Tellurium and Nano-Tellurium: Medicine or Poison?
Abstract
:1. Introduction
2. Discovery of Tellurium
3. Tellurium’s Occurrence, Forms in Nature, and Characterization
3.1. Tellurium’s Occurrence
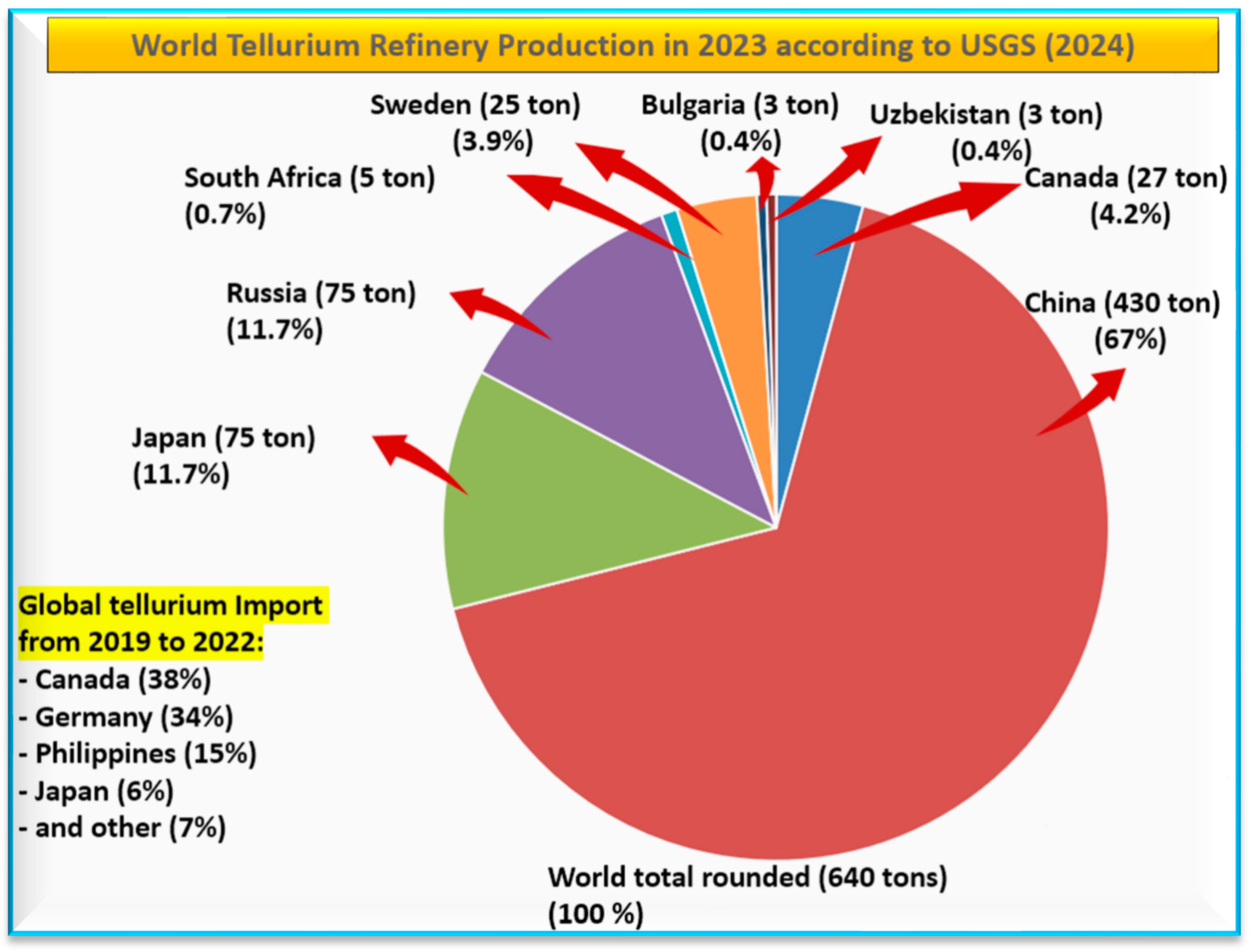
3.2. Global Tellurium Production
3.3. Tellurium Forms in Nature
3.4. Tellurium Characterization
4. Nano-Tellurium and Its Production
5. Applications of Tellurium and Nano-Tellurium
5.1. Pharmaceutical Applications
5.2. Biomedical Applications
6. Toxicity of Tellurium and Nano-Tellurium
6.1. Clinical Signs
6.2. Biogeochemistry
6.3. Environmental Effects
6.4. Toxicity of Nano-Tellurium
6.5. Tellurium Toxicology and Safety
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, G.; Li, X.; Abbas, M.; Fu, C.; Su, Z.; Tang, R.; Chen, S.; Fan, P.; Liang, G. Tellurium Doping Inducing Defect Passivation for Highly Effective Antimony Selenide Thin Film Solar Cell. Nanomaterials 2023, 13, 1240. [Google Scholar] [CrossRef]
- Yang, M.; Yang, M.; Li, Y.; Chen, Y.; Song, Y.; Jia, J.; Su, T. Optimizing Thermoelectric Performance of Tellurium via Doping with Antimony and Selenium. Molecules 2023, 28, 7287. [Google Scholar] [CrossRef] [PubMed]
- Rezaul Karim, S.; Khan, S.; Ansari, G.F.; Mishra, D.; Kumar, S.; Ashiq, M. X-ray Attenuation Performance of a Newly Synthesized Tellurium Based Lead-Free Radiation Shielding Glass System. Radiat. Phys. Chem. 2024, 216, 111477. [Google Scholar] [CrossRef]
- Pale, P.; Mamane, V. Chalcogen Bonding Catalysis: Tellurium, the Last Frontier? Chem. Eur. J. 2023, 29, e202302755. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Guo, X.; Li, D.; Tian, Q.; Zhu, L. Selective Recovery of Sb and Te from the Sodium Sulfide Leach Solution of Te-Bearing Alkaline Skimming Slag by Drop-Wise H2O2 Addition Followed by Na2S–Na2SO3 Precipitation. Hydrometallurgy 2020, 191, 105219. [Google Scholar] [CrossRef]
- Lian, Q.; Liu, W.; Ma, D.; Liang, Z.; Tang, Z.; Cao, J.; He, C.; Xia, D. Precisely Orientating Atomic Array in One-Dimension Tellurium Microneedles Enhances Intrinsic Piezoelectricity for an Efficient Piezo-Catalytic Sterilization. ACS Nano 2023, 17, 8755–8766. [Google Scholar] [CrossRef] [PubMed]
- PubChem Tellurium. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6327182 (accessed on 17 March 2024).
