Cement/Sulfur for Lithium–Sulfur Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cement/Sulfur Cathode Composite
2.2. Materials and Chemical Characterization
2.3. Electrochemical and Cell Performance Characterization
3. Results and Discussion
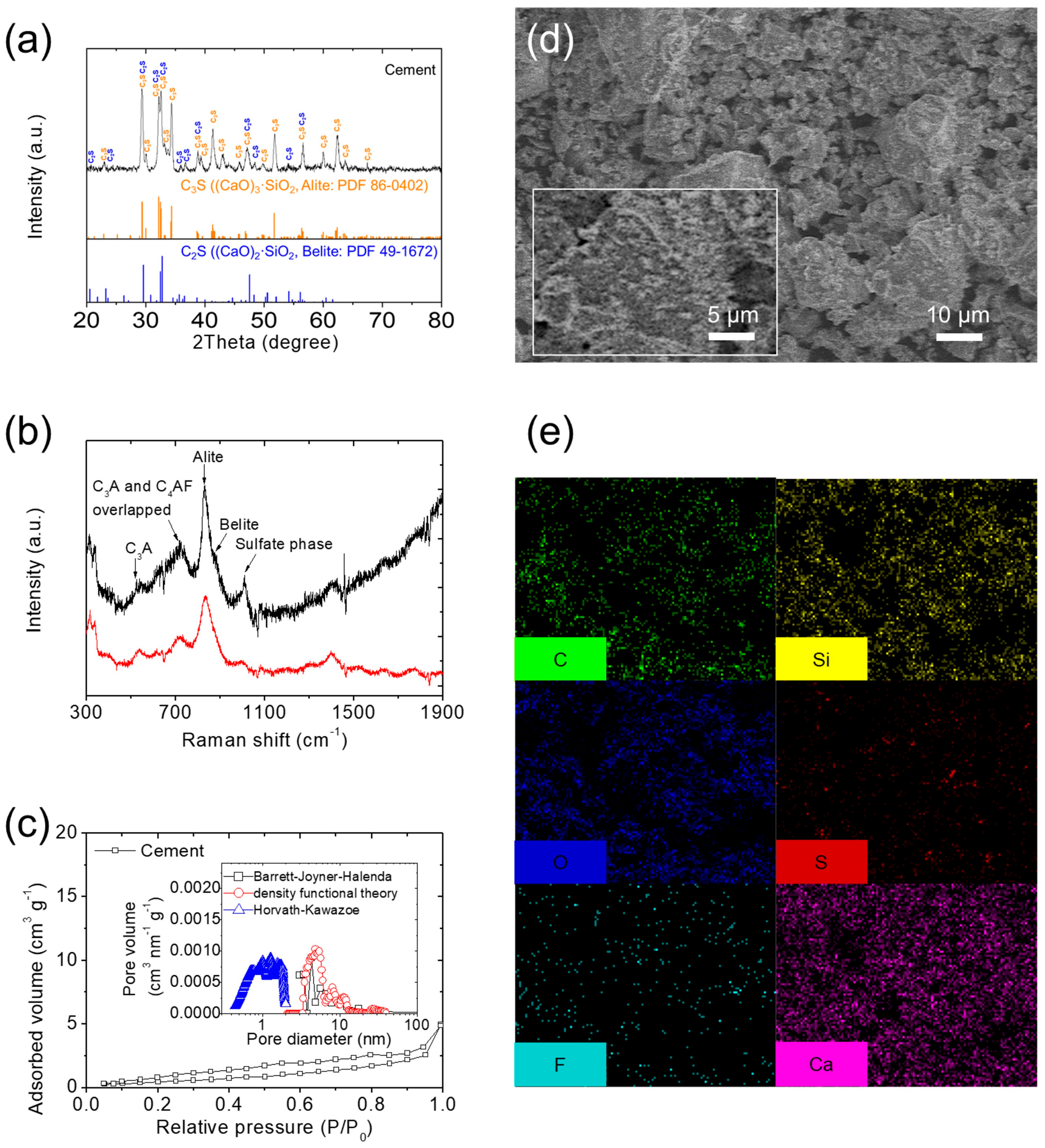
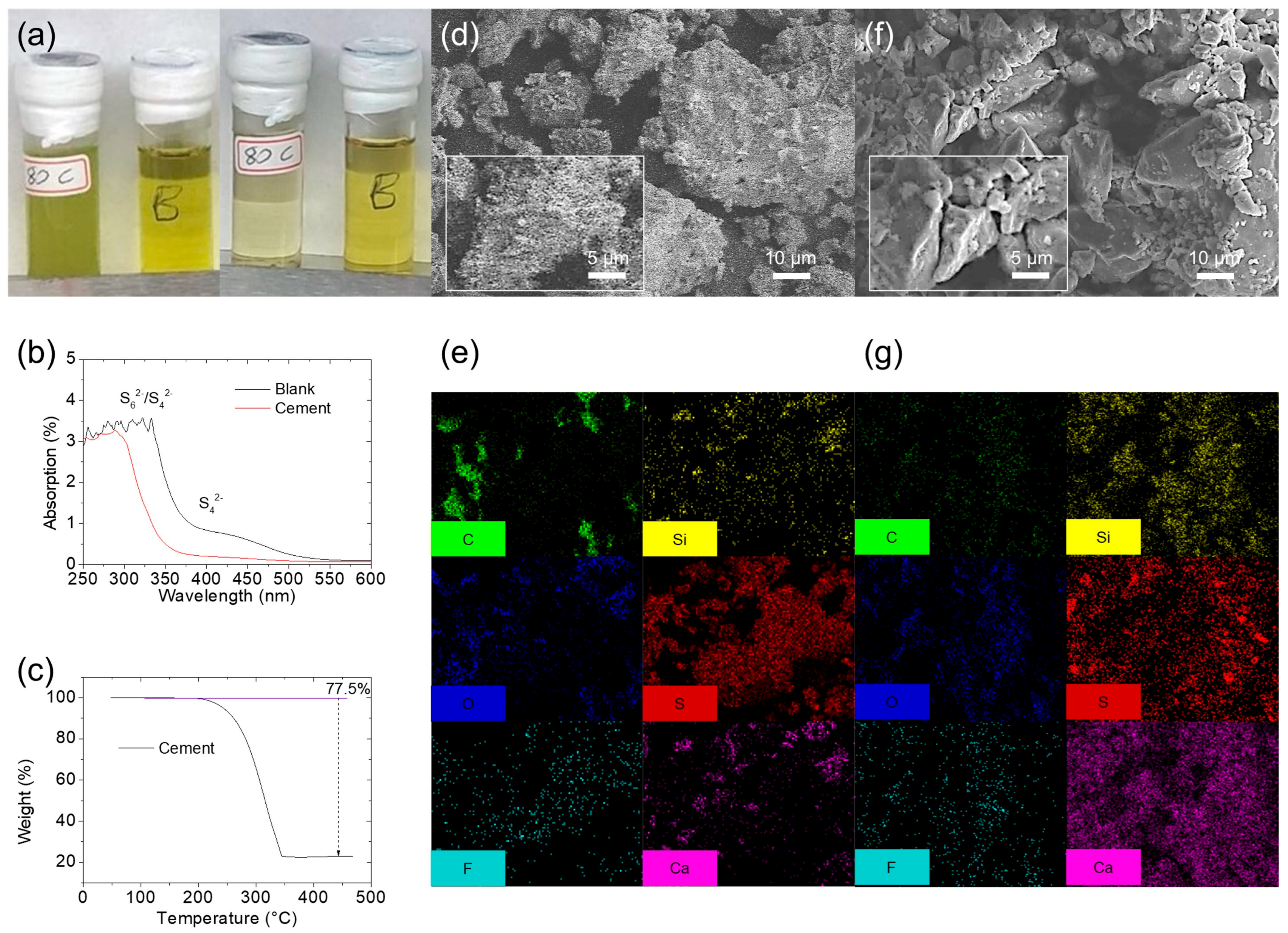
3.1. Material Characteristics
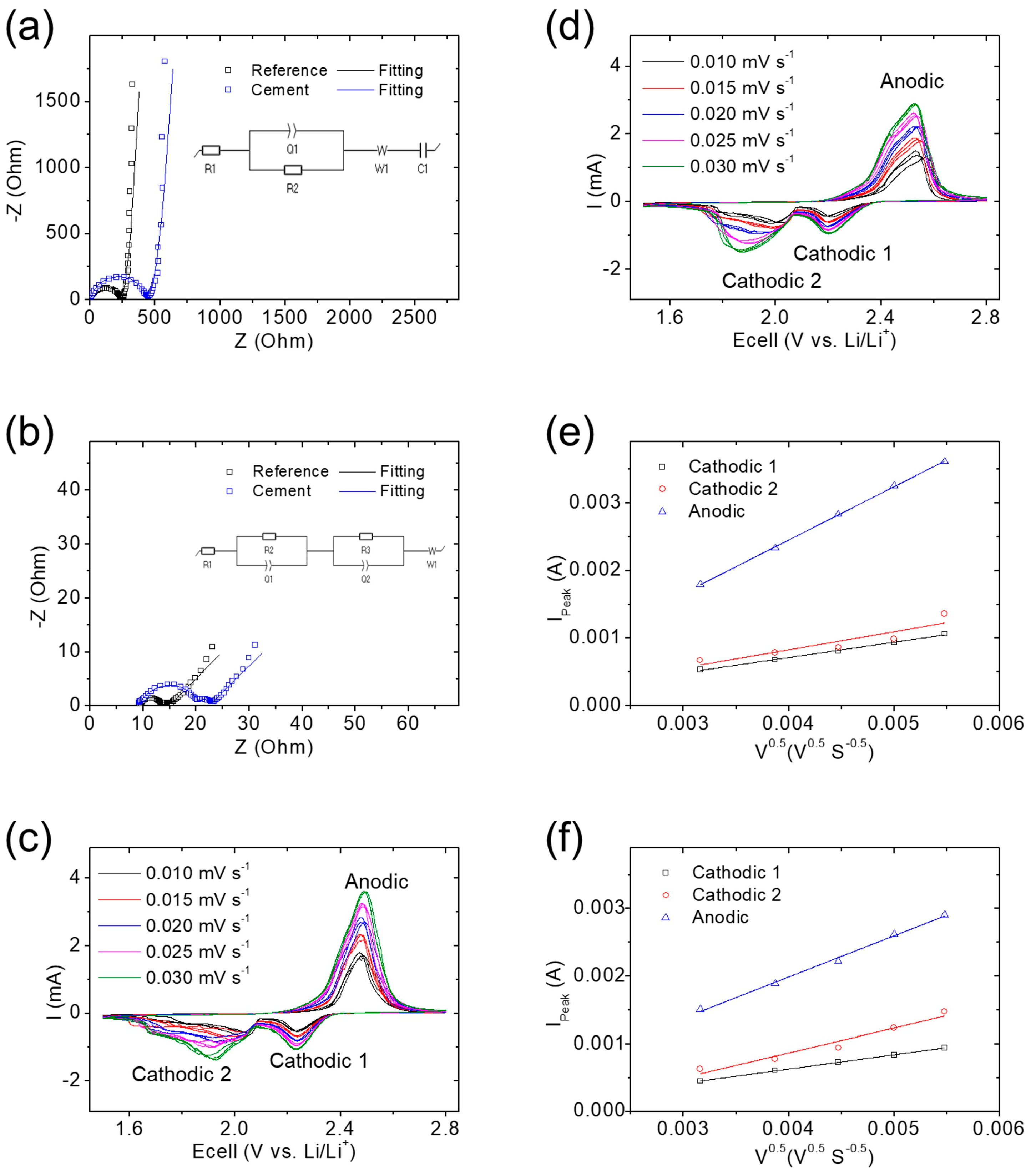
3.2. Electrochemical Analysis
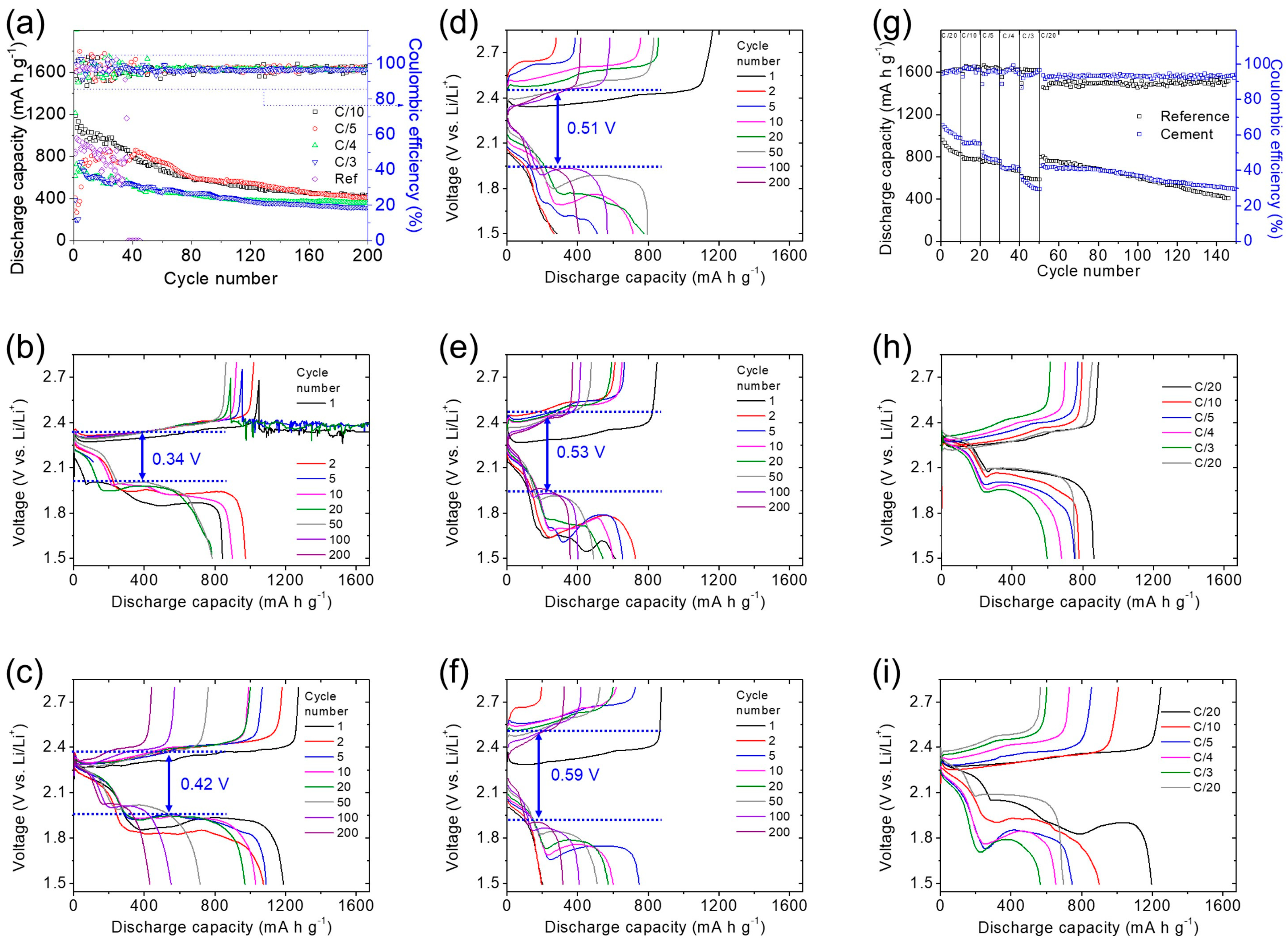
3.3. Lithium–Sulfur Cell Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manthiram, A.; Lutkenhaus, J.L.; Fu, Y.; Bai, P.; Kim, B.G.; Lee, S.W.; Okonkwo, E.; Penner, R.M. Technological Pathways toward Sustainable Batteries. One Earth 2022, 5, 203–206. [Google Scholar] [CrossRef]
- Salama, M.; Rosy; Attias, R.; Yemini, R.; Gofer, Y.; Aurbach, D.; Noked, M. Metal–Sulfur Batteries: Overview and Research Methods. ACS Energy Lett. 2019, 4, 436–446. [Google Scholar] [CrossRef]
- Sultanov, F.; Mentbayeva, A.; Kalybekkyzy, S.; Zhaisanova, A.; Myung, S.-T.; Bakenov, Z. Advances of Graphene-based Aerogels and Their Modifications in Lithium-Sulfur Batteries. Carbon 2023, 201, 679–702. [Google Scholar] [CrossRef]
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium sulfur batteries, a mechanistic review. Energy Environ. Sci. 2015, 8, 3477–3494. [Google Scholar] [CrossRef]
- Kaskel, S.; Huang, J.-Q.; Sakaebe, H. Lithium-Sulfur Batteries: Current Achievements and Further Development. Batter. Supercaps 2022, 5, e202200467. [Google Scholar] [CrossRef]
- Dong, S.; Liu, H.; Hu, Y.; Chong, S. Cathode Materials for Rechargeable Lithium-Sulfur Batteries: Current Progress and Future Prospects. ChemElectroChem 2022, 9, e202101564. [Google Scholar] [CrossRef]
