Nitrogen-Rich Triazine-Based Covalent Organic Frameworks as Efficient Visible Light Photocatalysts for Hydrogen Peroxide Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TBP-COF and TTP-COF
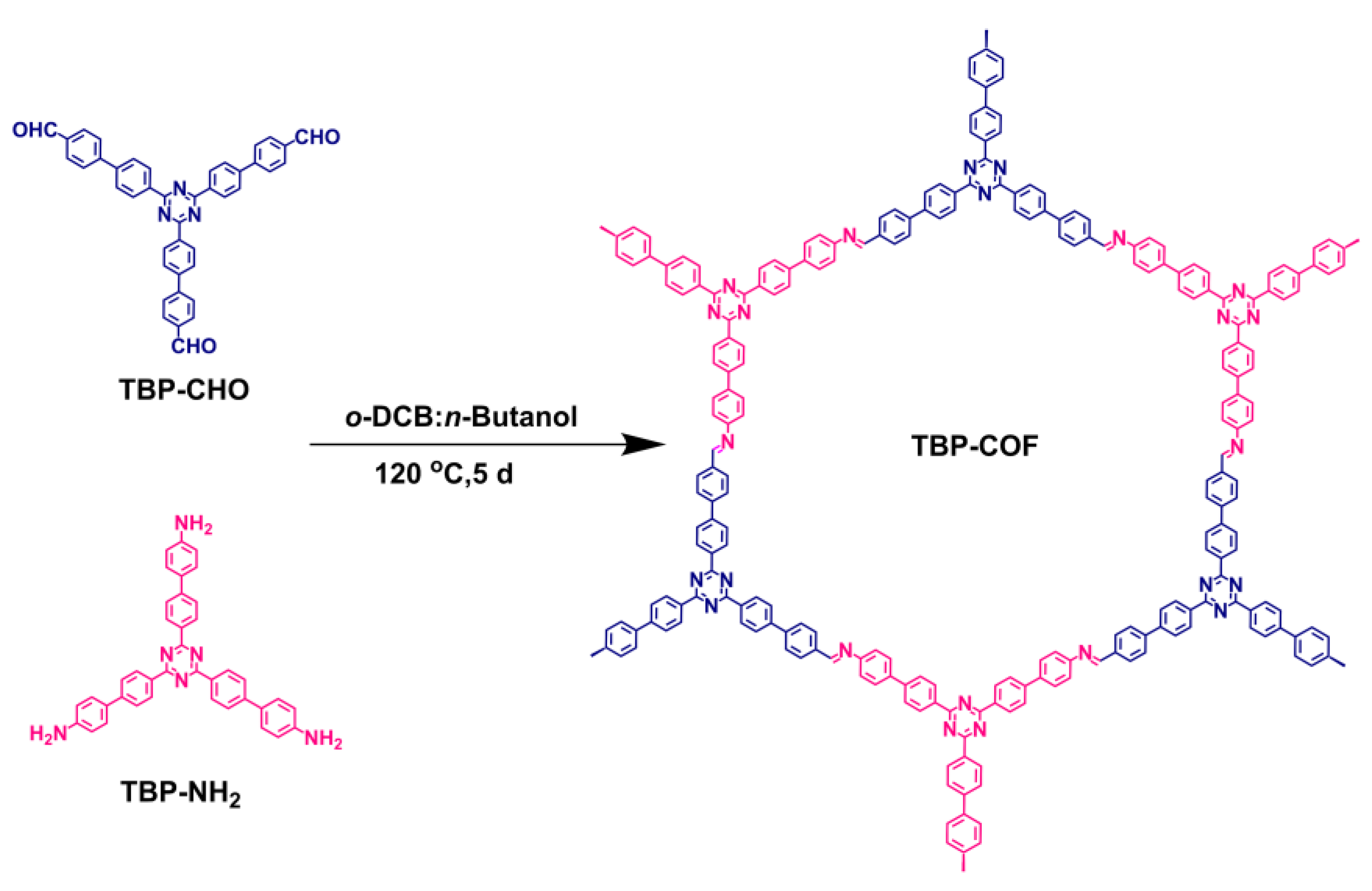
2.2.1. Synthesis of TBP-COF
- 2,4,6-tris(4-bromophenyl)-1,3,5-triazine (TBP-Br): As shown in Figure S1a, in a round bottom flask, 3.54 mL (6 g, 40 mmol) of trifluoromethanesulfonic acid was slowly added to 2 g (11 mmol) of 4-Bromobenzonitrile at 0 °C and stirred for 30 min. The mixture was then stirred at room temperature overnight. After completion of the reaction, the resultant mixture was washed with 100 mL of deionized water, yielding a large amount of white solid precipitate, which was then filtered and collected. The product was purified to yield 1.95 g of a white solid (yield, 99%) [28].
- 4′,4‴,4′′′′′-(1,3,5-triazine-2,4,6-triyl) tris (([1,1′-biphenyl]-4-amine)) (TBP-NH2): As shown in Figure S1b, TBP-Br (0.546 g, 1.0 mmol), 4-aminophenylboronic acid pinacol ester (0.788 g, 3.6 mmol), Pd(PPh3)4 (119.0 mg, 0.1 mmol), and K2CO3 (0.513 g, 3.72 mmol) were added to a two-neck flask and subjected to a vacuum for 15 min. The mixture was then heated at 110 °C for 14 h with the addition of dioxane (37 mL) and H2O (5 mL). The solution was poured into a stirring beaker containing ice cubes and H2O. The solid was separated through suction filtration, placed in a beaker, and treated with a small amount of methanol (MeOH). Following suction filtration, the undissolved solid was oven-dried for 12 h, yielding 0.472 g of green solid (yield, 81%).
- 4′,4‴,4′′′′′–(1,3,5-triazine–2,4,6-triyl) tris (([1,1′-biphenyl]-4-carbaldehyde)) (TBP-CHO): As shown in Figure S1c, TBP-Br (0.546 g, 1.0 mmol), 4-formylphenylboronic acid (0.788 g, 3.6 mmol), Pd(PPh3)4 (119.0 mg, 0.1 mmol), and K2CO3 (0.513 g, 3.72 mmol) were added in a two-neck flask and placed under vacuum for 15 min. Following the addition of dioxane (37 mL) and H2O (5 mL), the mixture was heated for 14 h at 110 °C. The mixture was transferred into a stirring beaker containing ice cubes and H2O. The solid was separated by suction filtration, placed in a beaker, and treated with a small amount of MeOH; the mixture was heated until it boiled, and then sonicated for 15 min. After suction filtration, the undissolved solid was treated with acetone and dried in an oven for 12 h, yielding 0.485 g of gray solid (yield, 78%) [29].
- TBP-COF: In Scheme 1, TBP-CHO (31 mg, 0.05 mmol) and TBP-NH2 (29 mg, 0.05 mmol) were mixed in 35 mL Pyrex tubes. The solvents o-dichlorobenzene (o-DCB) and n-butanol were added in a 1:1 volume ratio (4 mL). Additionally, acetic acid (6 M, 0.50 mL) was included. The mixture was subjected to ultrasonication for 15 min to achieve uniform dispersion, sealed in a pressure-resistant glass tube, and reacted in an oil bath at 120 °C for 5 d. After the reaction was completed, the reaction tube was cooled to room temperature, and then the crude product was collected and washed three times with N,N-Dimethylformamide (DMF), acetone, and tetrahydrofuran (THF). After that, it was dried for 24 h at 100 °C to obtain the green TBP-COF compound (41 mg), with a 68% yield.