- Basu, A.; Schilling, K.; Halliday, A.N.; Wasserman, N.; Johnson, T.M. Te(IV) Immobilization by Siderite: Reaction Kinetics, Mechanism, and Te Isotopic Fractionation. Chem. Geol. 2022, 612, 121123. [Google Scholar] [CrossRef]
- Chivers, T.; Laitinen, R.S. Tellurium: A Maverick among the Chalcogens. Chem. Soc. Rev. 2015, 44, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Missen, O.P.; Lausberg, E.R.; Brugger, J.; Etschmann, B.; Mills, S.J.; Momma, K.; Ram, R.; Maruyama, M.; Fang, X.-Y.; Melchiorre, E.; et al. Natural Nanoparticles of the Critical Element Tellurium. J. Hazard. Mater. Lett. 2022, 3, 100053. [Google Scholar] [CrossRef]
- Abed, N.N.; Abou El-Enain, I.M.M.; El-Husseiny Helal, E.; Yosri, M. Novel Biosynthesis of Tellurium Nanoparticles and Investigation of Their Activity against Common Pathogenic Bacteria. J. Taibah Univ. Med. Sci. 2023, 18, 400–412. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, S.; Guo, Q.; Missen, O.P.; Xia, X. Microbial Mechanisms to Transform the Super-Trace Element Tellurium: A Systematic Review and Discussion of Nanoparticulate Phases. World J. Microbiol. Biotechnol. 2023, 39, 262. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, L.; Wang, K.; Liu, H.; Zhang, J.; Yan, S. Progress in the Synthesis and Application of Tellurium Nanomaterials. Nanomaterials 2023, 13, 2057. [Google Scholar] [CrossRef] [PubMed]
- Mithun, K.P.; Tripathi, S.; Roy, A.; Ravishankar, N.; Sood, A.K. Ultrafast Time-Resolved Carrier Dynamics in Tellurium Nanowires Using Optical Pump Terahertz Probe Spectroscopy. Nanoscale 2023, 15, 12670–12678. [Google Scholar] [CrossRef] [PubMed]
- Bhol, P.; Jagdale, P.B.; Jadhav, A.H.; Saxena, M.; Samal, A.K. All-Solid-State Supercapacitors Based on Cobalt Magnesium Telluride Microtubes Decorated with Tellurium Nanotubes. ChemSusChem 2024, e202301009. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, J.; Chu, J.; Yang, L.; Zhao, X.; Zhang, Y.; Liu, T.; Lu, Y.; Chen, C.; Hou, X.; et al. Tailoring the Epitaxial Growth of Oriented Te Nanoribbon Arrays. iScience 2023, 26, 106177. [Google Scholar] [CrossRef] [PubMed]
- Zambonino, M.C.; Quizhpe, E.M.; Jaramillo, F.E.; Rahman, A.; Santiago Vispo, N.; Jeffryes, C.; Dahoumane, S.A. Green Synthesis of Selenium and Tellurium Nanoparticles: Current Trends, Biological Properties and Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Ghany, M.N.; Hamdi, S.A.; Korany, S.M.; Elbaz, R.M.; Farahat, M.G. Biosynthesis of Novel Tellurium Nanorods by Gayadomonas Sp. TNPM15 Isolated from Mangrove Sediments and Assessment of Their Impact on Spore Germination and Ultrastructure of Phytopathogenic Fungi. Microorganisms 2023, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Abdalla, N.; Sári, D.; Ferroudj, A.; Muthu, A.; Prokisch, J.; Fawzy, Z.F.; Brevik, E.C.; Solberg, S.Ø. Nanofarming: Promising Solutions for the Future of the Global Agricultural Industry. Agronomy 2023, 13, 1600. [Google Scholar] [CrossRef]
- El-Ramady, H.; Prokisch, J.; Mansour, H.; Bayoumi, Y.A.; Shalaby, T.A.; Veres, S.; Brevik, E.C. Review of Crop Response to Soil Salinity Stress: Possible Approaches from Leaching to Nano-Management. Soil Syst. 2024, 8, 11. [Google Scholar] [CrossRef]
- Longa, I. A three times discovered tellurium or geologists in the magic flute. Ipjogvéd. És Szerzői Jogi Szle. 2006, 11, 276. [Google Scholar]
- 12 October 1825—Death of Franz-Joseph Müller, Discoverer of Tellurium. Rincón Educ. Available online: https://rinconeducativo.org/en/anniversaries/12-october-1825-death-of-franz-joseph-muller-discoverer-of-tellurium/#:~:text=Cooperative%20learning-,12%20October%201825%20%2D%20Death%20of%20Franz%2DJoseph%20M%C3%BCller%2C%20discoverer,specialized%20in%20mineralogy%20and%20chemistry (accessed on 5 February 2024).
- Klaproth, Martin Heinrich. Available online: https://www.vilaglex.hu/Lexikon/Html/Klaproth_htm (accessed on 17 March 2024).
- Kitaibel Pál. Available online: https://mek.oszk.hu/02000/02060/html/kitaibel.htm (accessed on 17 March 2024).