- Tang, S.; Li, X.; Fan, Q.; Zhang, X.; Wang, D.-Y.; Guo, W.; Fu, Y. Review—Advances in Rechargeable Li-S Full Cells. J. Electrochem. Soc. 2022, 169, 040525. [Google Scholar] [CrossRef]
- Weret, M.A.; Su, W.-N.; Hwang, B.J. Strategies towards High Performance Lithium-Sulfur Batteries. Batter. Supercaps 2022, 5, e202200059. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide Shuttle Study in the Li/S Battery System. J. Electrochem. Soc. 2004, 151, A1969. [Google Scholar] [CrossRef]
- Nakamura, N.; Ahn, S.; Momma, T.; Osaka, T. Future Potential for Lithium-Sulfur Batteries. J. Power Sources 2023, 558, 232566. [Google Scholar] [CrossRef]
- Kim, A.; Oh, S.H.; Adhikari, A.; Sathe, B.R.; Kumar, S.; Patel, R. Recent Advances in Modified Commercial Separators for Lithium–Sulfur Batteries. J. Mater. Chem. A 2023, 11, 7833–7866. [Google Scholar] [CrossRef]
- Wang, T.; He, J.; Zhu, Z.; Cheng, X.-B.; Zhu, J.; Li, B.; Wu, Y. Heterostructures Regulating Lithium Polysulfides for Advanced Lithium-Sulfur Batteries. Adv. Mater. 2023, 35, 2303520. [Google Scholar] [CrossRef]
- Yao, W.; Xu, J.; Ma, L.; Lu, X.; Luo, D.; Qian, J.; Zhan, L.; Manke, I.; Yang, C.; Adelhelm, P.; et al. Recent Progress for Concurrent Realization of Shuttle-Inhibition and Dendrite-Free Lithium–Sulfur Batteries. Adv. Mater. 2023, 35, 2212116. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Z.; Lim, Y.V.; Wang, Y.; Li, Y.; Zhang, D.; Yang, H.Y. Recent Advances in Heterostructure Engineering for Lithium–Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2003689. [Google Scholar] [CrossRef]
- Xiang, Y.; Lu, L.; Kottapalli, A.G.P.; Pei, Y. Status and Perspectives of Hierarchical Porous Carbon Materials in Terms of High-performance Lithium–Sulfur Batteries. Carbon Energy 2022, 4, 346–398. [Google Scholar] [CrossRef]
