosphere" Nanomaterials 14, no. 16: 1343. https://doi.org/10.3390/nano14161343
APA StyleYan, J. (2024). Low-Temperature Fabrication of BiFeO3 Films on Aluminum Foils under a N2-Rich Atmosphere. Nanomaterials, 14(16), 1343. https://doi.org/10.3390/nano14161343