- Christy, A.G.; Mills, S.J.; Kampf, A.R. A Review of the Structural Architecture of Tellurium Oxycompounds. Mineral. Mag. 2016, 80, 415–545. [Google Scholar] [CrossRef]
- Cohen, B.L. Anomalous Behavior of Tellurium Abundances. Geochim. Cosmochim. Acta 1984, 48, 203–205. [Google Scholar] [CrossRef]
- Goldfarb, R.J.; Berger, B.R.; George, M.W.; Seal II, R.R. Tellurium; Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply; US Geological Survey: Reston, VA, USA, 2017; pp. R1–R27.
- Wiklund, J.A.; Kirk, J.L.; Muir, D.C.G.; Carrier, J.; Gleason, A.; Yang, F.; Evans, M.; Keating, J. Widespread Atmospheric Tellurium Contamination in Industrial and Remote Regions of Canada. Environ. Sci. Technol. 2018, 52, 6137–6145. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xiong, Y.; Song, Y.; Zhang, G.; Zhang, F.; Yang, Y.; Hua, Z.; Tian, Y.; You, J.; Zhao, Z. Recycling of Copper Telluride from Copper Anode Slime Processing: Toward Efficient Recovery of Tellurium and Copper. Hydrometallurgy 2020, 196, 105436. [Google Scholar] [CrossRef]
- US Geological Survey Mineral Commodity Summaries 2024. 2024. Available online: https://pubs.usgs.gov/periodicals/mcs2024/mcs2024-tellurium.pdf (accessed on 5 February 2024).
- Taylor, A. Biochemistry of Tellurium. Biol. Trace Elem. Res. 1996, 55, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Takahashi, Y. Origin of the Difference in the Distribution Behavior of Tellurium and Selenium in a Soil–Water System. Geochim. Cosmochim. Acta 2008, 72, 1281–1294. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A.; Halliday, A.N. Global Occurrence of Tellurium-Rich Ferromanganese Crusts and a Model for the Enrichment of Tellurium. Geochim. Cosmochim. Acta 2003, 67, 1117–1127. [Google Scholar] [CrossRef]
- Belzile, N.; Chen, Y.-W. Tellurium in the Environment: A Critical Review Focused on Natural Waters, Soils, Sediments and Airborne Particles. Appl. Geochem. 2015, 63, 83–92. [Google Scholar] [CrossRef]
- Vávrová, S.; Struhárňanská, E.; Turňa, J.; Stuchlík, S. Tellurium: A Rare Element with Influence on Prokaryotic and Eukaryotic Biological Systems. Int. J. Mol. Sci. 2021, 22, 5924. [Google Scholar] [CrossRef] [PubMed]
- Anan, Y.; Yoshida, M.; Hasegawa, S.; Katai, R.; Tokumoto, M.; Ouerdane, L.; Łobiński, R.; Ogra, Y. Speciation and Identification of Tellurium-Containing Metabolites in Garlic, Allium Sativum. Metallomics 2013, 5, 1215. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.W.; Haider, S.I.; Solangi, A.R.; Memon, A.F. Toxicity of Tellurium and Its Compounds. Phys. Sci. Rev. 2023, 8, 4375–4390. [Google Scholar] [CrossRef]
- Medina-Cruz, D.; Tien-Street, W.; Vernet-Crua, A.; Zhang, B.; Huang, X.; Murali, A.; Chen, J.; Liu, Y.; Garcia-Martin, J.M.; Cholula-Díaz, J.L.; et al. Tellurium, the Forgotten Element: A Review of the Properties, Processes, and Biomedical Applications of the Bulk and Nanoscale Metalloid. In Racing for the Surface; Li, B., Moriarty, T.F., Webster, T., Xing, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 723–783. ISBN 978-3-030-34470-2. [Google Scholar]
- Vernet Crua, A.; Medina, D.; Zhang, B.; González, M.U.; Huttel, Y.; García-Martín, J.M.; Cholula Díaz, J.L.; Webster, T.J. Comparison of Cytocompatibility and Anticancer Properties of Traditional and Green Chemistry-Synthesized Tellurium Nanowires. Int. J. Nanomed. 2019, 14, 3155–3176. [Google Scholar] [CrossRef] [PubMed]
- Mahalakhsmi, A.; Baskar, G. Greener Synthesis and Characterization of Cadmium-Tellurium Quantum Dots Using Aqueous Extract of Waste Orange Peel. Indian J. Biochem. Biophys. 2021, 58, 56–61. [Google Scholar] [CrossRef]
- Gómez-Gómez, B.; Sanz-Landaluce, J.; Pérez-Corona, M.T.; Madrid, Y. Fate and Effect of In-House Synthesized Tellurium Based Nanoparticles on Bacterial Biofilm Biomass and Architecture. Challenges for Nanoparticles Characterization in Living Systems. Sci. Total Environ. 2020, 719, 137501. [Google Scholar] [CrossRef] [PubMed]
- Barabadi, H.; Kobarfard, F.; Vahidi, H. Biosynthesis and Characterization of Biogenic Tellurium Nanoparticles by Using Penicillium Chrysogenum PTCC 5031: A Novel Approach in Gold Biotechnology. Iran. J. Pharm. Res. IJPR 2018, 17, 87–97. [Google Scholar] [PubMed]
- Beleneva, I.A.; Kharchenko, U.V.; Kukhlevsky, A.D.; Boroda, A.V.; Izotov, N.V.; Gnedenkov, A.S.; Egorkin, V.S. Biogenic Synthesis of Selenium and Tellurium Nanoparticles by Marine Bacteria and Their Biological Activity. World J. Microbiol. Biotechnol. 2022, 38, 188. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.; Lashani, E.; Moghimi, H. Simultaneous Bioremediation of Phenol and Tellurite by Lysinibacillus Sp. EBL303 and Characterization of Biosynthesized Te Nanoparticles. Sci. Rep. 2023, 13, 1243. [Google Scholar] [CrossRef] [PubMed]