- Kim, H.; Hwang, J.-Y.; Bang, S.; Jung, H.-G.; Sun, Y.-K. Geometrical Engineering of a SPAN–graphene Composite Cathode for Practical Li–S Batteries. J. Mater. Chem. A 2022, 10, 10844–10853. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, M.; Dong, C.; Wang, H.; Shen, C.; Wu, X.; An, A.; Chang, G.; Xu, X.; Mai, L. The Continuous Efficient Conversion and Directional Deposition of Lithium (Poly)sulfides Enabled by Bimetallic Site Regulation. Nano Energy 2022, 98, 107332. [Google Scholar] [CrossRef]
- Dong, C.; Zhou, C.; Li, Y.; Yu, Y.; Zhao, T.; Zhang, G.; Chen, X.; Yan, K.; Mai, L.; Xu, X. Ni Single Atoms on MoS2 Nanosheets Enabling Enhanced Kinetics of Li-S Batteries. Small 2023, 19, 2205855. [Google Scholar] [CrossRef]
- Tomer, V.K.; Malik, R.; Tjong, J.; Sain, M. State and Future Implementation Perspectives of Porous Carbon-based Hybridized Matrices for Lithium Sulfur Battery. Coord. Chem. Rev. 2023, 481, 215055. [Google Scholar] [CrossRef]
- Wang, C.-C.; Lee, S.-M.; Huang, Y.-H. Nitrogen-doped 3-D Porous Carbon Network Derived from Lotus Leaves as Interlayer for Lithium Sulfur Batteries. Mater. Chem. Phys. 2023, 300, 127565. [Google Scholar] [CrossRef]
- Wang, C.-C.; Lin, Y.-W.; Lee, S.-M. Manipulation of Surface Microstructures of Porous Carbon Derived from Waste Tea Leaves for Lithium Sulfur Batteries. Surf. Coat. Technol. 2020, 400, 126228. [Google Scholar] [CrossRef]
- Cho, C.-S.; Chang, J.-Y.; Li, C.-C. Highly Symmetric Gigaporous Carbonmicrosphere as Conductive Host for Sulfur to Achieve High Areal Capacity for Lithium–Sulfur Batteries. J. Power Sources 2020, 451, 227818. [Google Scholar] [CrossRef]
- Borchardt, L.; Oschatz, M.; Kaskel, S. Carbon Materials for Lithium Sulfur Batteries—Ten Critical Questions. Chem.-Eur. J. 2016, 22, 7324–7351. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-L.; Kim, J.-K.; Kang, K. Carbon Nanomaterials for Advanced Lithium Sulfur Batteries. Nano Today 2018, 19, 84–107. [Google Scholar] [CrossRef]
