Effects of the In Situ Growth of CNTs on Ti-Coated Diamond Surfaces on the Mechanical Properties of Diamond/Aluminum Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CNT−Ti−Diamond Multiscale Architectures
2.3. Fabrication of Diamond/Al Composite
2.4. Characterization
3. Results
3.1. Morphology of Modified Diamond Particles
3.2. Microstructure of Diamond/Al Composites
3.3. Mechanical and Thermal Properties
4. Conclusions
- (1)
- CNTs have been successfully grown on the surface of Ti-coated diamond particles using the PECVD method, with a length of approximately 1 μm.
- (2)
- The CNT-modified Ti-coated diamond/aluminum composite achieves an increase in the bending strength through the interfacial reaction during the infiltration process, which makes the diamond and aluminum closely connected through TiC and Al4C3. The bending strength of the CNT-modified Ti-coated diamond aluminum composite is increased by about 9% compared with the uncoated diamond/aluminum composite.
- (3)
- The thermal conductivity of composites is not only contributed by interface bonding, but it is also closely related to the interfacial structure. The thermal conductivity of Ti-coated diamond/aluminum composites and CNT-modified Ti-coated diamond/aluminum composites is lower than that of uncoated diamond/aluminum composites, and this is attributed to the formation of large-sized Al3Ti and the generation of thicker interface phases, respectively.
- (4)