- Vaigankar, D.C.; Dubey, S.K.; Mujawar, S.Y.; D’Costa, A.; Shyama, S.K. Tellurite Biotransformation and Detoxification by Shewanella Baltica with Simultaneous Synthesis of Tellurium Nanorods Exhibiting Photo-Catalytic and Anti-Biofilm Activity. Ecotoxicol. Environ. Saf. 2018, 165, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Ao, B.; He, F.; Lv, J.; Tu, J.; Tan, Z.; Jiang, H.; Shi, X.; Li, J.; Hou, J.; Hu, Y.; et al. Green Synthesis of Biogenetic Te(0) Nanoparticles by High Tellurite Tolerance Fungus Mortierella Sp. AB1 with Antibacterial Activity. Front. Microbiol. 2022, 13, 1020179. [Google Scholar] [CrossRef] [PubMed]
- Abo Elsoud, M.M.; Al-Hagar, O.E.A.; Abdelkhalek, E.S.; Sidkey, N.M. Synthesis and Investigations on Tellurium Myconanoparticles. Biotechnol. Rep. 2018, 18, e00247. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Zhang, X.; Wang, M.-H. Biosynthesis of Gallic Acid Fabricated Tellurium Nanoparticles (GA-Te NPs) for Enhanced Antibacterial, Antioxidant, and Cytotoxicity Applications. Environ. Res. 2024, 240, 117461. [Google Scholar] [CrossRef] [PubMed]
- Rachitha, P.; Krupashree, K.; Kandikattu, H.K.; Nagaraj, G.; Alahmadi, T.A.; Alharbi, S.A.; Shanmuganathan, R.; Brindhadevi, K.; Raghavendra, V.B. Nanofabrication of Cobalt-Tellurium Using Allium Sativum Extract and Its Protective Efficacy against H2O2-Induced Oxidative Damage in HaCaT Cells. Environ. Res. 2023, 226, 115659. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J.; Borghese, R.; Zannoni, D. Microbial Processing of Tellurium as a Tool in Biotechnology. Biotechnol. Adv. 2012, 30, 954–963. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, F.; Tian, Q.; Yue, X.; Zhang, T. Production and Recovery of Tellurium from Metallurgical Intermediates and Electronic Waste-A Comprehensive Review. J. Clean. Prod. 2022, 366, 132796. [Google Scholar] [CrossRef]
- Shi, Z.; Cao, R.; Khan, K.; Tareen, A.K.; Liu, X.; Liang, W.; Zhang, Y.; Ma, C.; Guo, Z.; Luo, X.; et al. Two-Dimensional Tellurium: Progress, Challenges, and Prospects. Nano-Micro Lett. 2020, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.I.; Kar, Y.B.; Doroody, C.; Kiong, T.S.; Rahman, K.S.; Harif, M.N.; Amin, N. A Comprehensive Review of Flexible Cadmium Telluride Solar Cells with Back Surface Field Layer. Heliyon 2023, 9, e21622. [Google Scholar] [CrossRef] [PubMed]
- Missen, O.P.; Ram, R.; Mills, S.J.; Etschmann, B.; Reith, F.; Shuster, J.; Smith, D.J.; Brugger, J. Love Is in the Earth: A Review of Tellurium (Bio)Geochemistry in Surface Environments. Earth-Sci. Rev. 2020, 204, 103150. [Google Scholar] [CrossRef]
- Tao, J.-J.; Jiang, J.; Zhao, S.-N.; Zhang, Y.; Li, X.-X.; Fang, X.; Wang, P.; Hu, W.; Lee, Y.H.; Lu, H.-L.; et al. Fabrication of 1D Te/2D ReS 2 Mixed-Dimensional van Der Waals p-n Heterojunction for High-Performance Phototransistor. ACS Nano 2021, 15, 3241–3250. [Google Scholar] [CrossRef] [PubMed]
- Poborchii, V.V.; Fokin, A.V.; Shklyaev, A.A. Optical Properties of Extreme Tellurium Nanowires Formed in Subnanometer-Diameter Channels. Nanoscale Adv. 2023, 5, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, R.; Zhang, Y. Tellurium Nanotubes and Chemical Analogues from Preparation to Applications: A Minor Review. Nanomaterials 2022, 12, 2151. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, H.; Lee, S.-H.; Yu, S.; Kim, W.; Bae, J.-S.; Ahn, C.-Y.; Shim, H.; Lee, J.E.; Yu, S.-H. Te Hexagonal Nanotubes with Fast 1-Dimensional Zn Ion Diffusion for High-Performance Zinc-Ion Battery Cathodes. Chem. Eng. J. 2024, 481, 148256. [Google Scholar] [CrossRef]
- Bošnjaković, M.; Santa, R.; Crnac, Z.; Bošnjaković, T. Environmental Impact of PV Power Systems. Sustainability 2023, 15, 11888. [Google Scholar] [CrossRef]
- Scarpulla, M.A.; McCandless, B.; Phillips, A.B.; Yan, Y.; Heben, M.J.; Wolden, C.; Xiong, G.; Metzger, W.K.; Mao, D.; Krasikov, D.; et al. CdTe-Based Thin Film Photovoltaics: Recent Advances, Current Challenges and Future Prospects. Sol. Energy Mater. Sol. Cells 2023, 255, 112289. [Google Scholar] [CrossRef]
- Dallaev, R.; Pisarenko, T.; Papež, N.; Holcman, V. Overview of the Current State of Flexible Solar Panels and Photovoltaic Materials. Materials 2023, 16, 5839. [Google Scholar] [CrossRef] [PubMed]
- National Food Institute—Technical University of Denmark; Doulgeridou, A.; Amlund, H.; Sloth, J.J.; Hansen, M. Review of Potentially Toxic Rare Earth Elements, Thallium and Tellurium in Plant-based Foods. EFSA J. 2020, 18, e181101. [Google Scholar] [CrossRef] [PubMed]
- Nassar, N.T.; Kim, H.; Frenzel, M.; Moats, M.S.; Hayes, S.M. Global Tellurium Supply Potential from Electrolytic Copper Refining. Resour. Conserv. Recycl. 2022, 184, 106434. [Google Scholar] [CrossRef]