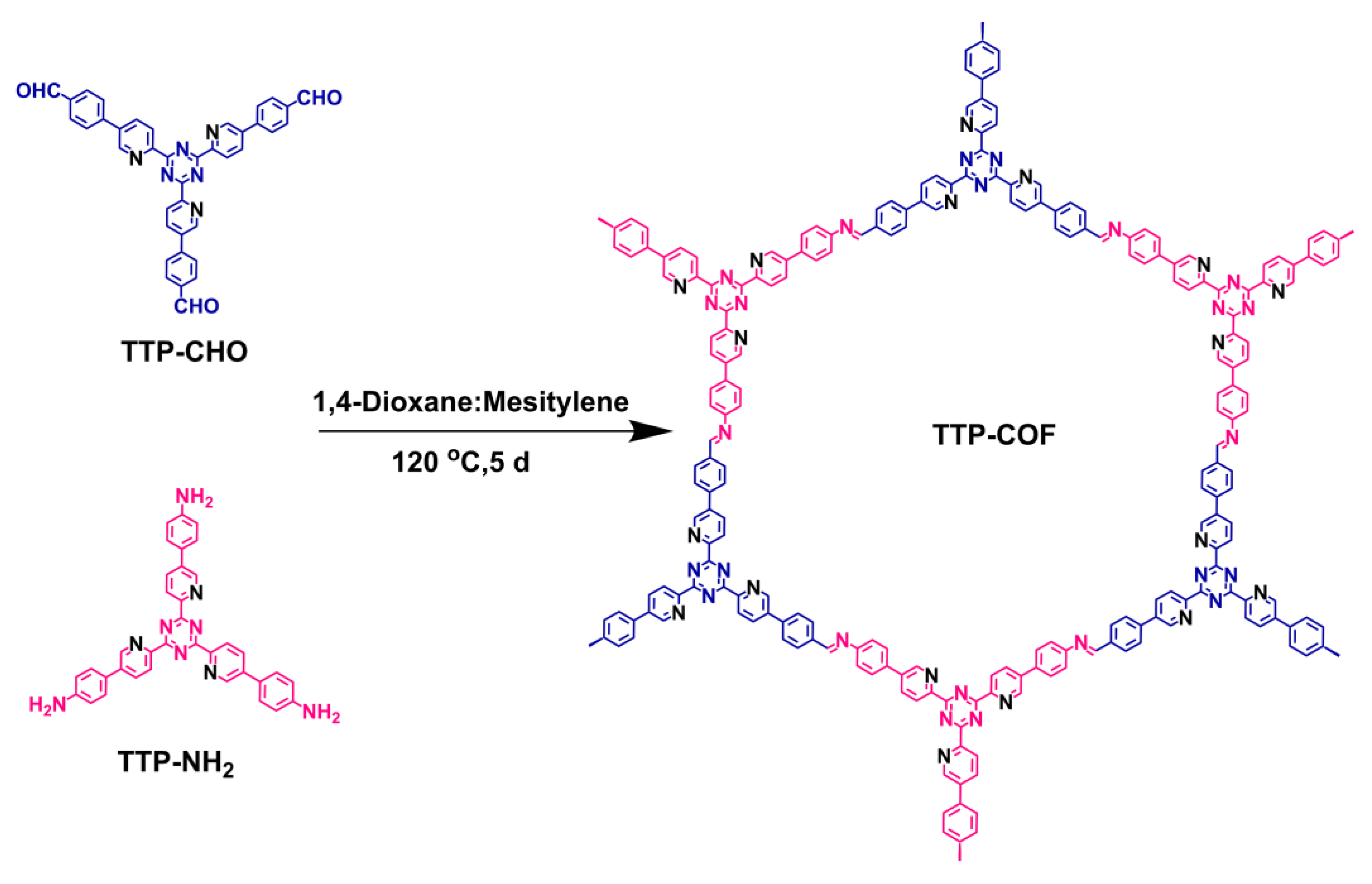
2.2.2. Synthesis of TTP-COF
- 2,4,6-tris(5-bromopyridin-2-yl)-1,3,5-triazine (TTP-Br): As shown in Figure S2a, NH4Br (65 mg) and diisopropylethylamine (113 μL, 0.66 mmol) were added into a round-bottom flask containing a suspension of 5-Bromopicolinonitrile (1.0 g) in 1-pentanol (5 mL). The suspension was heated while being stirred for 12 h in an oil bath at 135 °C. After the reaction, the mixture was cooled to room temperature, resulting in a significant amount of solid precipitation in the flask. The yellow–white solid (0.8 g) was obtained (yield, 82%) after the solid was collected and filtered, then thoroughly washed with acetonitrile [30].
- 4,4′,4″-((1,3,5-triazine-2,4,6-triyl) tris (pyridine-6,3-diyl)) trianiline (TTP-NH2): As shown in Figure S2b, TTP-Br (0.549 g, 1.0 mmol), 4-aminophenylboronic acid pinacol ester (0.788 g, 3.6 mmol), Pd(PPh3)4 (119.0 mg, 0.1 mmol), CsF (0.547 g, 3.6 mmol), and Cs2CO3 (1.212 g, 3.72 mmol) were added to a two-neck flask and kept under vacuum for 15 min. The mixture was then heated at 110 °C for 14 h after adding dioxane (37 mL) and H2O (5 mL). The solution was poured into a stirring beaker containing ice cubes and H2O. The solid was separated by suction filtration, transferred to a beaker, and treated with a small amount of MeOH. After suction filtration, the undissolved solid was oven-dried for 12 h, yielding 0.422 g of orange solid (yield, 72%).
- 1H NMR (Figure S10) (600 MHz, DMSO-d6) δ = 9.15 (s, 3H), 8.76 (d, J = 8.3 Hz, 3H), 8.26 (d, J = 5.9 Hz, 3H), 7.63 (d, J = 8.5 Hz, 6H), 6.74 (d, J = 8.5 Hz, 6H), 5.56 (s, 6H) (Figure S10).
- 13C NMR (Figure S11) (151 MHz, DMSO-d6) δ = 171.78, 150.47, 150.27, 147.34, 139.05, 133.17, 128.36, 125.42, 123.16, 114.80, 39.58 (Figure S11).
- HRMS m/z [M+] calculated for C36H28N9: 586.24622; found: 586.24648.
- 4,4′,4″–((1,3,5–triazine–2,4,6-triyl) tris (pyridine–6,3–diyl)) tribenzaldehyde (TTP-CHO): As shown in the Figure S2c, TTP- Br (0.549 g, 1.0 mmol), 4-formylphenylboronic acid (0.788 g, 3.6 mmol), Pd(PPh3)4 (119.0 mg, 0.1 mmol), CsF (0.547 g, 3.6 mmol), and Cs2CO3 (1.212 g, 3.72 mmol) were added in a two-neck flask and subjected to a vacuum for 15 min. Afterwards, 37 mL of dioxane and 5 mL of H2O were added, and the mixture was heated to 110 °C for 14 h. The solution was poured into a stirring beaker containing ice cubes and H2O. The solid was separated by suction filtration, placed in a beaker, and treated with a small amount of MeOH; the mixture was heated till boiling, and then sonicated for 15 min. Following suction filtration, the undissolved solid was dried in an oven for 12 h, yielding 0.487 g of yellow solid (yield, 78%).