- CNT-modified Ti-coated diamond/aluminum composites achieve a balance between improved mechanical properties and acceptable thermal conductivity. This study provides a promising strategy for the design and preparation of high-performance diamond/metal using the interface configuration design method.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, J.; Momin, S.A.; Mariatti, M. A review on advanced carbon-based thermal interface materials for electronic devices. Carbon 2020, 168, 65–112. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, Y.; Feng, W. Three-dimensional interconnected networks for thermally conductive polymer composites: Design, preparation, properties, and mechanisms. Mater. Sci.Eng. R Rep. 2020, 142, 100580. [Google Scholar] [CrossRef]
- Hao, X.; Peng, B.; Xie, G.; Chen, Y. Efficient on-chip hotspot removal combined solution of thermoelectric cooler and mini-channel heat sink. Appl. Therm. Eng. 2016, 100, 170–178. [Google Scholar] [CrossRef]
- Zhou, H.; Ran, M.; Li, Y.; Yin, Z.; Tang, Y.; Zhang, W.; Zheng, W.; Liu, J. Improvement of thermal conductivity of diamond/Al composites by optimization of liquid-solid separation process. J. Mater. Process. Technol. 2021, 297, 117267. [Google Scholar] [CrossRef]
- Tan, Z.; Xiong, D.; Fan, G.; Chen, Z.; Guo, Q.; Guo, C.; Ji, G.; Li, Z.; Zhang, D. Enhanced thermal conductivity of diamond/aluminum composites through tuning diamond particle dispersion. J. Mater. Sci. 2018, 53, 6602–6612. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Y.J.; Zhang, Y.; Wang, X.T.; Wu, H.J.; Zhao, L.D.; Zhang, H.L. Realizing ultrahigh thermal conductivity in bimodal-diamond/Al composites via interface engineering. Mater. Today Phys. 2022, 28, 11. [Google Scholar] [CrossRef]
