Erythritol as a Saccharide Multifunctional Electrolyte Additive for Highly Reversible Zinc Anode
Abstract
:1. Introduction
2. Results and Discussion
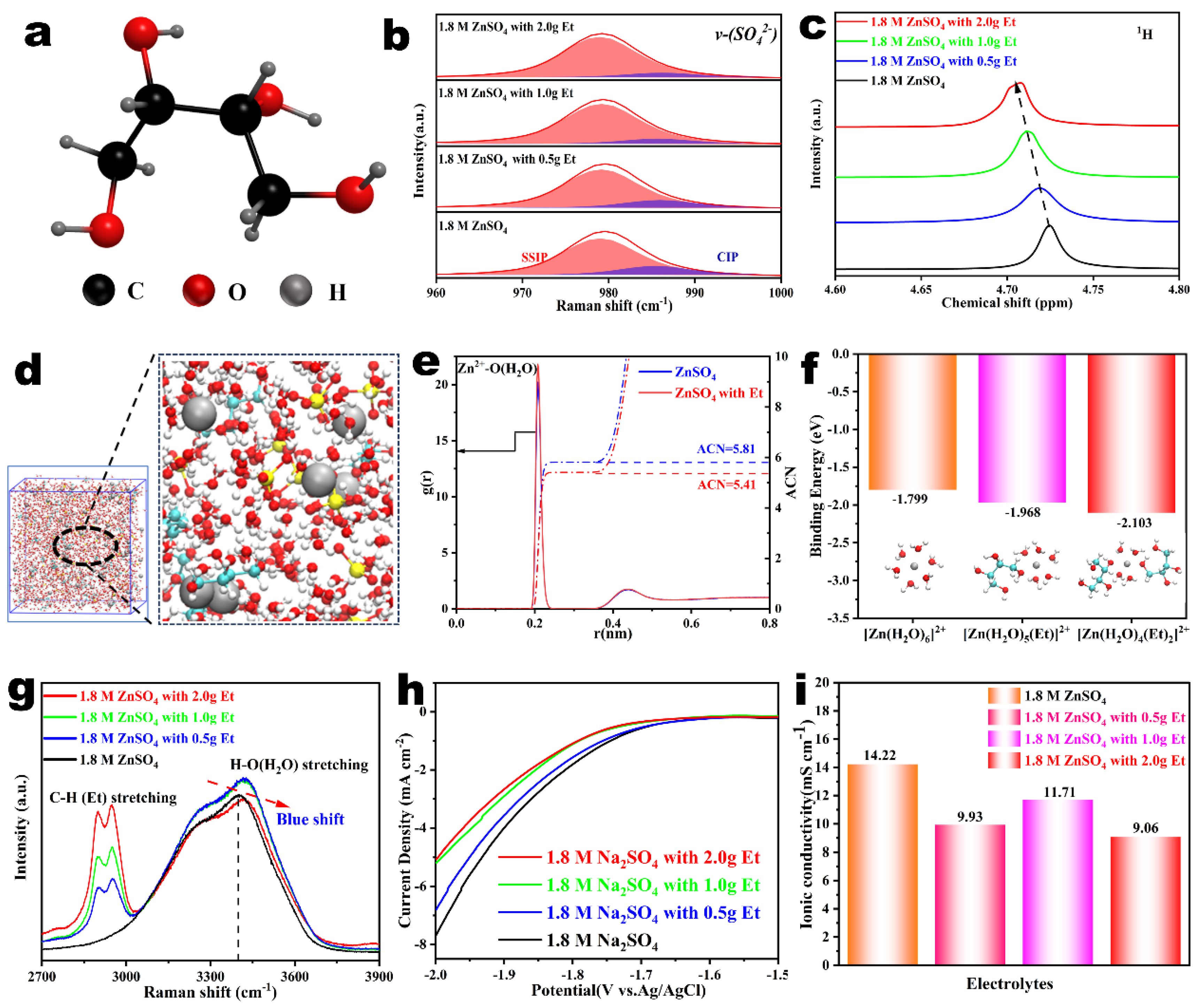
2.1. The Structure of Zn2+ Solvation Sheath Analysis with the Et Additives
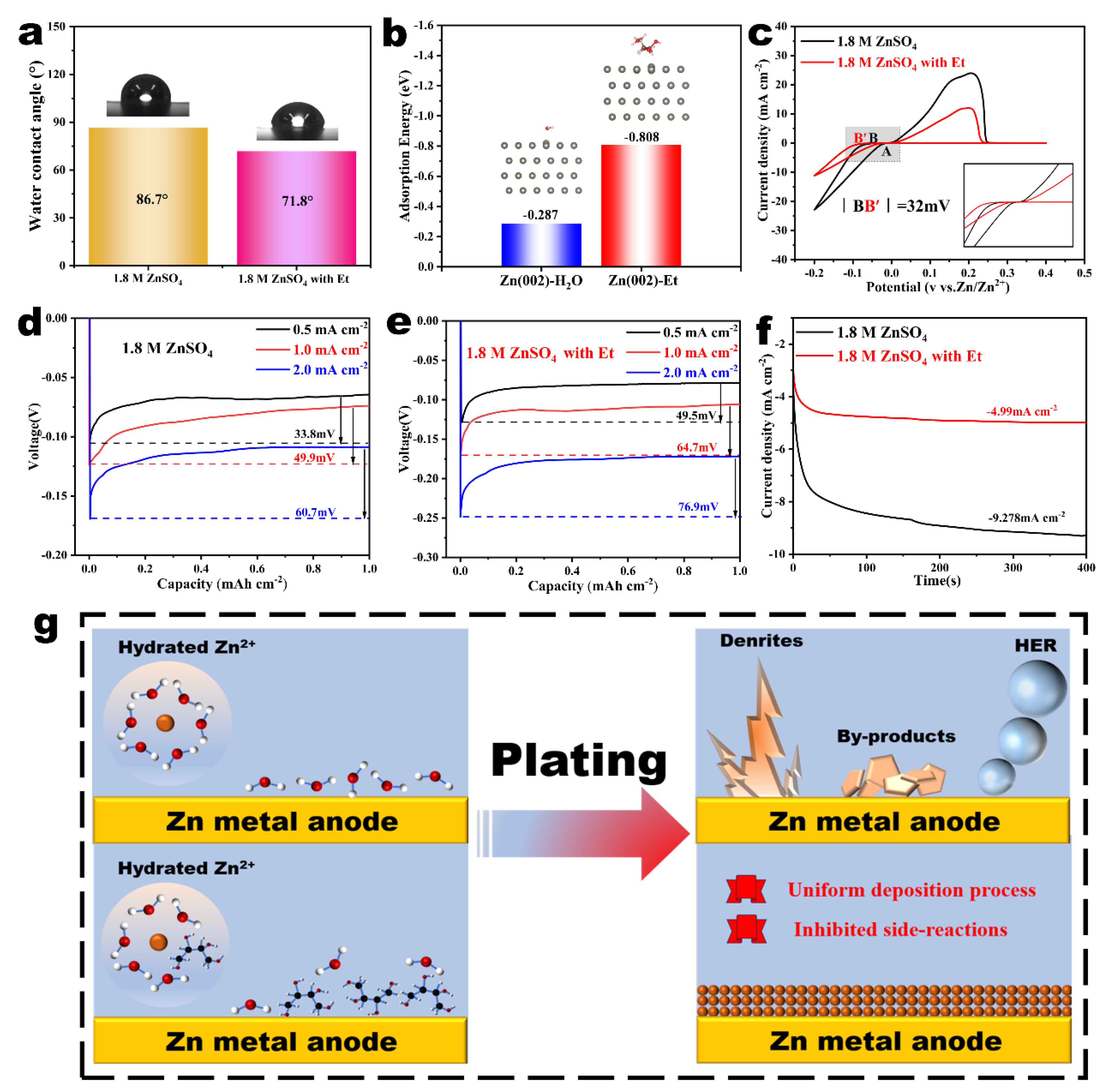
2.2. Effect of Et Additives on Deposition and Diffusion Kinetics of Zn2+
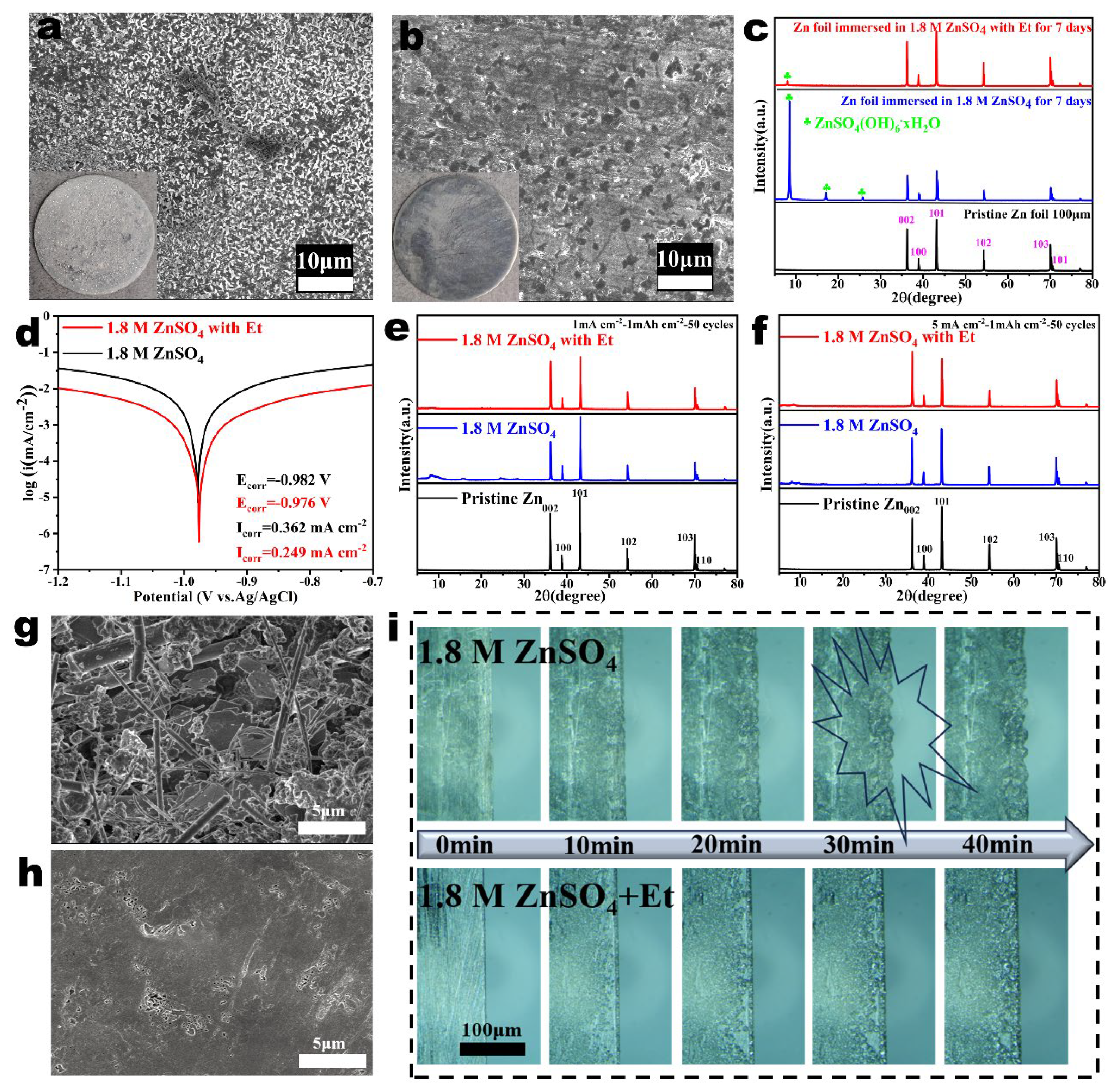
2.3. The Impact of Et Additives on the Morphology Evolution of Zn Anodes
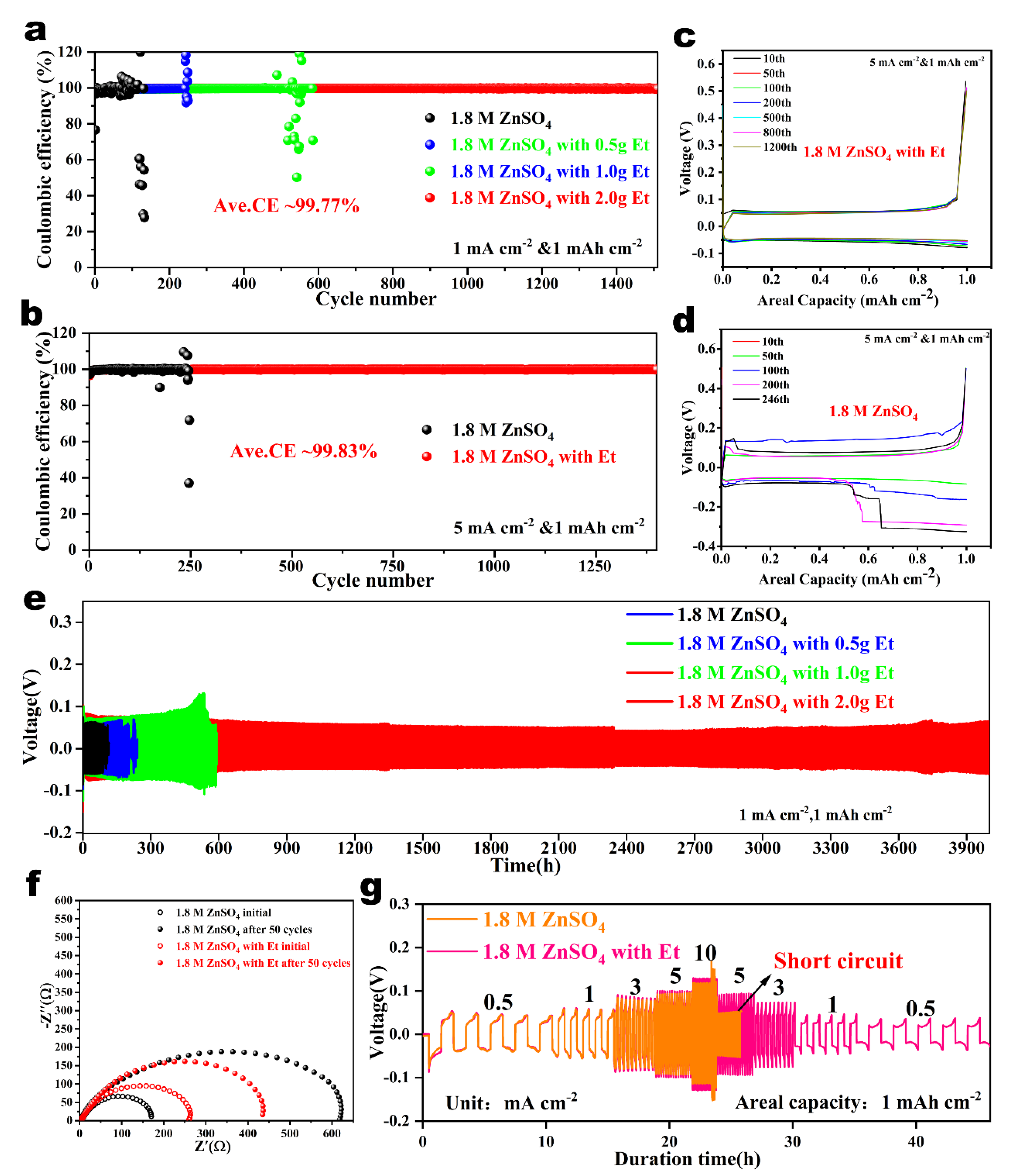
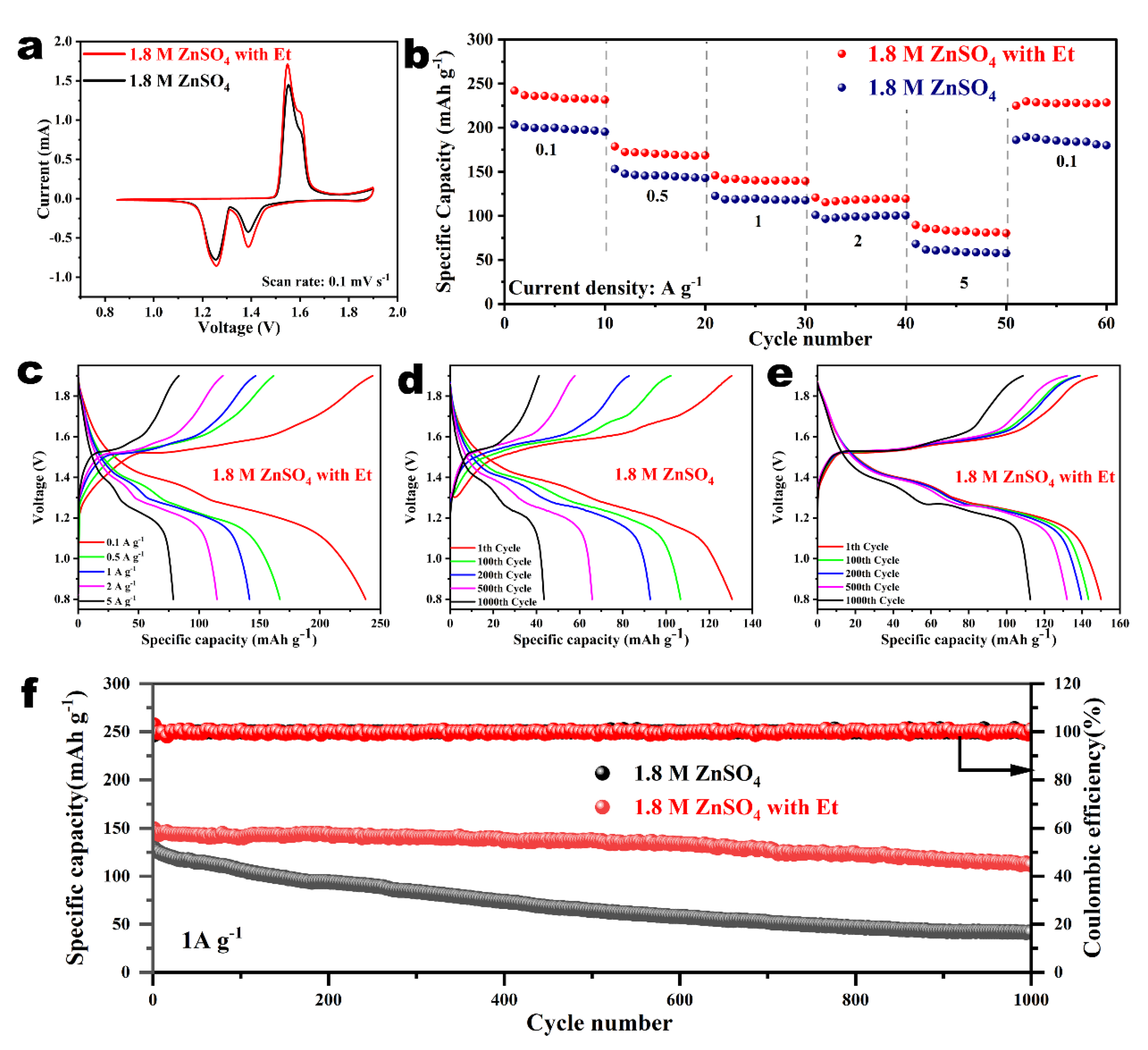
2.4. Reversibility and Stability Comparison of the Zn Anode in Various Electrolytes
2.5. Electrochemical Performance Comparison of AZIBs Equipped with Different Electrolytes
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Zhang, M.; Zou, P.; Sun, B.; Tao, S. Historical development and novel concepts on electrolytes for aqueous rechargeable batteries. Energy Environ. Sci. 2022, 15, 1805–1839. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Wang, G.; Wang, F.; Yang, S.; Zhu, F.; Zhuang, X.; Schmidt, O.G.; Feng, X. Zn-Ion Hybrid Micro-Supercapacitors with Ultrahigh Areal Energy Density and Long-Term Durability. Adv. Mater. 2019, 31, 1806005. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Wang, G.; Wang, D.; Wang, M.; Gao, X.; Bai, Z.; Wang, N.; Yang, J.; Xing, Z.; Dou, S. Recent Progress on Zn Anodes for Advanced Aqueous Zinc-Ion Batteries. Adv. Energy Mater. 2023, 13, 2300606. [Google Scholar] [CrossRef]
- Li, S.; Wang, K.; Zhang, G.; Li, S.; Xu, Y.; Zhang, X.; Zhang, X.; Zheng, S.; Sun, X.; Ma, Y. Fast Charging Anode Materials for Lithium-Ion Batteries: Current Status and Perspectives. Adv. Funct. Mater. 2022, 32, 2200796. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, T.; Chen, D.; Tan, Y.; Zhou, W.; Li, L.; Li, W.; Li, G.; Han, W.; Fan, H.; et al. Protocol in Evaluating Capacity of Zn–Mn Aqueous Batteries: A Clue of pH. Adv. Mater. 2023, 35, 2300053. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yang, J.; Xu, Z.; Nuli, Y.; Wang, J. Recent progress of aqueous and organic/aqueous hybrid electrolytes for low-temperature rechargeable metal-ion batteries and supercapacitors. Energy Storage Mater. 2023, 54, 382–402. [Google Scholar] [CrossRef]