- Azzouz, L.; Halit, M.; Charifi, Z.; Matta, C.F. Tellurium Doping and the Structural, Electronic, and Optical Properties of NaYS 2(1–x) Te 2 x Alloys. ACS Omega 2019, 4, 11320–11331. [Google Scholar] [CrossRef]
- Gao, Y.; Cui, T.; Li, D. Unexpected D−p Orbital Covalent Interaction Between the Non-d-Block Main-Group Metal Tellurium and Fluorine at High Pressure. Fundam. Res. 2023, S2667325823001279. [Google Scholar] [CrossRef]
- Jiang, B.; Xue, H.; Wang, P.; Du, H.; Kang, Y.; Zhao, J.; Wang, S.; Zhou, W.; Bian, Z.; Li, H.; et al. Noble-Metal–Metalloid Alloy Architectures: Mesoporous Amorphous Iridium–Tellurium Alloy for Electrochemical N2 Reduction. J. Am. Chem. Soc. 2023, 145, 6079–6086. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shi, T.; Li, Y.; Yang, B.; Tian, Y.; Xu, B.; Yang, H.; Chen, X.; Chen, C. Selective Sulfidation-Vacuum Volatilization Processes for Tellurium and Bismuth Recovery from Bismuth Telluride Waste Thermoelectric Material. J. Environ. Manag. 2023, 327, 116845. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zha, W.; Yuan, R.; Lou, B.; Sa, R. Indirect-to-Direct Band Gap Transition and Optical Properties of Metal Alloys of Cs2 Te1−x Tix I6: A Theoretical Study. RSC Adv. 2020, 10, 36734–36740. [Google Scholar] [CrossRef]
- Faizan, M.; Xie, J.; Murtaza, G.; Echeverría-Arrondo, C.; Alshahrani, T.; Bhamu, K.C.; Laref, A.; Mora-Seró, I.; Haidar Khan, S. A First-Principles Study of the Stability, Electronic Structure, and Optical Properties of Halide Double Perovskite Rb2Sn1−xTexI6 for Solar Cell Applications. Phys. Chem. Chem. Phys. 2021, 23, 4646–4657. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, Q.; Zhang, W.; Huang, S. Amorphous Tellurium-Embedded Hierarchical Porous Carbon Nanofibers as High-Rate and Long-Life Electrodes for Potassium-Ion Batteries. Small 2022, 18, 2202750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, H.; Freschi, D.J.; Liu, J. High-Performance Potassium-Tellurium Batteries Stabilized by Interface Engineering. Small 2022, 18, 2200085. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Mu, Z.; Qian, K.; Guo, C.; Li, M.; Li, J. Biochar-Derived Hierarchical Porous Carbon as Tellurium Host for High-Performance Potassium-Tellurium Batteries. Chem. Eur. J. 2023, 29, e202302121. [Google Scholar] [CrossRef] [PubMed]
- Hellier, K.; Stewart, D.A.; Read, J.; Sfadia, R.; Abbaszadeh, S. Tuning Amorphous Selenium Composition with Tellurium to Improve Quantum Efficiency at Long Wavelengths and High Applied Fields. ACS Appl. Electron. Mater. 2023, 5, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Vahidi, H.; Kobarfard, F.; Alizadeh, A.; Saravanan, M.; Barabadi, H. Green Nanotechnology-Based Tellurium Nanoparticles: Exploration of Their Antioxidant, Antibacterial, Antifungal and Cytotoxic Potentials against Cancerous and Normal Cells Compared to Potassium Tellurite. Inorg. Chem. Commun. 2021, 124, 108385. [Google Scholar] [CrossRef]
- Morena, A.G.; Bassegoda, A.; Hoyo, J.; Tzanov, T. Hybrid Tellurium–Lignin Nanoparticles with Enhanced Antibacterial Properties. ACS Appl. Mater. Interfaces 2021, 13, 14885–14893. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Rahimi-Nasrabadi, M.; Pourmasud, S.; Eghbali-Arani, M.; Banafshe, H.R.; Ahmadi, F.; Ganjali, M.R.; Sobhani Nasab, A. CdTe Quantum Dots Prepared Using Herbal Species and Microorganisms and Their Anti-Cancer, Drug Delivery and Antibacterial Applications; a Review. Ceram. Int. 2020, 46, 9979–9989. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, K.; Sharma, M.; Modi, S.K.; Nenavathu, B.P. Novel Tellurium Doped CeO2 Nano Wools as a next Generation Antibacterial Therapeutic Agent. Mater. Chem. Phys. 2023, 307, 128172. [Google Scholar] [CrossRef]
- Gautam, M.; Kim, J.O.; Yong, C.S. Fabrication of Aerosol-Based Nanoparticles and Their Applications in Biomedical Fields. J. Pharm. Investig. 2021, 51, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Dutton, W.A.; Cooper, W.C. The Oxides and Oxyacids of Tellurium. Chem. Rev. 1966, 66, 657–675. [Google Scholar] [CrossRef]
- Avila, D.S.; Soares, A.; Salgueiro, W.G. Toxicology and Pharmacology of Organotellurium Compounds. In Tellurium: Properties, Uses and Research; Grey, D., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017; pp. 137–169. [Google Scholar]
- Sahoo, B.M.; Banik, B.K.; Tiwari, A.; Tiwari, V.; Jain, A.; Borah, P. Synthesis and Application of Organotellurium Compounds. Phys. Sci. Rev. 2023, 8, 4435–4460. [Google Scholar] [CrossRef]
- Tripathi, A.; Khan, A.; Kiran, P.; Shetty, H.; Srivastava, R. Screening of AS101 Analog, Organotellurolate (IV) Compound 2 for Its in Vitro Biocompatibility, Anticancer, and Antibacterial Activities. Amino Acids 2023, 55, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Álvarez, E.; Rácz, B.; Marć, M.A.; Nasim, M.J.; Szemerédi, N.; Viktorová, J.; Jacob, C.; Spengler, G. Selenium and Tellurium in the Development of Novel Small Molecules and Nanoparticles as Cancer Multidrug Resistance Reversal Agents. Drug Resist. Updat. 2022, 63, 100844. [Google Scholar] [CrossRef] [PubMed]