- Feng, H.; Yu, J.K.; Tan, W. Microstructure and thermal properties of diamond/aluminum composites with TiC coating on diamond particles. Mater. Chem. Phys. 2010, 124, 851–855. [Google Scholar] [CrossRef]
- Yang, W.; Chen, G.; Wang, P.; Qiao, J.; Hu, F.; Liu, S.; Zhang, Q.; Hussain, M.; Dong, R.; Wu, G. Enhanced thermal conductivity in Diamond/Aluminum composites with tungsten coatings on diamond particles prepared by magnetron sputtering method. J. Alloys Compd. 2017, 726, 623–631. [Google Scholar] [CrossRef]
- Molina-Jorda, J.M. Thermal conductivity of metal matrix composites with coated inclusions: A new modelling approach for interface engineering design in thermal management. J. Alloys Compd. 2018, 745, 849–855. [Google Scholar] [CrossRef]
- Monje, I.E.; Louis, E.; Molina, J.M. Optimizing thermal conductivity in gas-pressure infiltrated aluminum/diamond composites by precise processing control. Compos. Part A Appl. Sci. Manuf. 2013, 48, 9–14. [Google Scholar] [CrossRef]
- Che, Z.; Li, J.; Wang, L.; Qi, Y.; Zhang, Y.; Zhang, H.; Wang, X.; Wang, J.; Kim, M.J. Effect of diamond surface chemistry and structure on the interfacial microstructure and properties of Al/diamond composites. RSC Adv. 2016, 6, 67252–67259. [Google Scholar] [CrossRef]
- Edtmaier, C.; Segl, J.; Rosenberg, E.; Liedl, G.; Pospichal, R.; Steiger-Thirsfeld, A. Microstructural characterization and quantitative analysis of the interfacial carbides in Al(Si)/diamond composites. J. Mater. Sci. 2018, 53, 15514–15529. [Google Scholar] [CrossRef]