- Zaini, M.S.M.; Anuar, N.F.; Al-Junid, S.A.M.; Syed-Hassan, S.S.A. Agricultural Biomass-based Carbon Cathode Materials for Lithium-Sulfur Batteries: A Systematic Review. Mater. Sci. Energy Technol. 2023, 6, 205–225. [Google Scholar]
- Li, C.; Li, Y.; Fan, Y.; Wang, F.; Lei, T.; Chen, W.; Hu, A.; Xue, L.; Huang, J.; Wu, C.; et al. Mapping Techniques for the Design of Lithium-Sulfur Batteries. Small 2022, 18, 2106657. [Google Scholar] [CrossRef]
- Cheng, C.-S.; Chung, S.-H. Nickel-plated Sulfur Nanocomposites for Electrochemically Stable High-loading Sulfur Cathodes in a Lean-electrolyte Lithium-Sulfur Cell. Chem. Eng. J. 2022, 429, 132257. [Google Scholar] [CrossRef]
- Wang, M.; Bai, Z.; Yang, T.; Nie, C.; Xu, X.; Wang, Y.; Yang, J.; Dou, S.; Wang, N. Advances in High Sulfur Loading Cathodes for Practical Lithium-Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2201585. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Zhang, L. Designing Principles of Advanced Sulfur Cathodes toward Practical Lithium-Sulfur Batteries. SusMat 2022, 2, 34–64. [Google Scholar] [CrossRef]
- Wu, C.-C.; Chung, S.-H. Material, Configuration, and Fabrication Designs for Lean-electrolyte Lithium–Sulfur Cell with a High-loading Sulfur Cathode. J. Power Sources 2023, 566, 232944. [Google Scholar] [CrossRef]
- Jeoun, Y.; Kim, M.-S.; Lee, S.-H.; Um, J.H.; Sung, Y.-E.; Yu, S.-H. Lean-electrolyte Lithium-Sulfur Batteries: Recent Advances in the Design of Cell Components. Chem. Eng. J. 2022, 450, 138209. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Chung, S.-H. Integrated High-sulfur-loading Polysulfide/Carbon Cathode in Lean-electrolyte Cell toward High-energy-density Lithium–Sulfur Cells with Stable Cyclability. J. Mater. Chem. A. 2023, 11, 9455. [Google Scholar] [CrossRef]