- Grey, D. (Ed.) Tellurium: Properties, Uses, and Research; Chemistry Research and Applications; Nova Science Publishers, Inc.: New York, NY, USA, 2017; ISBN 978-1-5361-0587-2. [Google Scholar]
- Zigman-Hoffman, E.; Sredni, B.; Meilik, B.; Naparstek, E.; Tartakovsky, B. Tellurium Compound Provides Pro-Apoptotic Signaling in Drug Resistant Multiple Myeloma. Leuk. Lymphoma 2021, 62, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Liu, T.; Chen, T. Near-Infrared Laser-Triggered Drug Release in a Tellurium Nanosystem for Simultaneous Chemo-Photothermal Cancer Therapy. Biomater. Sci. 2021, 9, 1767–1778. [Google Scholar] [CrossRef]
- Matharu, R.K.; Charani, Z.; Ciric, L.; Illangakoon, U.E.; Edirisinghe, M. Antimicrobial Activity of Tellurium-loaded Polymeric Fiber Meshes. J. Appl. Polym. Sci. 2018, 135, 46368. [Google Scholar] [CrossRef]
- Zare, B.; Nami, M.; Shahverdi, A.-R. Tracing Tellurium and Its Nanostructures in Biology. Biol. Trace Elem. Res. 2017, 180, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Liu, C.; Li, Y.; Yang, Y.; Li, W.; Feng, C.; Li, L. Ultrathin Tellurium Nanosheets for Simultaneous Cancer Thermo-Chemotherapy. Bioact. Mater. 2022, 13, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Naderi, S.; Zare, H.; Taghavinia, N.; Irajizad, A.; Aghaei, M.; Panjehpour, M. Cadmium Telluride Quantum Dots Induce Apoptosis in Human Breast Cancer Cell Lines. Toxicol. Ind. Health 2018, 34, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Ke, H.; Wang, Q.; Tang, Y.; Deng, Y.; Yang, H.; Yang, X.; Yang, P.; Ling, D.; Chen, C.; et al. Bifunctional Tellurium Nanodots for Photo-Induced Synergistic Cancer Therapy. ACS Nano 2017, 11, 10012–10024. [Google Scholar] [CrossRef] [PubMed]
- Najimi, S.; Shakibaie, M.; Jafari, E.; Ameri, A.; Rahimi, N.; Forootanfar, H.; Yazdanpanah, M.; Rahimi, H.R. Acute and Subacute Toxicities of Biogenic Tellurium Nanorods in Mice. Regul. Toxicol. Pharmacol. 2017, 90, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Missen, O.P.; Etschmann, B.; Mills, S.J.; Sanyal, S.K.; Ram, R.; Shuster, J.; Rea, M.A.D.; Raudsepp, M.J.; Fang, X.-Y.; Lausberg, E.R.; et al. Tellurium Biogeochemical Transformation and Cycling in a Metalliferous Semi-Arid Environment. Geochim. Cosmochim. Acta 2022, 321, 265–292. [Google Scholar] [CrossRef]
- Muthu, A.; Sári, D.; Ferroudj, A.; El-Ramady, H.; Béni, Á.; Badgar, K.; Prokisch, J. Microbial-Based Biotechnology: Production and Evaluation of Selenium-Tellurium Nanoalloys. Appl. Sci. 2023, 13, 11733. [Google Scholar] [CrossRef]
- Blackadder, E.S.; Manderson, W.G. Occupational Absorption of Tellurium: A Report of Two Cases. Occup. Environ. Med. 1975, 32, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Toxocology—International Occupational Safety & Health Information Centre. Available online: https://www.ilo.org/static/english/protection/safework/cis/products/safetytm/toxic.htm (accessed on 17 March 2024).
- Tellurium—ESPI Metals. Available online: https://www.espimetals.com/index.php/msds/285-Tellurium (accessed on 17 March 2024).
- Tellurium Dioxide CAS 7446-07-3|112356. Available online: https://www.emdmillipore.com/US/en/product/Tellurium-dioxide,MDA_CHEM-112356?bd=1 (accessed on 17 March 2024).
- Gerhardsson, L. Tellurium. In Handbook on the Toxicology of Metals; Elsevier: Amsterdam, The Netherlands, 2007; pp. 815–825. ISBN 978-0-12-369413-3. [Google Scholar]
- Hayes, S.M.; Ramos, N.A. Surficial Geochemistry and Bioaccessibility of Tellurium in Semiarid Mine Tailings. Environ. Chem. 2019, 16, 251. [Google Scholar] [CrossRef]
- Kowalczyk, E.; Givelet, L.; Amlund, H.; Sloth, J.J.; Hansen, M. Risk Assessment of Rare Earth Elements, Antimony, Barium, Boron, Lithium, Tellurium, Thallium and Vanadium in Teas. EFSA J. 2022, 20, e200410. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.C.; Srivastava, R.; Srivastava, T.N.; Jain, S.P. Effect of Organo-Tellurium Compounds on the Enzymatic Alterations in Rats. Toxicol. Lett. 1983, 16, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F. Toxicity of Glutathione-Binding Metals: A Review of Targets and Mechanisms. Toxics 2015, 3, 20–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chai, L.; Xie, Z.; Zhang, H. Recent Advance of Tellurium for Biomedical Applications. Chem. Res. Chin. Univ. 2020, 36, 551–559. [Google Scholar] [CrossRef]
- Jia, Y.; Nitz, M. Bio-Incorporation of TePhe, a Tellurium-Containing Phenylalanine Analogue, Preserves Protein Structure & Stability. Available online: https://sciforum.net/manuscripts/10192/slides.pdf (accessed on 5 February 2024).