- Che, Z.; Zhang, Y.; Li, J.; Zhang, H.; Wang, X.; Sun, C.; Wang, J.; Kim, M.J. Nucleation and growth mechanisms of interfacial Al4C3 in Al/diamond composites. J. Alloys Compd. 2016, 657, 81–89. [Google Scholar] [CrossRef]
- Ruch, P.W.; Beffort, O.; Kleiner, S.; Weber, L.; Uggowitzer, P.J. Selective interfacial bonding in Al(Si)–diamond composites and its effect on thermal conductivity. Compos. Sci. Technol. 2006, 66, 2677–2685. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Zhao, L.; Wang, X. Optimisation of high thermal conductivity Al/diamond composites produced by gas pressure infiltration by controlling infiltration temperature and pressure. J. Mater. Sci. 2014, 50, 688–696. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, X.; Zhang, Y.; Wang, J.; Kim, M.J.; Zhang, H. Aluminum carbide hydrolysis induced degradation of thermal conductivity and tensile strength in diamond/aluminum composite. J. Compos. Mater. 2018, 52, 2709–2717. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, Q.; Xia, Y.; Sun, K.; Lin, X.; Gou, H.; Shil’ko, S.; Wu, G. Effect of Nanoscale W Coating on Corrosion Behavior of Diamond/Aluminum Composites. Nanomaterials 2023, 13, 307. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Wang, L.; Li, J.; Li, H.; Zhang, H. Interfacial characteristic and thermal conductivity of Al/diamond composites produced by gas pressure infiltration in a nitrogen atmosphere. Mater. Des. 2016, 92, 643–648. [Google Scholar] [CrossRef]
- Monachon, C.; Weber, L. Effect of diamond surface orientation on the thermal boundary conductance between diamond and aluminum. Diam. Relat. Mater. 2013, 39, 8–13. [Google Scholar] [CrossRef]