- Raulo, A.; Jalilvand, G. Advances in Fibrous Materials for High-Capacity Lithium Sulfur Batteries. Nano Energy 2024, 122, 109265. [Google Scholar] [CrossRef]
- Chen, Z.-X.; Zhao, M.; Hou, L.-P.; Zhang, X.-Q.; Li, B.-Q.; Huang, J.-Q. Toward Practical High-Energy-Density Lithium–Sulfur Pouch Cells: A Review. Adv. Mater. 2022, 34, 2201555. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Hung, C.-C.; Chung, S.-H. High-loading Polysulfide/Cement Cathode for the Manufacture of Dynamically and Statically Stable Lean-electrolyte Lithium–Sulfur Batteries. Ceram. Int. 2023, 49, 11846–11853. [Google Scholar] [CrossRef]
- Hirsch, T.; Matschei, T.; Stephan, D. The Hydration of Tricalcium Aluminate (Ca3Al2O6) in Portland Cement-related Systems: A Review. Cem. Concr. Res. 2023, 168, 107150. [Google Scholar] [CrossRef]
- Jiang, F.-N.; Yang, S.-J.; Liu, H.; Cheng, X.-B.; Liu, L.; Xiang, R.; Zhang, Q.; Kaskel, S.; Huang, J.-Q. Mechanism Understanding for Stripping Electrochemistry of Li Metal Anode. SusMat 2021, 1, 506–536. [Google Scholar] [CrossRef]
- Mori, R. Cathode Materials for Lithium-Sulfur Battery: A Review. J. Solid State Electrochem. 2023, 27, 813. [Google Scholar] [CrossRef]
- Capkova, D.; Knap, V.; Fedorkova, A.S.; Stroe, D.-I. Analysis of 3.4 Ah Lithium-Sulfur Pouch Cells by Electrochemical Impedance Spectroscopy. J. Energy Chem. 2022, 72, 318–325. [Google Scholar] [CrossRef]
- Kilic, A.; Eroglu, D. Characterization of the Effect of Cell Design on Li−S Battery Resistance Using Electrochemical Impedance Spectroscopy. ChemElectroChem 2021, 8, 963–971. [Google Scholar] [CrossRef]