- 1H NMR (Figure S12) (600 MHz, TFA) δ = 10.11 (s, 3H), 9.74 (d, J = 2.2 Hz, 3H), 9.66 (d, J = 8.4 Hz, 3H), 9.28 (dd, J = 8.4, 2.2 Hz, 3H), 8.32 (d, J = 8.4 Hz, 6H), 8.12 (d, J = 8.4 Hz, 6H) (Figure S12).
- 13C NMR (Figure S13) (151 MHz, TFA) δ = 192.24, 162.12, 141.98, 140.87, 138.77, 137.31, 134.33, 132.96, 127.66, 124.55, 124.12 (Figure S13).
- HRMS m/z [M+] calculated for C39H25O3N6: 625.19827; found: 625.19880.
- TTP-COF: As shown in Scheme 2, TTP-CHO (31 mg, 0.05 mmol) and TTP-NH2 (29 mg, 0.05 mmol) were introduced into 35 mL Pyrex tubes. Subsequently, a mixture of mesitylene:1, 4-dioxane solvents (4 mL, v:v = 1:1), and acetic acid (6 M, 0.5 mL) was added. After 15 min of ultrasonication to ensure uniform dispersion, the mixture was sealed in a pressure-resistant glass tube and reacted for 5 d at 120 °C in an oil bath. After the reaction was completed, the reaction tube was cooled to room temperature, and then the crude product was collected and washed three times with DMF, acetone, and THF. It was then dried at 100 °C for 24 h to give the croci TTP-COF compound (49 mg), with a yield of 82%.
2.3. Characterization
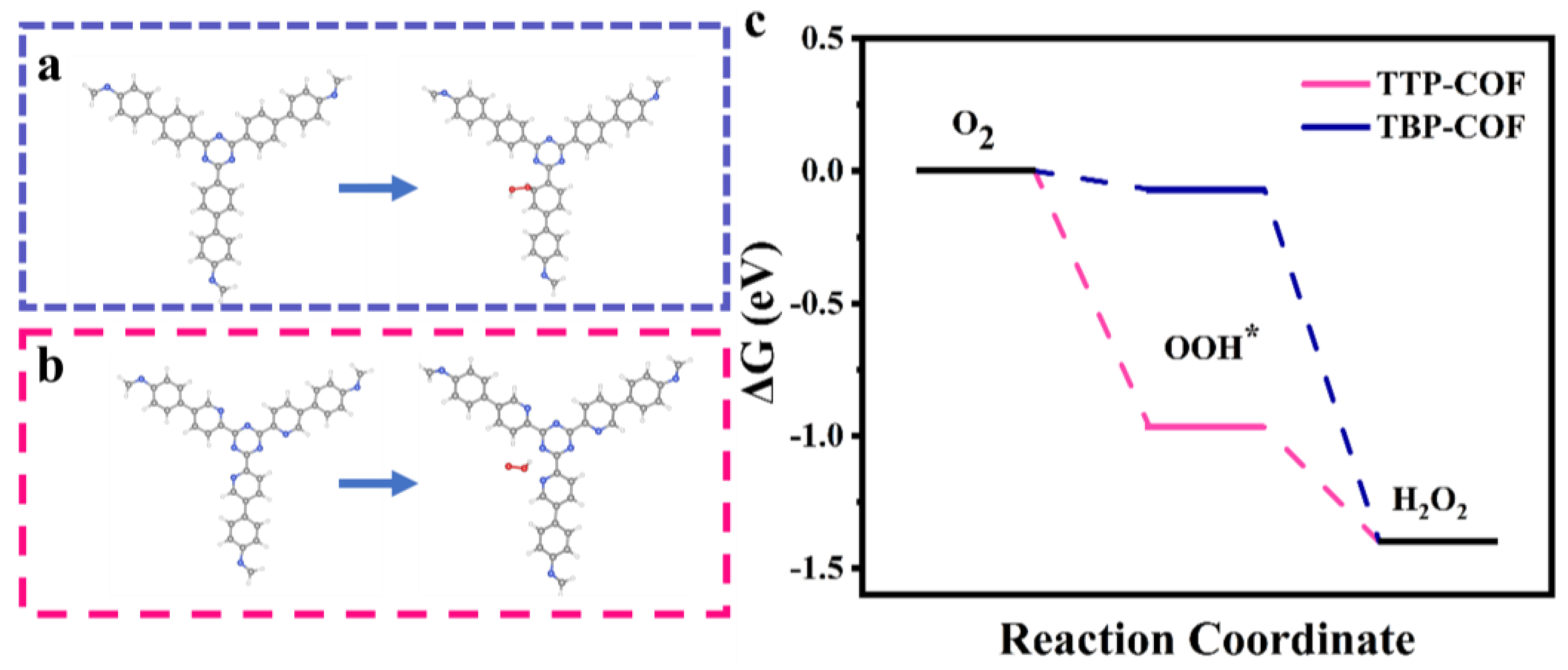
2.4. Theoretical Calculation Details
2.5. H2O2 Detection Methods
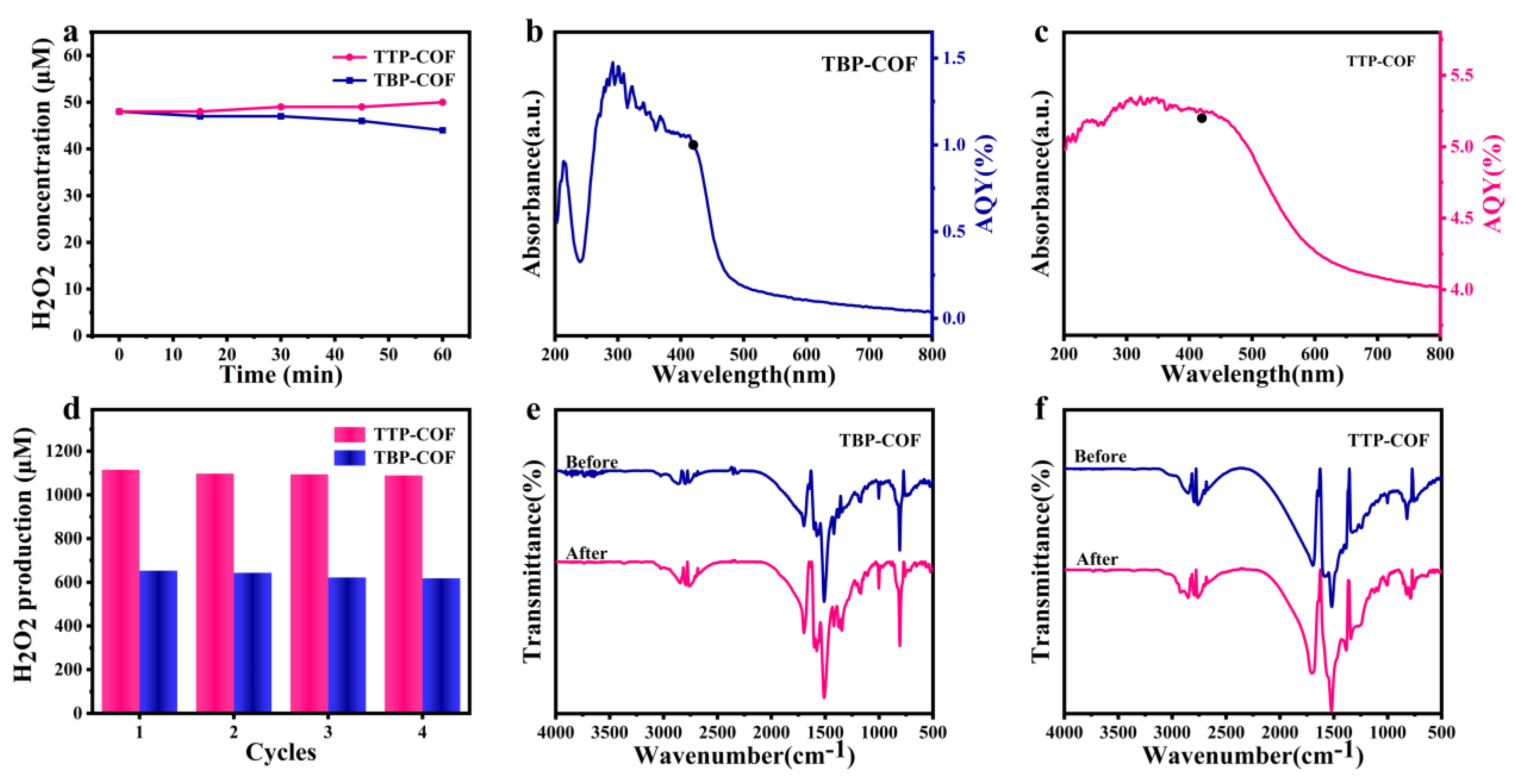
2.6. AQY Measurements
3. Results
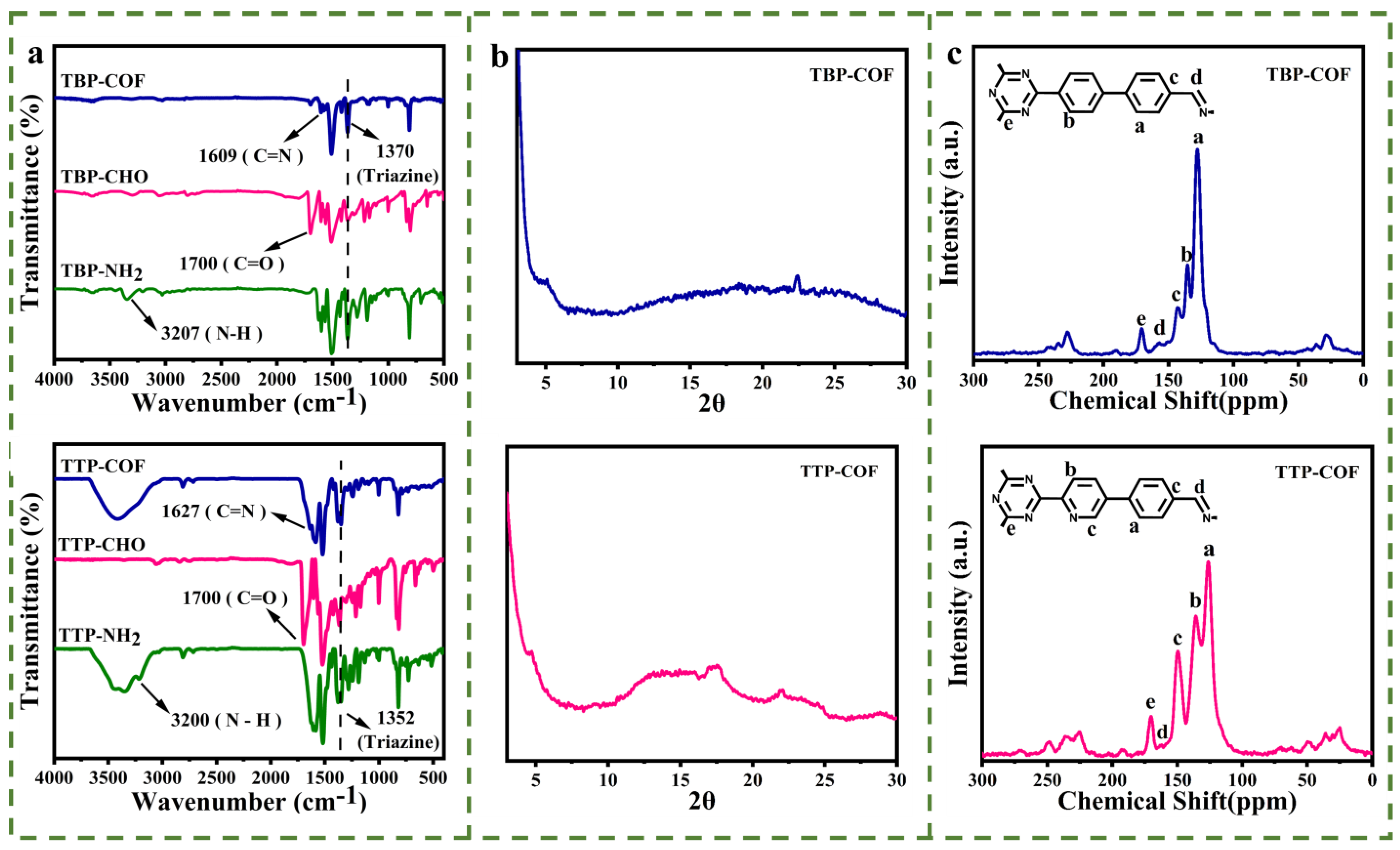
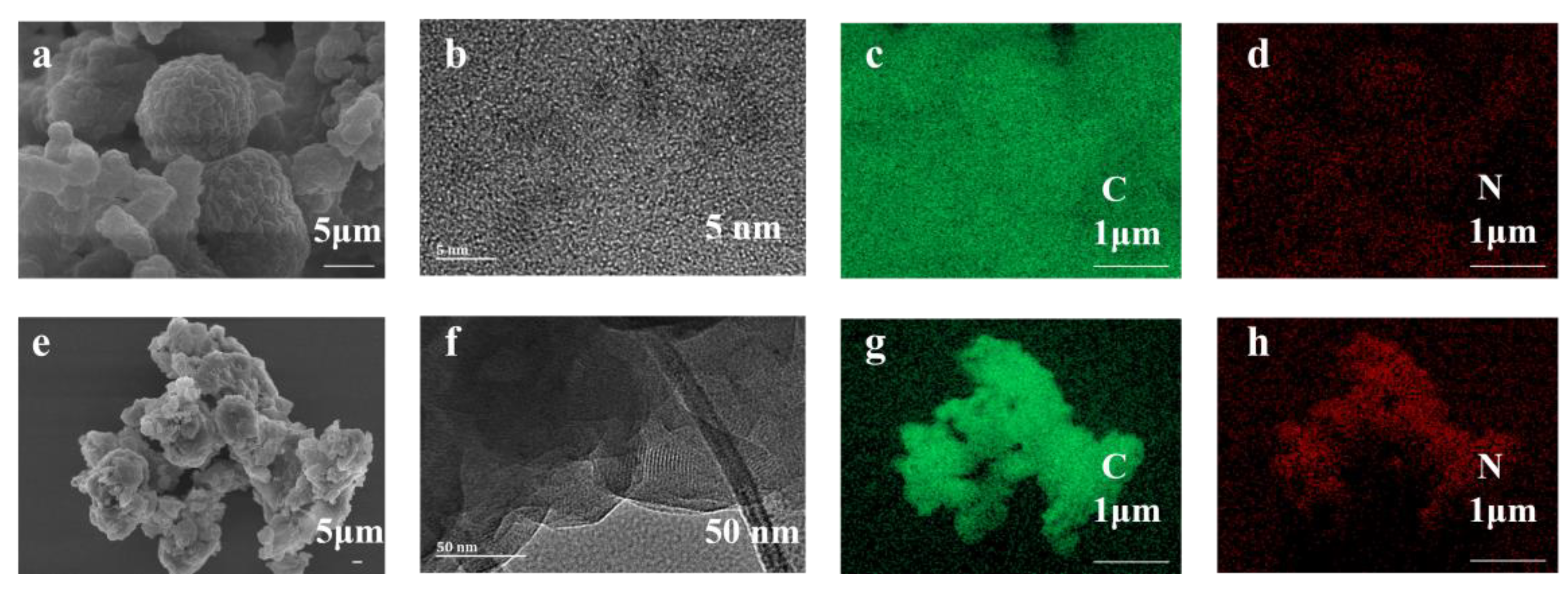
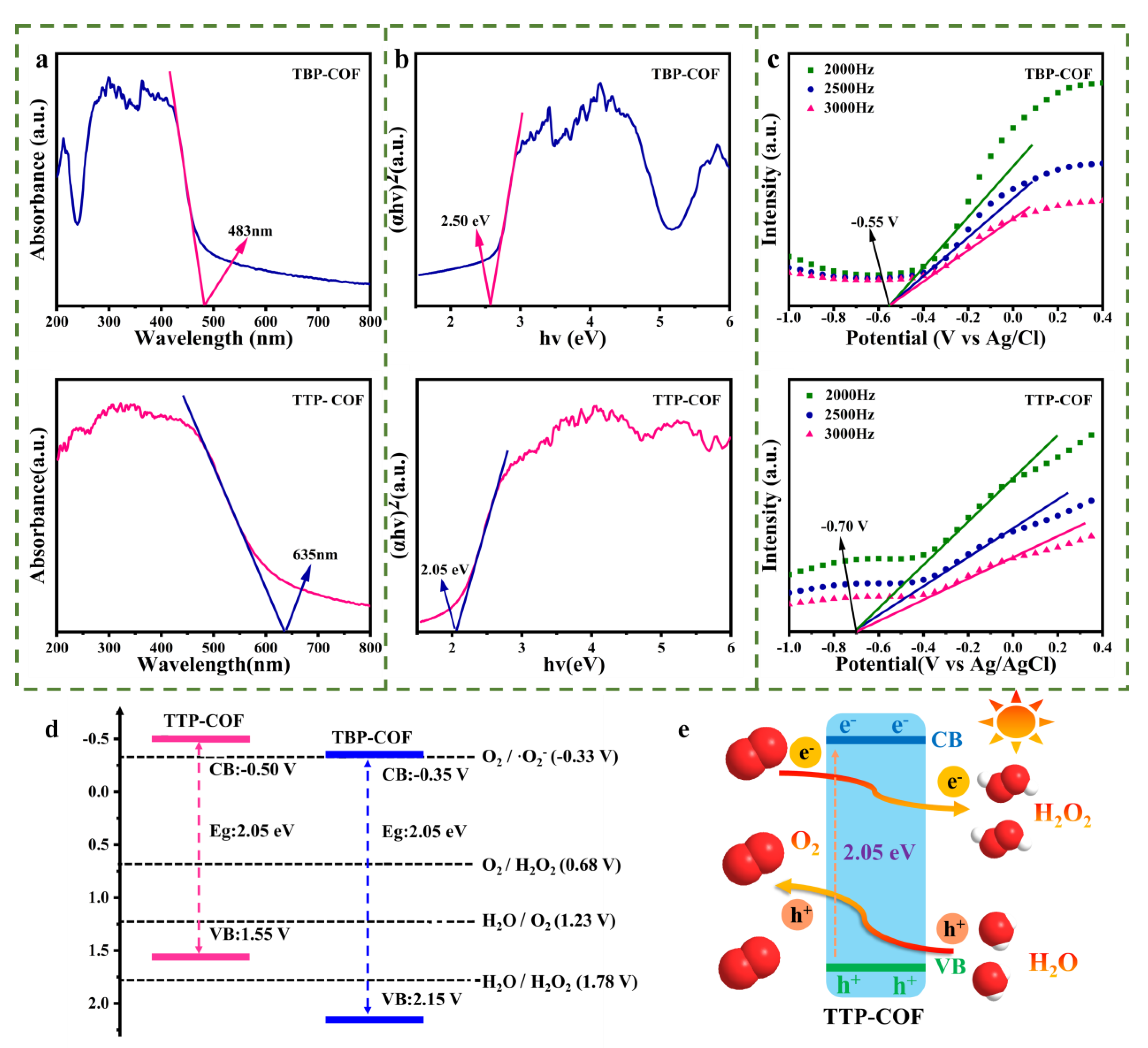
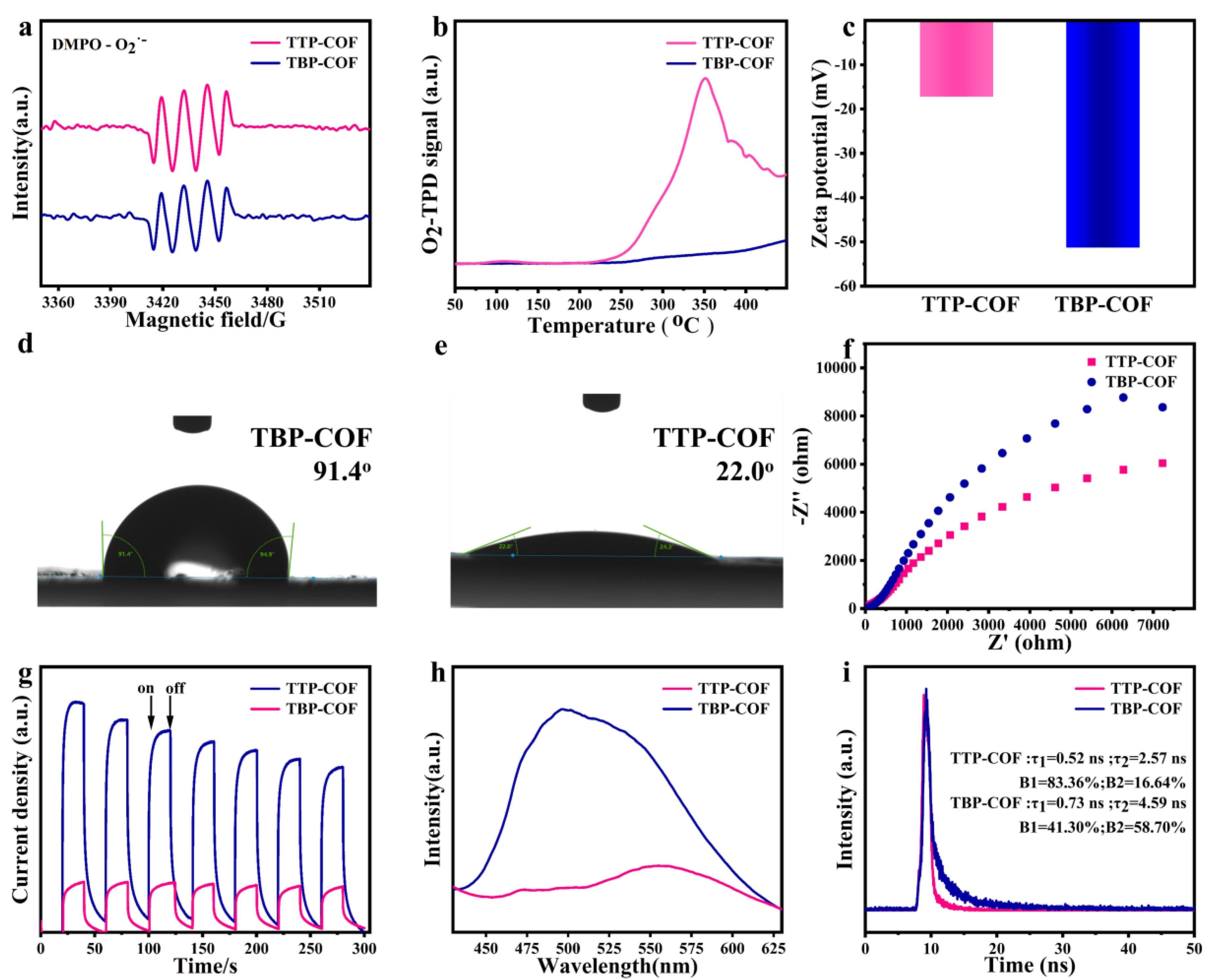
3.1. Structural Analysis of TBP-COF and TTP-COF
3.2. Photocatalytic H2O2 Production Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Li, X.K.; Fan, X.; Wang, H.S.; Liu, Y.L.; Chen, Y.Y.; Yang, T.Y.; Ye, J.; Huang, H.; Li, H.T.; et al. Small-Molecule Catalyzed H2O2 Production via a Phase-Transfer Photocatalytic Process. Appl. Catal. B-Environ. 2022, 314, 121499. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Dai, C.N.; Lei, Z.G.; Chen, B.H.; Fang, X.C. Synthesis of Hydrogen Peroxide over Pd/SiO/COR Monolith Catalysts by Anthraquinone Method. Catal. Today 2016, 276, 36–45. [Google Scholar] [CrossRef]
- Urban, S.; Weltin, A.; Flamm, H.; Kieninger, J.; Deschner, B.J.; Kraut, M.; Dittmeyer, R.; Urban, G.A. Electrochemical Multisensor System for Monitoring Hydrogen Peroxide, Hydrogen and Oxygen in Direct Synthesis Microreactors. Sens. Actuat. B-Chem. 2018, 273, 973–982. [Google Scholar] [CrossRef]