- Monachon, C.; Weber, L. Influence of diamond surface termination on thermal boundary conductance between Al and diamond. J. Appl. Phys. 2013, 113, 183504. [Google Scholar] [CrossRef]
- Guo, C.; He, X.; Ren, S.; Qu, X. Effect of (0–40) wt.% Si addition to Al on the thermal conductivity and thermal expansion of diamond/Al composites by pressure infiltration. J. Alloys Compd. 2016, 664, 777–783. [Google Scholar] [CrossRef]
- Sang, J.; Chen, Q.; Yang, W.; Zhu, J.; Fu, L.; Li, D.; Zhou, L. Architecting micron SiC particles on diamond surface to improve thermal conductivity and stability of Al/diamond composites. Surf. Interfaces 2022, 31, 102019. [Google Scholar] [CrossRef]
- Ji, G.; Tan, Z.; Lu, Y.; Schryvers, D.; Li, Z.; Zhang, D. Heterogeneous interfacial chemical nature and bonds in a W-coated diamond/Al composite. Mater. Charact. 2016, 112, 129–133. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, N.; Shi, C.; Liu, E.; He, C.; He, F.; Ma, L. Mo2C coating on diamond: Different effects on thermal conductivity of diamond/Al and diamond/Cu composites. Appl. Surf. Sci. 2017, 402, 372–383. [Google Scholar] [CrossRef]
- Chen, G.; Yang, W.; Xin, L.; Wang, P.; Liu, S.; Qiao, J.; Hu, F.; Zhang, Q.; Wu, G. Mechanical properties of Al matrix composite reinforced with diamond particles with W coatings prepared by the magnetron sputtering method. J. Alloys Compd. 2018, 735, 777–786. [Google Scholar] [CrossRef]
- Xue, C.; Yu, J.K. Enhanced thermal conductivity in diamond/aluminum composites: Comparison between the methods of adding Ti into Al matrix and coating Ti onto diamond surface. Surf. Coat. Technol. 2013, 217, 46–50. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Liu, X.Y.; Wang, D.; Wang, W.; Xiao, B.; Ma, Z.Y. Effect of Nano-SiC coating on the thermal properties and microstructure of diamond/Al composites. Compos. Commun. 2023, 40, 101564. [Google Scholar] [CrossRef]