- Yang, W.; Han, Q.; Li, W.; Wu, M.; Yao, J.; Zhao, M.; Lu, X. Ti3C2Tx MXene as Janus separators for redox-enhanced electrochemical capacitors with reduced self-discharge. Energy Storage Mater. 2022, 52, 29–39. [Google Scholar] [CrossRef]
- Cao, Z.; Zhuang, P.; Zhang, X.; Ye, M.; Shen, J.; Ajayan, P.M. Strategies for Dendrite-Free Anode in Aqueous Rechargeable Zinc Ion Batteries. Adv. Energy Mater. 2020, 10, 2001599. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Zhang, X.; Zeng, Z.; Qin, J.; Huang, Y. Strategies of regulating Zn2+solvation structures for dendrite-free and side reaction-suppressed zinc-ion batteries. Energy Environ. Sci. 2022, 15, 499–528. [Google Scholar] [CrossRef]
- Minakshi, M. Alkaline-Earth Oxide Modified MnO2 Cathode: Enhanced Performance in an Aqueous Rechargeable Battery. Ind. Eng. Chem. Res. 2011, 50, 8792–8795. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Z.; Pan, Z.; Wang, S.; Chen, Y.; Zhuang, Q.; Ju, Z.; Zhang, X. Recent Progress and Prospects on Dendrite-free Engineerings for Aqueous Zinc Metal Anodes. Energy Environ. Mater. 2022, 6, e12410. [Google Scholar] [CrossRef]
- Guo, N.; Huo, W.; Dong, X.; Sun, Z.; Lu, Y.; Wu, X.; Dai, L.; Wang, L.; Lin, H.; Liu, H.; et al. A Review on 3D Zinc Anodes for Zinc Ion Batteries. Small Methods 2022, 6, 2200597. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, D.; Hu, E.; Xu, J.; Deng, T.; Ma, L.; Wang, Y.; Yang, X.Q.; Wang, C. Solvation Structure Design for Aqueous Zn Metal Batteries. J. Am. Chem. Soc. 2020, 142, 21404–21409. [Google Scholar] [CrossRef]