- Chen, C.; Huang, Z. Tellurium-Modified Nucleosides, Nucleotides, and Nucleic Acids with Potential Applications. Molecules 2022, 27, 8379. [Google Scholar] [CrossRef] [PubMed]
- Wieslander, E.; Engman, L.; Svensjö, E.; Erlansson, M.; Johansson, U.; Linden, M.; Andersson, C.-M.; Brattsand, R. Antioxidative Properties of Organotellurium Compounds in Cell Systems. Biochem. Pharmacol. 1998, 55, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Trindade, C.; Juchem, A.L.M.; Guecheva, T.N.; De Oliveira, I.M.; Dos Santos Silveira, P.; Vargas, J.E.; Puga, R.; Pessoa, C.Ó.; Henriques, J.A.P. Diphenyl Ditelluride: Redox-Modulating and Antiproliferative Properties. Oxid. Med. Cell. Longev. 2019, 2019, 2510936. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Kumar, M.; Bendi, A.; Garg, S. Theoretical and Experimental In-vitro Studies of Novel Thiophene Based Organotellurium(IV) Complexes. Chem. Biodivers. 2024, 21, e202301544. [Google Scholar] [CrossRef] [PubMed]
- Juchem, A.L.M.; Trindade, C.; Da Silva, J.B.; Machado, M.D.S.; Guecheva, T.N.; Rocha, J.C.; Saffi, J.; De Oliveira, I.M.; Henriques, J.A.P.; Escargueil, A. Diphenyl Ditelluride Anticancer Activity and DNA Topoisomerase I Poisoning in Human Colon Cancer HCT116 Cells. Oncotarget 2023, 14, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Takada, S.; Kumagai, K.; Kobayashi, K.; Hokura, A.; Ogra, Y. Elucidation of Tellurium Biogenic Nanoparticles in Garlic, Allium Sativum, by Inductively Coupled Plasma-Mass Spectrometry. J. Trace Elem. Med. Biol. 2020, 62, 126628. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Zschiesche, W.; Steffen, H.M.; Schaller, K.H. Tellurium-Intoxication. Klin. Wochenschr. 1989, 67, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.A.; Buckman, J.; Balassa, J.J. Abnormal Trace Elements in Man: Tellurium. J. Chronic Dis. 1967, 20, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Czapla, M.; Grygoyć, K. Speciation and Fractionation of Less-StudiedTechnology-Critical Elements (Nb, Ta, Ga, In, Ge, Tl, Te): A Review. Pol. J. Environ. Stud. 2021, 30, 1477–1486. [Google Scholar] [CrossRef]
- Filella, M.; Reimann, C.; Biver, M.; Rodushkin, I.; Rodushkina, K. Tellurium in the Environment: Current Knowledge and Identification of Gaps. Environ. Chem. 2019, 16, 215. [Google Scholar] [CrossRef]
- Dickson, R.S.; Glowa, G.A. Tellurium Behaviour in the Fukushima Dai-Ichi Nuclear Power Plant Accident. J. Environ. Radioact. 2019, 204, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Bajpai, S. Accessing the Environmental Impact of Tellurium Metal. Phys. Sci. Rev. 2023, 8, 4903–4913. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, Y.-M.; Wu, D.-D.; Yao, Y.; Liang, Z.-L.; Tan, H.W.; Lau, A.T.Y. Acute and Chronic Cadmium Telluride Quantum Dots-Exposed Human Bronchial Epithelial Cells: The Effects of Particle Sizes on Their Cytotoxicity and Carcinogenicity. Biochem. Biophys. Res. Commun. 2018, 495, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, A.; Fortier, M.; Gagne, F.; Gagnon, C.; Turcotte, P.; Tayabali, A.; Davis, T.L.; Auffret, M.; Fournier, M. Size Distribution Effects of Cadmium Tellurium Quantum Dots (CdS/CdTe) Immunotoxicity on Aquatic Organisms. Environ. Sci. Process. Impacts 2013, 15, 596. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Maysinger, D.; Jain, M.; Röder, B.; Hackbarth, S.; Winnik, F.M. Long-Term Exposure to CdTe Quantum Dots Causes Functional Impairments in Live Cells. Langmuir 2007, 23, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.C.; Seligy, V.L.; Tayabali, A.F. Cadmium Telluride Quantum Dot Nanoparticle Cytotoxicity and Effects on Model Immune Responses to Pseudomonas aeruginosa. Nanotoxicology 2013, 7, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Suminda, G.G.D.; Heo, Y.; Kim, M.; Ghosh, M.; Son, Y.-O. Metal-Based Nanoparticles and Their Relevant Consequences on Cytotoxicity Cascade and Induced Oxidative Stress. Antioxidants 2023, 12, 703. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, M.; Feng, Z.; Xu, X.; Chen, L.; Ma, Z.; Li, L. Biocompatible Tellurium Nanoneedles with Long-Term Stable Antibacterial Activity for Accelerated Wound Healing. Mater. Today Bio 2022, 15, 100271. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Liang, Y.; Wei, T.; Huang, X.; Zhang, T.; Tang, M. Protein Corona Exacerbated Inflammatory Response in Macrophages Elicited by CdTe Quantum Dots. NanoImpact 2024, 33, 100494. [Google Scholar] [CrossRef] [PubMed]
- Pedram Jarf, M.; Kamali, A.; Khara, H.; Pourang, N.; Shekarabi, S.P.H. Microplastic Pollution and Heavy Metal Risk Assessment in Perca Fluviatilis from Anzali Wetland: Implications for Environmental Health and Human Consumption. Sci. Total Environ. 2024, 907, 167978. [Google Scholar] [CrossRef] [PubMed]
- Abidin, A.U.; Maziya, F.B.; Susetyo, S.H.; Yoneda, M.; Matsui, Y. Heavy Metal Air Pollution in an Indonesian Landfill Site: Characterization, Sources, and Health Risk Assessment for Informal Workers. Environ. Adv. 2024, 15, 100512. [Google Scholar] [CrossRef]
- Mwelwa, S.; Chungu, D.; Tailoka, F.; Beesigamukama, D.; Tanga, C.M. Data to Understand the Biotransfer of Heavy Metals along the Soil-Plant-Edible Insect-Human Food Chain in Africa. Data Brief 2023, 49, 109434. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Nandimandalam, J.R. Environmental Health Risk Assessment and Source Apportion of Heavy Metals Using Chemometrics and Pollution Indices in the Upper Yamuna River Basin, India. Chemosphere 2024, 346, 140570. [Google Scholar] [CrossRef] [PubMed]