- Li, N.; Wang, L.; Dai, J.; Wang, X.; Wang, J.; Kim, M.J.; Zhang, H. Interfacial products and thermal conductivity of diamond/Al composites reinforced with ZrC-coated diamond particles. Diam. Relat. Mater. 2019, 100, 107565. [Google Scholar] [CrossRef]
- Xu, B.; Hung, S.W.; Hu, S.; Shao, C.; Guo, R.; Choi, J.; Kodama, T.; Chen, F.-R.; Shiomi, J. Scalable monolayer-functionalized nanointerface for thermal conductivity enhancement in copper/diamond composite. Carbon 2021, 175, 299–306. [Google Scholar] [CrossRef]
- Cao, H.; Tan, Z.; Lu, M.H.; Ji, G.; Yan, X.J.; Di, C.; Yuan, M.; Guo, Q.; Su, Y.; Addad, A.; et al. Graphene interlayer for enhanced interface thermal conductance in metal matrix composites: An approach beyond surface metallization and matrix alloying. Carbon 2019, 150, 60–68. [Google Scholar] [CrossRef]
- Wu, J.H.; Zhang, H.L.; Zhang, Y.; Li, J.W.; Wang, X.T. The role of Ti coating in enhancing tensile strength of Al/diamond composites. Mater. Sci. Eng. A 2013, 565, 33–37. [Google Scholar] [CrossRef]
- Chen, C.Y.; Xie, Y.C.; Yan, X.C.; Ahmed, M.; Lupoi, R.; Wang, J.; Ren, Z.M.; Liao, H.L.; Yin, S. Tribological properties of Al/diamond composites produced by cold spray additive manufacturing. Addit. Manuf. 2020, 36, 101434. [Google Scholar] [CrossRef]