- Zeng, X.; Mao, J.; Hao, J.; Liu, J.; Liu, S.; Wang, Z.; Wang, Y.; Zhang, S.; Zheng, T.; Liu, J.; et al. Electrolyte Design for In Situ Construction of Highly Zn2+-Conductive Solid Electrolyte Interphase to Enable High-Performance Aqueous Zn-Ion Batteries under Practical Conditions. Adv. Mater. 2021, 33, 2007416. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Yuan, L.; Ye, C.; Chao, D.; Davey, K.; Guo, Z.; Qiao, S. Boosting Zinc Electrode Reversibility in Aqueous Electrolytes by Using Low-Cost Antisolvents. Angew. Chem. Int. Ed. 2021, 60, 7366–7375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Luan, J.; Fu, L.; Wu, S.; Tang, Y.; Ji, X.; Wang, H. The Three-Dimensional Dendrite-Free Zinc Anode on a Copper Mesh with a Zinc-Oriented Polyacrylamide Electrolyte Additive. Angew. Chem. Int. Ed. 2019, 58, 15841–15847. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.; Li, T.; Li, R.; Wang, S.; Yin, Y.; Zhang, H.; Li, X. An aqueous hybrid electrolyte for low-temperature zinc-based energy storage devices. Energy Environ. Sci. 2020, 13, 3527–3535. [Google Scholar] [CrossRef]
- Liu, S.; Mao, J.; Pang, W.; Vongsvivut, J.; Zeng, X.; Thomsen, L.; Wang, Y.; Liu, J.; Li, D.; Guo, Z. Tuning the Electrolyte Solvation Structure to Suppress Cathode Dissolution, Water Reactivity, and Zn Dendrite Growth in Zinc-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2104281. [Google Scholar] [CrossRef]
- Xin, T.; Zhou, R.; Xu, Q.; Yuan, X.; Zheng, Z.; Li, Y.; Zhang, Q.; Liu, J. 15-Crown-5 ether as efficient electrolyte additive for performance enhancement of aqueous Zn-ion batteries. Chem. Eng. J. 2023, 452, 139572. [Google Scholar] [CrossRef]
- Wang, G.; Dou, Q.; Xiong, P.; Liu, Q.; Min, D.; Park, H.S. Surface-preferred crystal plane induced by supramolecular cyclodextrin additive for highly reversible aqueous zinc metal batteries. Chem. Eng. J. 2023, 457, 141250. [Google Scholar] [CrossRef]
- Zhang, L.; Miao, L.; Xin, W.; Peng, H.; Yan, Z.; Zhu, Z. Engineering zincophilic sites on Zn surface via plant extract additives for dendrite-free Zn anode. Energy Storage Mater. 2022, 44, 408–415. [Google Scholar] [CrossRef]