- Tan, D.; Zhuang, R.; Chen, R.; Ban, M.; Feng, W.; Xu, F.; Chen, X.; Wang, Q. Covalent Organic Frameworks Enable Sustainable Solar to Hydrogen Peroxide. Adv. Funct. Mater. 2023, 34, 202311655. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Sun, M.H.; Zhu, Y.B.; Li, P.Z.; Zhang, Y.R.; Wang, M.K.; Shen, Y. A Thioether-Decorated Triazine-Based Covalent Organic Framework Towards Overall H2O2 Photosynthesis without Sacrificial Agents. Appl. Catal. B-Environ. 2023, 334, 122862. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Kondo, Y.; Kuwahara, Y.; Mori, K.; Yamashita, H. Hydrophobic and Visible-Light Responsive Tio as an Efficient Photocatalyst for Promoting Hydrogen Peroxide Production in a Two-Phase System. Catal. Today 2024, 425, 114350. [Google Scholar] [CrossRef]
- Mahvelati-Shamsabadi, T.; Fattahimoghaddam, H.; Lee, B.K.; Bae, S.; Ryu, J. Synthesis of Hexagonal Rosettes of g-C3N4 with Boosted Charge Transfer for the Enhanced Visible-Light Photocatalytic Hydrogen Evolution and Hydrogen Peroxide Production. J. Colloid Interf. Sci. 2021, 597, 345–360. [Google Scholar] [CrossRef]
- Yang, D.; Li, Y.; Chen, R.; Wang, X.; Li, Z.; Xing, T.; Wei, L.; Xu, S.; Dai, P.; Wu, M. Flower-Like Superstructure of Boron Carbon Nitride Nanosheets with Adjustable Band Gaps for Photocatalytic Hydrogen Peroxide Production. J. Mater. Sci. Technol 2024, 183, 23–31. [Google Scholar] [CrossRef]
- Yang, C.; Qian, C.; Yu, M.; Liao, Y. Manipulation of Band Gap and Hydrophilicity in Vinylene-Linked Covalent Organic Frameworks for Improved Visible-Light-Driven Hydrogen Evolution by End-Capping Strategy. Chem. Eng. J. 2023, 454, 140341. [Google Scholar] [CrossRef]
- Sun, K.; Shen, J.; Liu, Q.; Tang, H.; Zhang, M.; Zulfiqar, S.; Lei, C. Synergistic Effect of Co(II)-Hole and Pt-Electron Cocatalysts for Enhanced Photocatalytic Hydrogen Evolution Performance of P-Doped g-C3N4. Chin. J. Catal. 2020, 41, 72–81. [Google Scholar] [CrossRef]
- Naberezhnyi, D.; Park, S.; Li, W.; Westphal, M.; Feng, X.; Dong, R.; Dementyev, P. Mass Transfer in Boronate Ester 2D COF Single Crystals. Small 2021, 17, e2104392. [Google Scholar] [CrossRef] [PubMed]
- Borkowska, M.; Mrówczyński, R. Triptycene Based 3D Covalent Organic Frameworks (COFs)—An Emerging Class of 3D Structures. Symmetry 2023, 15, 1803. [Google Scholar] [CrossRef]
- Mow, R.E.; Russell-Parks, G.A.; Redwine, G.E.B.; Petel, B.E.; Gennett, T.; Braunecker, W.A. Polymer-Coated Covalent Organic Frameworks as Porous Liquids for Gas Storage. Chem. Mater. 2024, 36, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Chen, J.; Chen, X.; Wang, J.; Qiu, H. In Situ Synthesis of a GO/COFs Composite with Enhanced Adsorption Performance for Organic Pollutants in Water. Environ. Sci. Nano 2022, 9, 554–567. [Google Scholar] [CrossRef]
- He, Y.; An, N.; Meng, C.; Xie, K.; Wang, X.; Dong, X.; Sun, D.; Yang, Y.; Hu, Z. High-Density Active Site COFs with a Flower-Like Morphology for Energy Storage Applications. J. Mater. Chem. A 2022, 10, 11030–11038. [Google Scholar] [CrossRef]