- Yu, G.-T.; Chung, S.-H. Rational Design of a High-Loading Polysulfide Cathode and a Thin-Lithium Anode for Developing Lean-Electrolyte Lithium–Sulfur Full Cells. Small 2023, 19, 2303490. [Google Scholar] [CrossRef]
- Yen, Y.-J.; Chung, S.-H. Lithium–Sulfur Cells with a Sulfide Solid Electrolyte/Polysulfide Cathode Interface. J. Mater. Chem. A 2023, 11, 4519–4526. [Google Scholar] [CrossRef]
- Jan, W.; Khan, A.D.; Iftikhar, F.J.; Ali, G. Recent Advancements and Challenges in Deploying Lithium Sulfur Batteries as Economical Energy Storage Devices. J. Energy Storage 2023, 72, 108559. [Google Scholar] [CrossRef]
- Li, Z.; Guan, B.Y.; Zhang, J.; Lou, X.W. A Compact Nanoconfined Sulfur Cathode for High-performance Lithium–Sulfur Batteries. Joule 2017, 1, 576–587. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, L.; Hao, Z.; Liu, X.; Xiang, J.; Zhang, Z.; Huang, Y.; Xie, J. Free-standing Mn3O4@CNF/S Paper Cathodes with High Sulfur Loading for Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 13406–13412. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Wu, J.; Yang, P.; Li, S.; Xu, J.; Song, K. Embedding S@TiO2 Nanospheres into MXene Layers as High Rate Cyclability Cathodes for Lithium–Sulfur Batteries. Electrochim. Acta 2019, 295, 1067–1074. [Google Scholar] [CrossRef]
- Shan, X.; Guo, Z.; Zhang, X.; Yang, J.; Duan, L. Mesoporous TiO2 Nanofiber as Highly Efficient Sulfur Host for Advanced Lithium–Sulfur Batteries. Chin. J. Mech. Eng. 2019, 32, 60. [Google Scholar] [CrossRef]
- Lee, J.; Moon, J.H. Polyhedral TiO2 Particle-based Cathode for Li–S Batteries with High Volumetric Capacity and High Performance in Lean Electrolyte. Chem. Eng. J. 2020, 399, 125670. [Google Scholar] [CrossRef]