- Qiu, M.; Ma, L.; Sun, P.; Wang, Z.; Cui, G.; Mai, W. Manipulating Interfacial Stability Via Absorption-Competition Mechanism for Long-Lifespan Zn Anode. Nano-Micro Lett. 2021, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, X.; Luo, M.; Cao, K.; Lu, Y.; Xu, B.B.; Pan, H.; Tao, K.; Jiang, Y. Amino Acid-Induced Interface Charge Engineering Enables Highly Reversible Zn Anode. Adv. Funct. Mater. 2021, 31, 2103514. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Zhong, X.; Wang, K.; Zhang, Y.; Liu, H.; Zhang, L.; Liang, J.; Wen, R. Interfacial chemistry regulation via dibenzenesulfonamide-functionalized additives enables high-performance Zn metal anodes. Energy Storage Mater. 2023, 58, 85–93. [Google Scholar] [CrossRef]
- Zhao, F.; Jing, Z.; Guo, X.; Li, J.; Dong, H.; Tan, Y.; Liu, L.; Zhou, Y.; Owen, R.; Shearing, P.R.; et al. Trace amounts of fluorinated surfactant additives enable high performance zinc-ion batteries. Energy Storage Mater. 2022, 53, 638–645. [Google Scholar] [CrossRef]
- Bayaguud, A.; Luo, X.; Fu, Y.; Zhu, C. Cationic Surfactant-Type Electrolyte Additive Enables Three-Dimensional Dendrite-Free Zinc Anode for Stable Zinc-Ion Batteries. ACS Energy Lett. 2020, 5, 3012–3020. [Google Scholar] [CrossRef]
- Wang, M.; Wu, X.; Yang, D.; Zhao, H.; He, L.; Su, J.; Zhang, X.; Yin, X.; Zhao, K.; Wang, Y.; et al. A colloidal aqueous electrolyte modulated by oleic acid for durable zinc metal anode. Chem. Eng. J. 2023, 451, 138589. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, T.; Li, X.; Cui, H.; Liang, G.; Yang, Q.; Chen, Z.; Chen, A.; Guo, Y.; Fan, J.; et al. Small-Dipole-Molecule-Containing Electrolytes for High-Voltage Aqueous Rechargeable Batteries. Adv. Mater. 2022, 34, 2106180. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Ma, L.; Zhou, W.; Qiu, M.; Wang, Z.; Chao, D.; Mai, W. Simultaneous Regulation on Solvation Shell and Electrode Interface for Dendrite-Free Zn Ion Batteries Achieved by a Low-Cost Glucose Additive. Angew. Chem. Int. Ed. 2021, 60, 18247–18255. [Google Scholar] [CrossRef]