- Chen, C.X.; Xiong, Y.Y.; Zhong, X.; Lan, P.C.; Wei, Z.W.; Pan, H.; Su, P.Y.; Song, Y.; Chen, Y.F.; Nafady, A.; et al. Enhancing Photocatalytic Hydrogen Production Via the Construction of Robust Multivariate Ti-MOF/COF Composites. Angew. Chem. Int. Ed. Engl. 2022, 61, e202114071. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lan, X.; Zhang, Y.; Li, H.; Bai, G. Rational Design of MoS2@COF Hybrid Composites Promoting C-C Coupling for Photocatalytic CO2 Reduction to Ethane. Appl. Catal. B 2023, 325, 122393. [Google Scholar] [CrossRef]
- Sun, R.X.; Tan, B.E. Covalent Triazine Frameworks(CTFs) for Photocatalytic Applications. Chem. Res. Chin. Univ. 2022, 38, 310–324. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Wan, Y.; Zhang, Y.; Qi, Z.; Wu, X.; Xu, H. Acetylene and Diacetylene Functionalized Covalent Triazine Frameworks as Metal-Free Photocatalysts for Hydrogen Peroxide Production: A New Two-Electron Water Oxidation Pathway. Adv. Mater. 2020, 32, e1904433. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Wang, Z.; Tang, L.; Zeng, G.; Xu, P.; Chen, M.; Xiong, T.; Zhou, C.; Li, X.; et al. Covalent Organic Framework Photocatalysts: Structures and Applications. Chem. Soc. Rev. 2020, 49, 4135–4165. [Google Scholar] [CrossRef]
- Tan, F.; Zheng, Y.; Zhou, Z.; Wang, H.; Dong, X.; Yang, J.; Ou, Z.; Qi, H.; Liu, W.; Zheng, Z.; et al. Aqueous Synthesis of Covalent Organic Frameworks as Photocatalysts for Hydrogen Peroxide Production. CCS Chem. 2022, 4, 3751–3761. [Google Scholar] [CrossRef]
- Wang, H.; Yang, C.; Chen, F.; Zheng, G.; Han, Q. A Crystalline Partially Fluorinated Triazine Covalent Organic Framework for Efficient Photosynthesis of Hydrogen Peroxide. Angew. Chem. Int. Ed. Engl. 2022, 61, e202202328. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Xie, Z.; Cui, C.-X.; Yang, X.; Xu, Q.; Ke, X.; Liu, M.; Qu, L.-B.; Chen, X.; Mi, L. Constructing Synergistic Triazine and Acetylene Cores in Fully Conjugated Covalent Organic Frameworks for Cascade Photocatalytic H2O2 Production. Chem. Mater. 2022, 34, 5232–5240. [Google Scholar] [CrossRef]
- Yang, L.; Song, Y.; Li, J.; Xu, W.; Peng, C.; Wang, L. S,N-Rich Luminous Covalent Organic Frameworks for Hg2+ Detection and Removal. Chemosphere 2023, 311, 136919. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shao, Y.; Mei, S.; Lu, Y.; Zhang, M.; Sun, J.-k.; Matyjaszewski, K.; Antonietti, M.; Yuan, J. Polymer-Derived Heteroatom-Doped Porous Carbon Materials. Chem. Rev. 2020, 120, 9363–9419. [Google Scholar] [CrossRef]
- Wood, K.N.; O’Hayre, R.; Pylypenko, S. Recent Progress on Nitrogen/Carbon Structures Designed for Use in Energy and Sustainability Applications. Energy Environ. Sci. 2014, 7, 1212–1249. [Google Scholar] [CrossRef]