- Qiu, S.Y.; Wang, C.; Jiang, Z.X.; Zhang, L.S.; Gu, L.L.; Wang, K.X.; Gao, J.; Zhu, X.-D.; Wu, G. Rational Design of MXene@TiO2 Nanoarray Enabling Dual Lithium Polysulfide Chemisorption towards High-performance Lithium–Sulfur Batteries. Nanoscale 2020, 12, 16678–16684. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, Z.; Liu, S.; Li, G.; Gao, X. High-Entropy Spinel Oxide Nanofibers as Catalytic Sulfur Hosts Promise the High Gravimetric and Volumetric Capacities for Lithium–Sulfur Batteries. Energy Environ. Mater. 2022, 5, 645–654. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, Z.; Liu, S.; Li, G.; Gao, X. High-entropy perovskite oxide nanofibers as efficient bidirectional electrocatalyst of liquid-solid conversion processes in lithium-sulfur batteries. Nano Energy 2023, 106, 108037. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ganguly, D.; Sundara, R.; Bhattacharya, S.S. Rare-Earth Doped Configurational Entropy Stabilized High Entropy Spinel Oxide as an Efficient Anchoring/Catalyst Functional Interlayer for High-Performance Lithium-Sulfur Battery. Batter. Supercaps 2023, 6, e202300082. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Wang, H.-M.; Jiang, Y.-C.; Li, G.-R.; Liu, S.; Gao, X.-P. High-Entropy Oxide Nanofibers as Catalytic Host Promising High Volumetric Capacity of Sulfur-Based Composites for Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2023, 6, 8377–8387. [Google Scholar] [CrossRef]
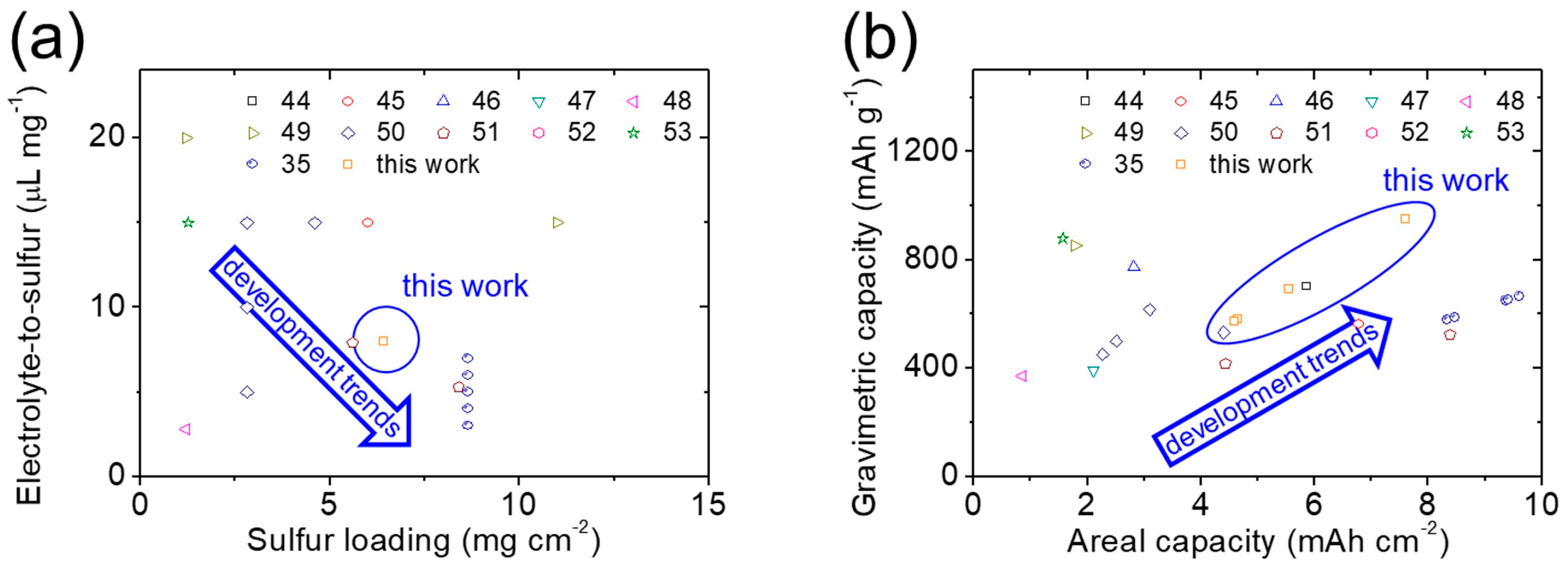
| Sulfur Loading (mg cm−2) and Sulfur Content (wt%) | Electrolyte-to-Sulfur Ratio (µL mg−1) | Peak Capacity (mA·h g−1) and Cycling Rate | Areal Capacity (mA·h cm−2) | Gravimetric Capacity (mA·h g−1) | Cycle Life | Additive | Ref. |
|---|---|---|---|---|---|---|---|
| 8.6/60 | 7 | 1280 @ 0.1C | 11.1 | 768 | 100 | cement | 35 |
| 8.6/60 | 6 | 981 @ 0.1C | 8.5 | 589 | 100 | cement | 35 |
| 8.6/60 | 5 | 1127 @ 0.1C | 9.7 | 676 | 100 | cement | 35 |
| 8.6/60 | 4 | 1097 @ 0.1C | 9.5 | 658 | 100 | cement | 35 |