- Chen, W.; Guo, S.; Qin, L.; Li, L.; Cao, X.; Zhou, J.; Luo, Z.; Fang, G.; Liang, S. Hydrogen Bond-Functionalized Massive Solvation Modules Stabilizing Bilateral Interfaces. Adv. Funct. Mater. 2022, 32, 2112609. [Google Scholar] [CrossRef]
- Rudolph, W.W.; Brooker, M.H.; Tremaine, P.R. Raman Spectroscopy of Aqueous ZnSO4 Solutions under Hydrothermal Conditions: Solubility, Hydrolysis, and Sulfate Ion Pairing. J. Solut. Chem. 1999, 28, 621–630. [Google Scholar] [CrossRef]
- Luo, M.; Wang, C.; Lu, H.; Lu, Y.; Xu, B.; Sun, W.; Pan, H.; Yan, M.; Jiang, Y. Dendrite-free zinc anode enabled by zinc-chelating chemistry. Energy Storage Mater. 2021, 41, 515–521. [Google Scholar] [CrossRef]
- Yang, H.; Chang, Z.; Qiao, Y.; Deng, H.; Mu, X.; He, P.; Zhou, H. Constructing a Super-Saturated Electrolyte Front Surface for Stable Rechargeable Aqueous Zinc Batteries. Angew. Chem. Int. Ed. 2020, 59, 9377–9381. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, P.; Wang, W.; Liu, J. Tuning the Electrode/Electrolyte Interface Enabled by a Trifunctional Inorganic Oligomer Electrolyte Additive for Highly Stable and High-Rate Zn Anodes. Small Methods 2023, 7, 2300546. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zheng, R.; Yang, W.; Han, X.; Hou, C.; Zhang, Q.; Li, Y.; Li, K.; Wang, H. Synergistic Solvation and Interface Regulations of Eco-Friendly Silk Peptide Additive Enabling Stable Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2112693. [Google Scholar] [CrossRef]
- Xu, J.; Lv, W.; Yang, W.; Jin, Y.; Jin, Q.; Sun, B.; Zhang, Z.; Wang, T.; Zheng, L.; Shi, X.; et al. In Situ Construction of Protective Films on Zn Metal Anodes via Natural Protein Additives Enabling High-Performance Zinc Ion Batteries. ACS Nano 2022, 16, 11392–11404. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhou, J.; Gong, H.; Qiao, L.; Li, Y.; Li, D.; Gao, M.; Xu, G.; Wang, M.; Liang, X.; et al. Environment-friendly degradable zinc-ion battery based on guar gum-cellulose aerogel electrolyte. Biomater. Sci. 2022, 10, 1476–1485. [Google Scholar] [CrossRef]