- Ismagilov, Z.R.; Shalagina, A.E.; Podyacheva, O.Y.; Ischenko, A.V.; Kibis, L.S.; Boronin, A.I.; Chesalov, Y.A.; Kochubey, D.I.; Romanenko, A.I.; Anikeeva, O.B.; et al. Structure and Electrical Conductivity of Nitrogen-Doped Carbon Nanofibers. Carbon 2009, 47, 1922–1929. [Google Scholar] [CrossRef]
- Liu, Z.; Su, Q.; Ju, P.; Li, X.; Li, G.; Wu, Q.; Yang, B. A Hydrophilic Covalent Organic Framework for Photocatalytic Oxidation of Benzylamine in Water. Chem. Commun. 2020, 56, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Zhang, Z.; Meng, F.; Wu, D.; Chen, J.S.; Zhang, F. Heteroatom-Embedded Approach to Vinylene-Linked Covalent Organic Frameworks with Isoelectronic Structures for Photoredox Catalysis. Angew. Chem. Int. Ed. Engl. 2021, 61, e202111627. [Google Scholar] [CrossRef]
- Frost, J.M.; Kobera, L.; Pialat, A.; Zhang, Y.; Southern, S.A.; Gabidullin, B.; Bryce, D.L.; Murugesu, M. From Discrete Molecule, to Polymer, to Mof: Mapping the Coordination Chemistry of Cdiiusing113cd Solid-State Nmr. Chem. Commun. 2016, 52, 10680–10683. [Google Scholar] [CrossRef]
- Zhou, S.; Hu, H.; Hu, H.; Jiang, Q.; Xie, H.; Li, C.; Gao, S.; Kong, Y.; Hu, Y. Unveiling the Latent Reactivity of Imines on Pyridine-Functionalized Covalent Organic Frameworks for H2O2 Photosynthesis. China Mater. 2023, 66, 1837–1846. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Jia, L.; Yu, X.; Wang, Q.; Zhang, Y.; Zhang, Z.; Liang, E.; Han, B.; Li, J. N-Doping and Structural Regularity of Covalent Organic Frameworks (COFs) Tune the Catalytic Activity for Knoevenagel Condensation Reaction. J. Solid State Chem. 2023, 328, 124352. [Google Scholar] [CrossRef]
- Zhai, S.; Lu, Z.; Ai, Y.; Jia, X.; Yang, Y.; Liu, X.; Tian, M.; Bian, X.; Lin, J.; He, S. High Performance Nanocomposite Proton Exchange Membranes Based on the Nanohybrids Formed by Chemically Bonding Phosphotungstic Acid with Covalent Organic Frameworks. J. Power Sources 2023, 554, 232332. [Google Scholar] [CrossRef]
- Han, B.; Song, J.; Liang, S.; Chen, W.; Deng, H.; Ou, X.; Xu, Y.-J.; Lin, Z. Hierarchical NiCo2O4 Hollow Nanocages for Photoreduction of Diluted Co2: Adsorption and Active Sites Engineering. Appl. Catal. B 2020, 260, 118208. [Google Scholar] [CrossRef]
- Kou, M.; Wang, Y.; Xu, Y.; Ye, L.; Huang, Y.; Jia, B.; Li, H.; Ren, J.; Deng, Y.; Chen, J.; et al. Molecularly Engineered Covalent Organic Frameworks for Hydrogen Peroxide Photosynthesis. Angew. Chem. Int. Ed. Engl. 2022, 61, e202200413. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yan, P.; Li, B.; Bahri, M.; Liu, L.; Zhou, X.; Clowes, R.; Browning, N.D.; Wu, Y.; Ward, J.W.; et al. Accelerated Synthesis and Discovery of Covalent Organic Framework Photocatalysts for Hydrogen Peroxide Production. J. Am. Chem. Soc. 2022, 144, 9902–9909. [Google Scholar] [CrossRef]