| 8.6/60 | 3 | 1115 @ 0.1C | 9.6 | 669 | 100 | cement | 35 |
| 5.0/60 | -- | 1172 @ 0.1C | 5.9 | 703 | 100 | TiO | 44 |
| 6.0/50 | 15 | 1130 @ 0.1C | 6.8 | 565 | 100 | Mn3O4 | 45 |
| 2.0/55 | -- | 1409 @ 0.2C | 2.8 | 773 | 50 | TiO2 | 46 |
| 3.0/56 | -- | 703 @ 0.1C | 2.1 | 394 | 200 | TiO2 | 47 |
| 1.2/52 | 2.8 | 714 @ 1.0C | 0.9 | 371 | 100 | TiO2 | 48 |
| 1.2/57 | 20 | 1481 @ 0.5C | 1.8 | 851 | 100 | MXene@TiO2 | 49 |
| 4.6/56 | 15 | 957 @ 0.1C | 4.4 | 533 | 100 | (Mg0.2Mn0.2Ni0.2Co0.2Zn0.2)Fe2O4 | 50 |
| 5.6/53 | 7.9 | 491 @ 0.1C | 4.3 | 417 | 1 | La0.8Sr0.2(Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)O3 | 51 |
| 8.4/53 | 5.3 | 998 @ 0.1C | 8.4 | 526 | 1 | La0.8Sr0.2(Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)O3 | 51 |
| 1.7/-- | -- | 1146 @ 0.1C | 1.9 | -- | 300 | Co0.08Mn0.08Ni0.08Fe1.96Mg0.08Nd0.01Gd0.01Sm0.01Pr0.01O4 | 52 |
| 1.3/70 | 15 | 1256 @ 0.1C | 1.6 | 879 | 100 | (Ni0.2Co0.2Mn0.2Cu0.2Zn0.2)WO4 | 53 |
| 6.4/80 | 8 | 1189 @ 0.1C | 7.6 | 951 | 200 | cement | This work |
| 6.4/80 | 8 | 866 @ 0.2C | 5.5 | 692 | 200 | cement | This work |
| 6.4/80 | 8 | 727 @ 0.25C | 4.7 | 582 | 200 | cement | This work |
| 6.4/80 | 8 | 716 @ 0.33C | 4.6 | 573 | 200 | cement | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, T.-M.; Wu, C.-C.; Hung, C.-C.; Chung, S.-H. Cement/Sulfur for Lithium–Sulfur Cells. Nanomaterials 2024, 14, 384. https://doi.org/10.3390/nano14040384
Hung T-M, Wu C-C, Hung C-C, Chung S-H. Cement/Sulfur for Lithium–Sulfur Cells. Nanomaterials. 2024; 14(4):384. https://doi.org/10.3390/nano14040384
Chicago/Turabian StyleHung, Tzu-Ming, Cheng-Che Wu, Chung-Chan Hung, and Sheng-Heng Chung. 2024. "Cement/Sulfur for Lithium–Sulfur Cells" Nanomaterials 14, no. 4: 384. https://doi.org/10.3390/nano14040384