- Qin, R.; Wang, Y.; Zhang, M.; Wang, Y.; Ding, S.; Song, A.; Yi, H.; Yang, L.; Song, Y.; Cui, Y.; et al. Tuning Zn2+ coordination environment to suppress dendrite formation for high-performance Zn-ion batteries. Nano Energy 2021, 80, 105478. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Luo, K.; Zhong, L.; Xiong, Y.; Li, G.; Zhong, S.; Yan, D. Erythritol-strengthen electrolytes effectively improve the electrochemical stability window of aqueous symmetric supercapacitor. Mater. Lett. 2023, 343, 134384. [Google Scholar] [CrossRef]
- Zhao, K.; Fan, G.; Liu, J.; Liu, F.; Li, J.; Zhou, X.; Ni, Y.; Yu, M.; Zhang, Y.; Su, H.; et al. Boosting the Kinetics and Stability of Zn Anodes in Aqueous Electrolytes with Supramolecular Cyclodextrin Additives. J. Am. Chem. Soc. 2022, 144, 11129–11137. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, Z.; Li, X.; Liu, Z.; Li, H.; Liang, G.; Wang, D.; Huang, Q.; Zhang, S.; Chen, S.; et al. A Wholly Degradable, Rechargeable Zn-Ti3C2 MXene Capacitor with Superior Anti-Self-Discharge Function. ACS Nano 2019, 13, 8275–8283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, R.; Xu, R.; Li, Y.; Tian, W.; Gao, M.; Wang, M.; Li, D.; Liang, X.; Xie, L.; et al. Super-Assembled Hierarchical Cellulose Aerogel-Gelatin Solid Electrolyte for Implantable and Biodegradable Zinc Ion Battery. Adv. Funct. Mater. 2022, 21, 2111406. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Xie, L.; Xu, R.; Zhang, R.; Gao, M.; Tian, W.; Li, D.; Qiao, L.; Wang, T.; et al. Humidity-sensitive, shape-controllable, and transient zinc-ion batteries based on plasticizing gelatin-silk protein electrolytes. Mater. Today Energy 2021, 21, 100712. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, X.; Liu, S.; Hao, Y.; Tang, Q.; Hu, A.; Liu, Z.; Chen, X. Stabilizing Zinc Anodes by Regulating the Electrical Double Layer with Saccharin Anions. Adv. Mater. 2021, 33, 2100445. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sun, J.; Hua, Y.; Krishna, B.N.V.; Xi, Q.; Ai, W.; Yu, J.S. Planar and dendrite-free zinc deposition enabled by exposed crystal plane optimization of zinc anode. Energy Storage Mater. 2022, 53, 273–304. [Google Scholar] [CrossRef]