- Li, L.; Xu, L.; Hu, Z.; Yu, J.C. Enhanced Mass Transfer of Oxygen through a Gas–Liquid–Solid Interface for Photocatalytic Hydrogen Peroxide Production. Adv. Funct. Mater. 2021, 31, 202106120. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, B.; Liu, C.; Xia, D.; Ou, X.; Cai, Y.; Zhou, Y.; Jiang, J.; Han, B. Sulfone-Modified Covalent Organic Frameworks Enabling Efficient Photocatalytic Hydrogen Peroxide Generation Via One-Step Two-Electron O2 Reduction. Angew. Chem. Int. Ed. Engl. 2023, 62, e202305355. [Google Scholar] [CrossRef]
- Chen, W.; Han, B.; Tian, C.; Liu, X.; Liang, S.; Deng, H.; Lin, Z. Mofs-Derived Ultrathin Holey Co3O4 Nanosheets for Enhanced Visible Light Co2 Reduction. Appl. Catal. B 2019, 244, 996–1003. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, J.Y.; Zhu, B.C.; Fedin, M.V.; Cheng, B.; Yu, J.G.; Zhang, L.Y. ZnO/COF S-Scheme Heterojunction for Improved Photocatalytic H2O2 Production Performance. Chem. Eng. J. 2022, 444, 136584. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, Y.; Cao, S.; Wang, R.; Jiao, W. A Novel Strategy for Loading Metal Cocatalysts onto Hollow Nano-TiO2 Inner Surface with Highly Enhanced H2 Production Activity. Green Energy Environ. 2023, 8, 509–518. [Google Scholar] [CrossRef]
- Zhou, S.; Shi, Y.; Chen, G.; Kong, W.; Hu, H.; Xie, H.; Li, C.; Qin, J.; Zhang, Z.; Peng, L.; et al. Fragmentation Engineering on the Edge of Hydroxy-Functional COFs for the Enhanced Photocatalytic Production of H2O2 and Direct Photo-Oxidation of Benzene to Phenol in Aqueous Systems. Chem. Eng. J. 2023, 477, 146946. [Google Scholar] [CrossRef]
- Ma, X.H.; Hou, H.G.; Yang, J.; Liu, J.L.; Liu, G.W.; Du, F.; Qiao, G.J. Porous Si Loaded with Ag Nanoparticles for Ultra-Broadband Infrared Absorption and Detection. Opt. Mater. 2023, 138, 113651. [Google Scholar] [CrossRef]
- Xu, X.; Sa, R.; Huang, W.; Sui, Y.; Chen, W.; Zhou, G.; Li, X.; Li, Y.; Zhong, H. Conjugated Organic Polymers with Anthraquinone Redox Centers for Efficient Photocatalytic Hydrogen Peroxide Production from Water and Oxygen under Visible Light Irradiation without Any Additives. ACS Catal. 2022, 12, 12954–12963. [Google Scholar] [CrossRef]
- He, Y.; Zhao, J.; Sham, Y.-T.; Gao, S.; Pan, M.; Chen, Q.; Huang, G.; Wong, P.K.; Bi, J. Efficient Hydrogen Peroxide Photosynthesis over Cds/COF for Water Disinfection: The S-Scheme Pathway, Oxygen Adsorption, and Reactor Design. ACS Sustain. Chem. Eng. 2023, 11, 17552–17563. [Google Scholar] [CrossRef]