- Bellucci, A.; Campanari, V.; Mastellone, M.; O’Keeffe, P.; Paladini, A.; Polini, R.; Trucchi, D.M. Optical characteristics of nanostructured aluminium/diamond composite systems in the visible range. Diam. Relat. Mater. 2023, 132, 109669. [Google Scholar] [CrossRef]
- Yang, C.Y.; Li, X.; Li, C.; Peng, Y.; Xing, Y.; Feng, Z.; Tan, J.; Tao, J.; Li, Z.; Wang, Y.; et al. Interface and strengthening mechanisms of Al matrix composites reinforced with in-situ CNTs grown on Ti particles. Mater. Des. 2023, 229, 111923. [Google Scholar] [CrossRef]
- Mizuuchi, K.; Inoue, K.; Agari, Y.; Morisada, Y.; Sugioka, M.; Tanaka, M.; Takeuchi, T.; Tani, J.-I.; Kawahara, M.; Makino, Y. Processing of diamond particle dispersed aluminum matrix composites in continuous solid–liquid co-existent state by SPS and their thermal properties. Compos. Part B Eng. 2011, 42, 825–831. [Google Scholar] [CrossRef]
- Kleiner, S.; Khalid, F.A.; Ruch, P.W.; Meier, S.; Beffort, O. Effect of diamond crystallographic orientation on dissolution and carbide formation in contact with liquid aluminium. Scr. Mater. 2006, 55, 291–294. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, Q.; Gou, H.S.; Wang, P.P.; Shao, P.Z.; Kobayashi, E.; Wu, G.H. First-principles calculation of diamond/Al interface properties and study of interface reaction. Acta Phys. Sin. 2021, 70, 178101. [Google Scholar] [CrossRef]
- Tan, Z.; Li, Z.; Xiong, D.B.; Fan, G.; Ji, G.; Zhang, D. A predictive model for interfacial thermal conductance in surface metallized diamond aluminum matrix composites. Mater. Des. 2014, 55, 257–262. [Google Scholar] [CrossRef]
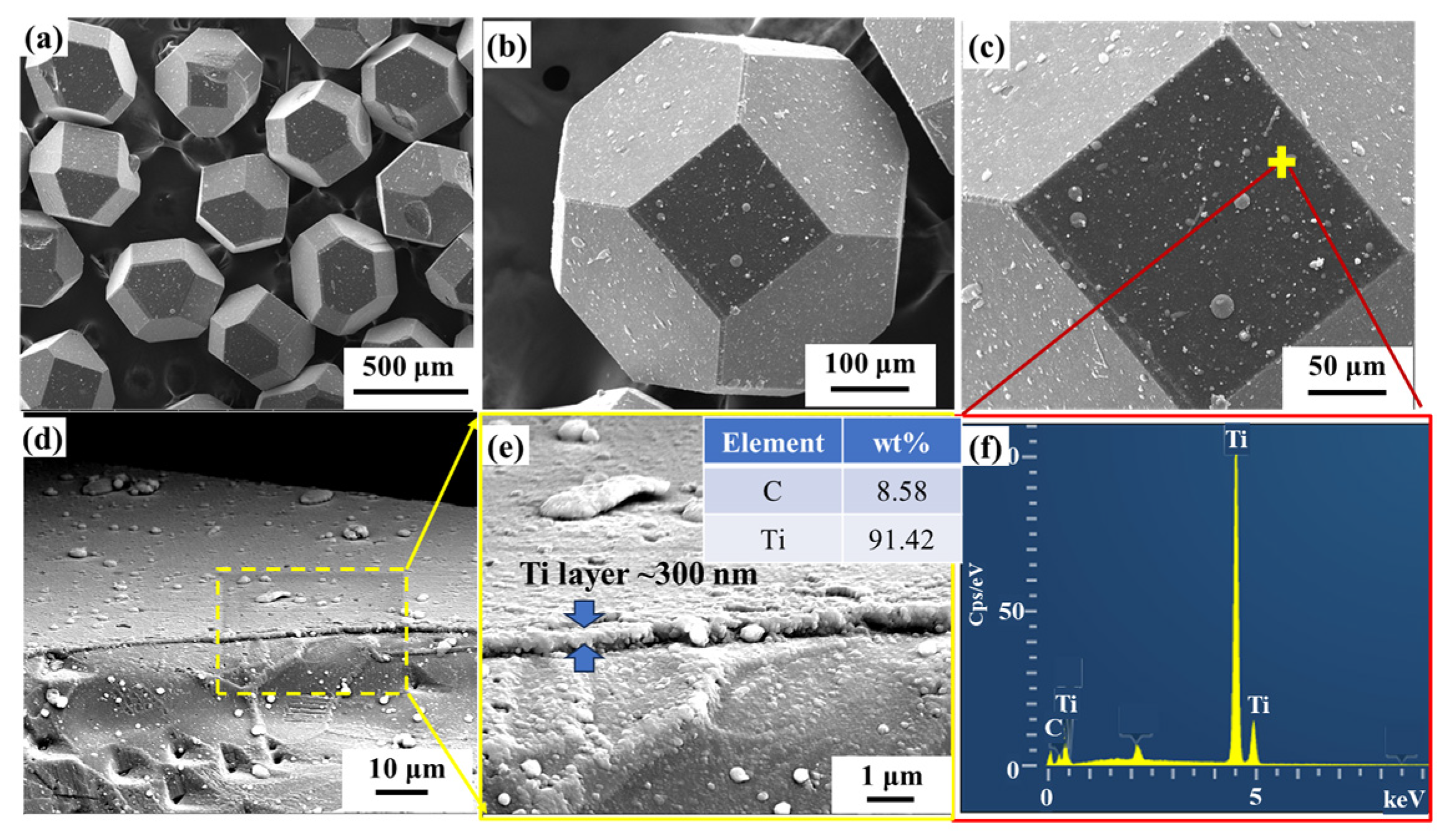

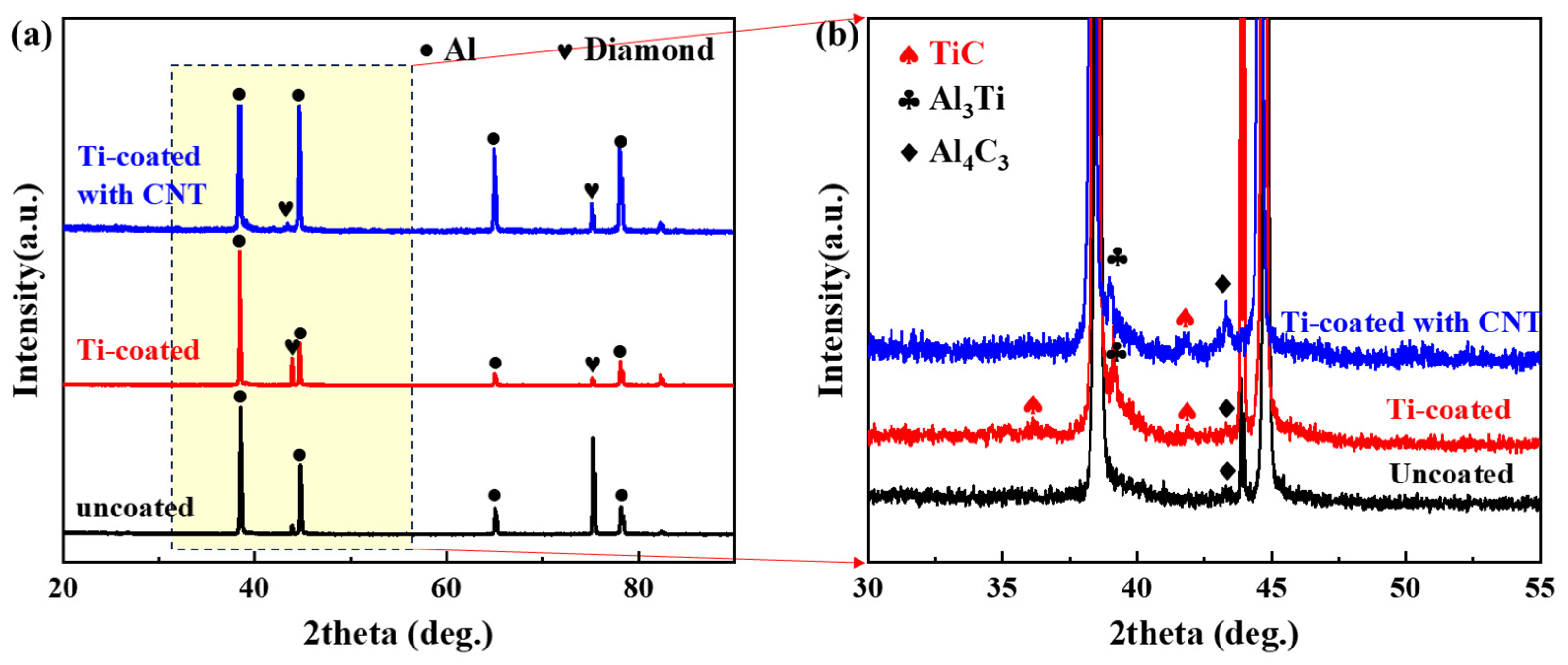