- Petersson, G.A.; Al-Laham, M.A. A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J. Chem. Phys. 1991, 94, 6081–6090. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Ribeiro, A.A.S.T.; Horta, B.A.C.; Alencastro, R.B.D. MKTOP: A program for automatic construction of molecular topologies. J. Braz. Chem. Soc. 2008, 19, 1433–1435. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; van der Spoel, D.; Drunen, R.V. GROMACS: A message-passing parallel molecular dynamics implementation Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Van Gunsteren, W.F.; Berendsen, H.J.C. A Leap-frog Algorithm for Stochastic Dynamics. Mol. Simul. 1988, 1, 173–185. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Tan, H.; Gao, Y.; Li, M.; Lu, Z.; Zhang, B. Tailoring desolvation kinetics enables stable zinc metal anodes. J. Mater. Chem. A 2020, 8, 19367–19374. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, H.; Che, Y.; Cheng, L.; Zhang, H.; Chen, J.; Xie, F.; Wang, N.; Jin, Y.; Meng, H. Arginine Cations Inhibiting Charge Accumulation of Dendrites and Boosting Zn Metal Reversibility in Aqueous Rechargeable Batteries. ACS Sustain. Chem. Eng. 2021, 9, 6855–6863. [Google Scholar] [CrossRef]
- Wang, N.; Zhai, S.; Ma, Y.; Tan, X.; Jiang, K.; Zhong, W.; Zhang, W.; Chen, N.; Chen, W.; Li, S.; et al. Tridentate citrate chelation towards stable fiber zinc-polypyrrole battery with hybrid mechanism. Energy Storage Mater. 2021, 43, 585–594. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, F.; Fan, G.; Liu, J.; Yu, M.; Yan, Z.; Zhang, N.; Cheng, F. Stabilizing Zinc Electrodes with a Vanillin Additive in Mild Aqueous Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 47650–47658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, M.; Wu, K.; Yu, F.; Wang, G.; Xu, G.; Wu, M.; Liu, H.; Dou, S.; Wu, C. An in-depth insight of a highly reversible and dendrite-free Zn metal anode in an hybrid electrolyte. J. Mater. Chem. A 2021, 9, 4253–4261. [Google Scholar] [CrossRef]