- You, D.; Pan, Z.; Cheng, Q. COFs-Ph@Cds S-Scheme Heterojunctions with Photocatalytic Hydrogen Evolution and Efficient Degradation Properties. J. Alloys Compd. 2023, 930, 167069. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Ouyang, S.; Lu, D.; Wang, X.; Wang, D.; Ye, J. In Situ Surface Alkalinized G-C3N4 toward Enhancement of Photocatalytic H2 Evolution under Visible-Light Irradiation. J. Mater. Chem. A 2016, 4, 2943–2950. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, H.; Wei, Y.; Liu, M.; Hong, J.; Gao, W.; Zhao, S.; Zhang, S.; Guo, S. Cu2O/2D COFs Core/Shell Nanocubes with Antiphotocorrosion Ability for Efficient Photocatalytic Hydrogen Evolution. ACS Nano 2023, 17, 5994–6001. [Google Scholar] [CrossRef]
- Hao, F.; Yang, C.; Lv, X.; Chen, F.; Wang, S.; Zheng, G.; Han, Q. Photo-Driven Quasi-Topological Transformation Exposing Highly Active Nitrogen Cation Sites for Enhanced Photocatalytic H2O2 Production. Angew. Chem. Int. Ed. Engl. 2023, 62, e202315456. [Google Scholar] [CrossRef]
- Vinogradov, K.Y.; Bulanova, A.V.; Shafigulin, R.V.; Tokranova, E.O.; Mebel, A.M.; Zhu, H. Density Functional Theory Study of the Oxygen Reduction Reaction Mechanism on Graphene Doped with Nitrogen and a Transition Metal. ACS Omega 2022, 7, 7066–7073. [Google Scholar] [CrossRef]
- Jung, S.; Senthil, R.A.; Min, A.; Kumar, A.; Moon, C.J.; Jeong, G.H.; Kim, T.W.; Choi, M.Y. Exploring Ir-Doped Nife-Ldh Nanosheets Via a Pulsed Laser for Oxygen Evolution Kinetics: In Situ Raman and Dft Insights. J. Mater. Chem. A 2024. [Google Scholar] [CrossRef]
- Li, G.; Fu, P.; Yue, Q.; Ma, F.; Zhao, X.; Dong, S.; Han, X.; Zhou, Y.; Wang, J. Boosting Exciton Dissociation by Regulating Dielectric Constant in Covalent Organic Framework for Photocatalysis. Chem Catalysis 2022, 2, 1734–1747. [Google Scholar] [CrossRef]


| Element | C% | N% | H% |
|---|---|---|---|
| Theoretical | 84.31 | 10.66 | 5.03 |
| Experiment | 79.17 | 10.31 | 2.37 |
| Element | C% | N% | H% |
|---|---|---|---|
| Theoretical | 77.82 | 17.68 | 4.50 |
| Experiment | 63.65 | 15.48 | 2.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zhi, K.; Zhang, Z.; Kerem, R.; Hong, Q.; Zhao, L.; Wu, W.; Wang, L.; Wang, D. Nitrogen-Rich Triazine-Based Covalent Organic Frameworks as Efficient Visible Light Photocatalysts for Hydrogen Peroxide Production. Nanomaterials 2024, 14, 643. https://doi.org/10.3390/nano14070643
Yang S, Zhi K, Zhang Z, Kerem R, Hong Q, Zhao L, Wu W, Wang L, Wang D. Nitrogen-Rich Triazine-Based Covalent Organic Frameworks as Efficient Visible Light Photocatalysts for Hydrogen Peroxide Production. Nanomaterials. 2024; 14(7):643. https://doi.org/10.3390/nano14070643
Chicago/Turabian StyleYang, Shu, Keke Zhi, Zhimin Zhang, Rukiya Kerem, Qiong Hong, Lei Zhao, Wenbo Wu, Lulu Wang, and Duozhi Wang. 2024. "Nitrogen-Rich Triazine-Based Covalent Organic Frameworks as Efficient Visible Light Photocatalysts for Hydrogen Peroxide Production" Nanomaterials 14, no. 7: 643. https://doi.org/10.3390/nano14070643