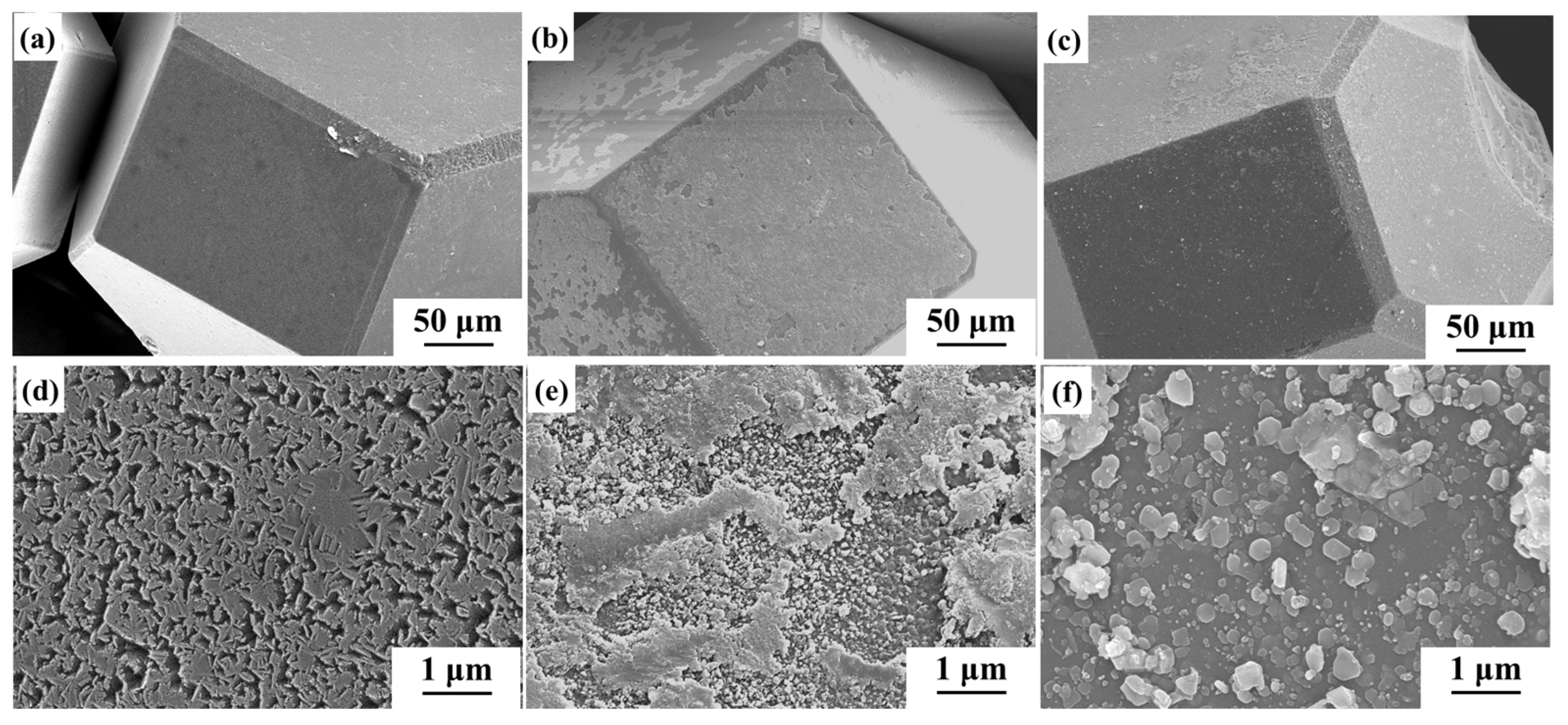
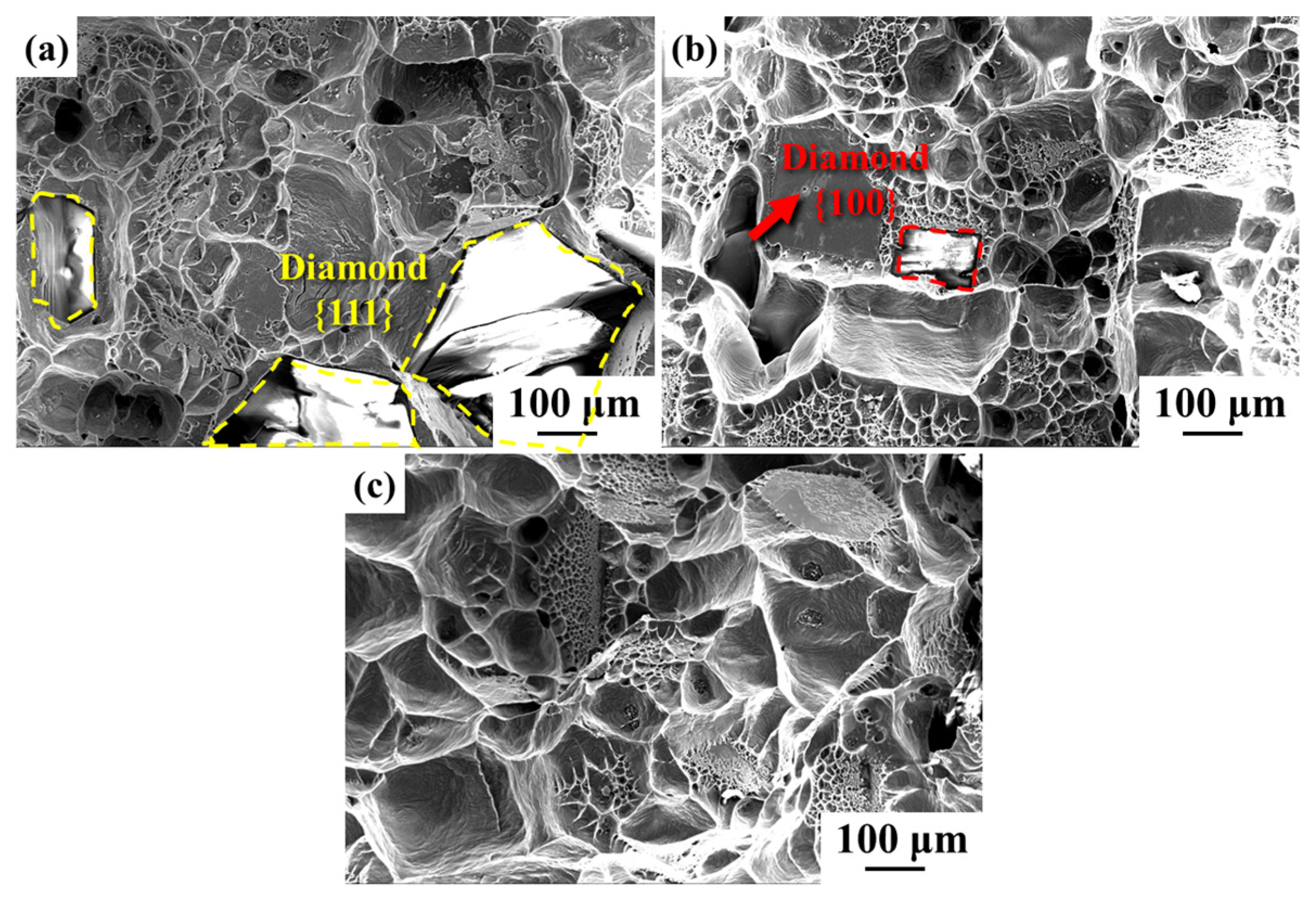
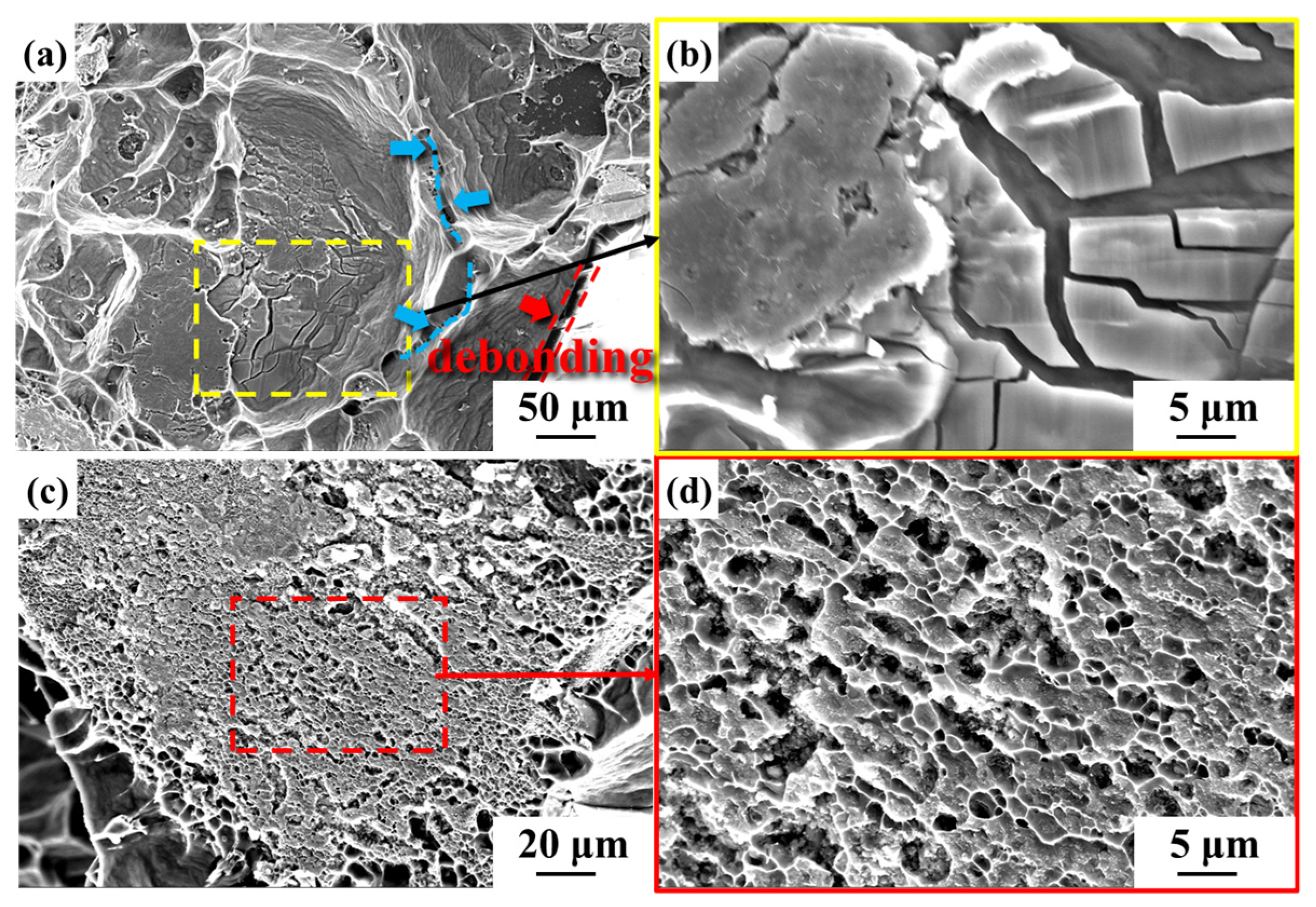
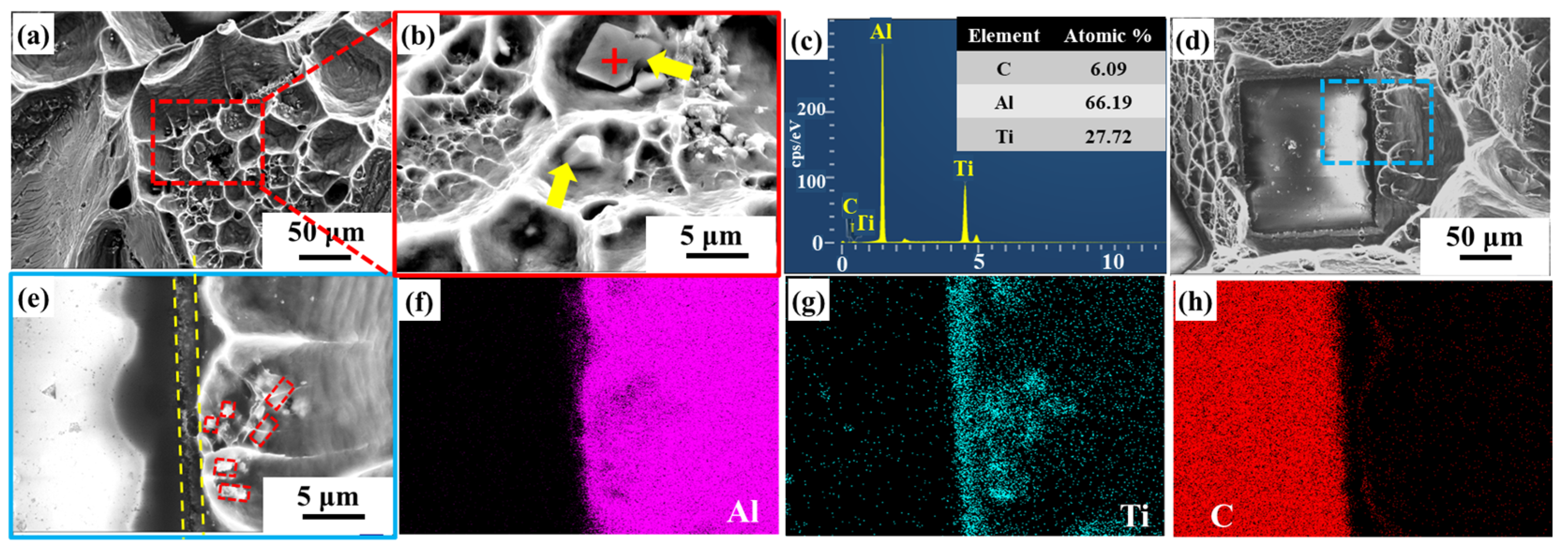
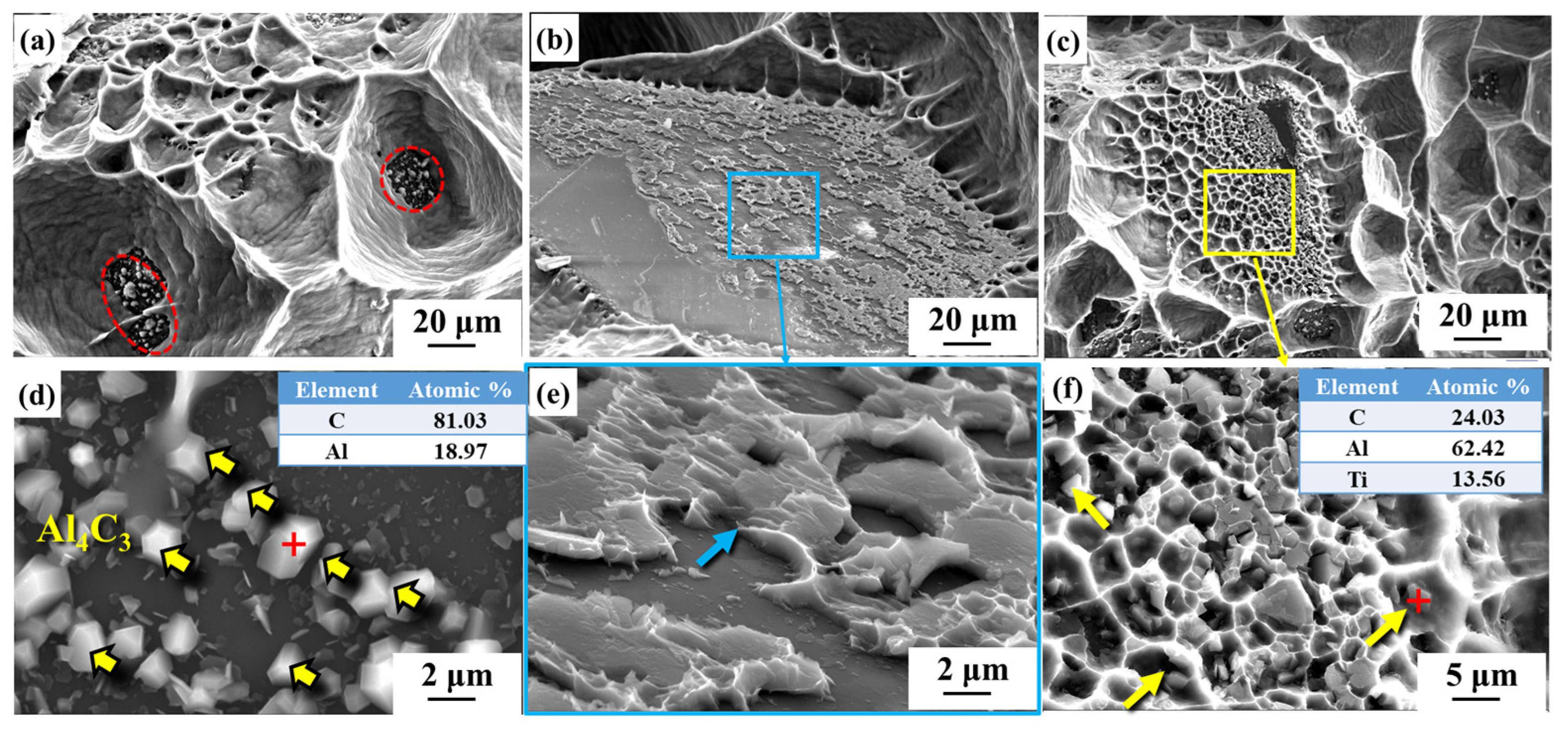

| Material | Thermal Conductivity (W·m−1·K−1) | Bending Strength (MPa) |
|---|---|---|
| Uncoated diamond/Al | 726 | 252 ± 9 |
| Ti-coated diamond/Al | 650 | 217 ± 14 |
| CNT-modified Ti-coated diamond/Al | 577 | 275 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Zhu, P.; Xia, Y.; Ma, Y.; Ding, J.; Gou, H.; Zhang, Q.; Yang, S.; Wu, G. Effects of the In Situ Growth of CNTs on Ti-Coated Diamond Surfaces on the Mechanical Properties of Diamond/Aluminum Composites. Nanomaterials 2024, 14, 640. https://doi.org/10.3390/nano14070640
Wu H, Zhu P, Xia Y, Ma Y, Ding J, Gou H, Zhang Q, Yang S, Wu G. Effects of the In Situ Growth of CNTs on Ti-Coated Diamond Surfaces on the Mechanical Properties of Diamond/Aluminum Composites. Nanomaterials. 2024; 14(7):640. https://doi.org/10.3390/nano14070640
Chicago/Turabian StyleWu, Hao, Ping Zhu, Yixiao Xia, Yifu Ma, Junyao Ding, Huasong Gou, Qiang Zhang, Sen Yang, and Gaohui Wu. 2024. "Effects of the In Situ Growth of CNTs on Ti-Coated Diamond Surfaces on the Mechanical Properties of Diamond/Aluminum Composites" Nanomaterials 14, no. 7: 640. https://doi.org/10.3390/nano14070640
APA StyleWu, H., Zhu, P., Xia, Y., Ma, Y., Ding, J., Gou, H., Zhang, Q., Yang, S., & Wu, G. (2024). Effects of the In Situ Growth of CNTs on Ti-Coated Diamond Surfaces on the Mechanical Properties of Diamond/Aluminum Composites. Nanomaterials, 14(7), 640. https://doi.org/10.3390/nano14070640