- Gou, Q.; Luo, H.; Zhang, Q.; Deng, J.; Zhao, R.; Odunmbaku, O.; Wang, L.; Li, L.; Zheng, Y.; Li, J.; et al. Electrolyte Regulation of Bio-Inspired Zincophilic Additive toward High-Performance Dendrite-Free Aqueous Zinc-Ion Batteries. Small 2023, 19, 2207502. [Google Scholar] [CrossRef]
- Ji, H.; Han, Z.; Lin, Y.; Yu, B.; Wu, D.; Zhao, L.; Wang, M.; Chen, J.; Ma, Z.; Guo, B.; et al. Stabilizing zinc anode for high-performance aqueous zinc ion batteries via employing a novel inositol additive. J. Alloys Compd. 2022, 914, 165231. [Google Scholar] [CrossRef]
- Cao, H.; Huang, X.; Liu, Y.; Hu, Q.; Zheng, Q.; Huo, Y.; Xie, F.; Zhao, J.; Lin, D. An efficient electrolyte additive of tetramethylammonium sulfate hydrate for Dendritic-Free zinc anode for aqueous Zinc-ion batteries. J. Colloid Interface Sci. 2022, 627, 367–374. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, R.; Ma, Q.; Wan, J.; Zhang, S.; Zhang, L.; Li, H.; Luo, Q.; Wu, J.; Zhou, T.; et al. A Dual-Functional Organic Electrolyte Additive with Regulating Suitable Overpotential for Building Highly Reversible Aqueous Zinc Ion Batteries. Adv. Funct. Mater. 2023, 34, 2214538. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Guo, Z.; Li, S.; Cao, P.; Du, W.; Feng, D.; Wei, W.; Xu, F.; Ye, C.; Yang, M.; et al. Erythritol as a Saccharide Multifunctional Electrolyte Additive for Highly Reversible Zinc Anode. Nanomaterials 2024, 14, 644. https://doi.org/10.3390/nano14070644
Li L, Guo Z, Li S, Cao P, Du W, Feng D, Wei W, Xu F, Ye C, Yang M, et al. Erythritol as a Saccharide Multifunctional Electrolyte Additive for Highly Reversible Zinc Anode. Nanomaterials. 2024; 14(7):644. https://doi.org/10.3390/nano14070644
Chicago/Turabian StyleLi, Linjie, Zongwei Guo, Shiteng Li, Piting Cao, Weidong Du, Deshi Feng, Wenhui Wei, Fengzhao Xu, Chuangen Ye, Mingzhi Yang, and et al. 2024. "Erythritol as a Saccharide Multifunctional Electrolyte Additive for Highly Reversible Zinc Anode" Nanomaterials 14, no. 7: 644. https://doi.org/10.3390/nano14070644