- Brevik, E.C. Soil, Food Security, and Human Health. In Soils, Plant Growth and Crop Production; Verheye, W.H., Ed.; EOLSS Publishers: Abu Dhabi, United Arab Emirates, 2010; Volume III, pp. 161–195. ISBN 978-1-84826-879-7. [Google Scholar]
- Niu, S.; Colosio, C.; Carugno, M.; Adisesh, A. Diagnostic and Exposure Criteria for Occupational Diseases Guidance Notes for Diagnosis and Prevention of the Diseases in the ILO List of Occupational Diseases (Revised 2010); International Labour Organization (ILO): Geneva, Switzerland, 2022. [Google Scholar]
- Bazaluk, O.; Tsopa, V.; Cheberiachko, S.; Deryugin, O.; Radchuk, D.; Borovytskyi, O.; Lozynskyi, V. Ergonomic Risk Management Process for Safety and Health at Work. Front. Public Health 2023, 11, 1253141. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fresneda, M.A.; Morales-Hidalgo, M.; Povedano-Priego, C.; Jroundi, F.; Hidalgo-Iruela, J.; Cano-Cano, M.; Pérez-Muelas, E.; Merroun, M.L.; Martín-Sanchez, I. Unlocking the Key Role of Bentonite Fungal Isolates in Tellurium and Selenium Bioremediation and Biorecovery: Implications in the Safety of Radioactive Waste Disposal. Sci. Total Environ. 2024, 912, 169242. [Google Scholar] [CrossRef] [PubMed]



| Term(s) | Definition or More Details |
|---|---|
| Nano-materials (NMs) | A material in which at least one dimension of measurement is between 1 and 100 nm. |
| Nano-tellurium | Nano-tellurium or elemental Te-nano-particles (Te-NPs) is the nano form (diameter ranges from 1 to 100 nm) of the Te metalloid that can be found naturally in regolith samples produced by chemical or biological approaches. |
| Te-NP synonyms | Tellurium nano-powder, nano-tellurium, nano-Te |
| Natural nano-materials | A nano-material made naturally through (bio)geochemical or mechanical processes, without a direct or indirect connection to a human activity or anthropogenic process |
| Engineered nano-materials | Chemical substances/materials that are purposely created by humans with particle sizes between 1 and 100 nm in at least one dimension |
| Nano-alloy of tellurium | Produced by combining Te with other elements to create desirable properties. Common examples include combining Te with Se using microbes (e.g., Lactobacillus casei NCAIM B 1147), Pd–Te nano-clusters, CdTe QDs, CdTe/CdS QD nano-sensors, etc. |
| Common tellurium nano-materials | Nano-Te structures, nano-Te wires, nano-Te tubes, nano-Te rods, and nano-Te ribbons |
| Quantum dots (QDs) | Quantum dots (QDs) are semi-conducting nano-crystals with unique optical properties. |
| Cadmium telluride quantum dots (CdTe QDs) | CdTe-based quantum dots (QDs) are colloidal structures that have unique luminescence and electronic properties and are used in diagnostic and biomedical research. CdTe QDs have a low excitation energy and small band gap compared to those of CdS and CdSe materials. |
| Nano-needles | Needles in the nano-size range, used to deliver therapeutics intracellularly. |
| Nano-Te structures | Nano-forms of Te compounds that have a unique van der Waals structure and intriguing chemical and physical properties, such as nano-wires, nano-tubes, nano-cables, belt-shaped structures, etc. |
| Nano-particles (NPs) | Individual particles that range from 1 to 100 nm in diameter. Also sometimes used for nano-wires and nano-tubes. |
| Nano-spheres | Spherical particles with a diameter between 1 and 100 nm. |
| Nano-wires (NWs) | Nano-wires are structures with a diameter of ∼10 nm and a much greater length. |
| Nano-rods (NRs) | Nano-rods have a typical nano-size between 1 and 100 nm with standard aspect ratios (length divided by width) of 3–5. |
| Nano-tubes (NTs) | Nano-tubes are NMs with a cylindrical shape around a hollow center; their diameters typically range from 200 to 600 nm and their lengths from 4 to 15 nm. |
| Nano-ribbons (NRs) | Nano-ribbons are rectangular in shape, very thin with an appreciably greater width, and a length that can be hundreds of nm. Two dimensions are less than 100 nm. |
| Nano-plates | Nano-plates are rectangular in shape with only one dimension in the nano-meter range. |
| Dimensional Te nano-structures | NMs come in a variety of dimensions. Nano-particles are considered zero-dimensional (0D); nano-rods, nano-tubes, and nano-wires are one-dimensional (1D); nano-ribbons are two-dimensional (2D); and flower-like three-dimensional (3D) Te NMs have been created. |
| Physical Parameter(s) | Value |
|---|---|
| Density in solid form at room T | 6.0 (amorphous) and 6.25 (crystalline) g cm−3 |
| Density in liquid form | 5.70 g cm−3 |
| Thermal conductivity | 1.0–3.4 W/(m·K) in a single crystal |
| Electrical resistivity | 1–50 mΩ·m |
| Electron affinity | 1.971 eV or 1.96 eV |
| Electronegativity | 2.1 (Pauling scale), 2.158 (Allen scale) |
| Van der Waals radius | 206 pm |
| Ionization energy | 9.010 eV |
| Band gap energy | 0.35 eV at room temperature |
| Molar volume | 2.05 × 10−5 m3 |
| Crystal structure | Trigonal, orthorhombic, or hexagonal form |
| Atomic radius | 123 pm |
| Covalent radius | 138 pm |
| Atomic number | 52 |
| Melting point | 449.5 °C (amorphous) and 452 °C (crystal) |
| Boiling point | 988 °C (amorphous) and 1390 °C (crystalline) |
| Heat of fusion | 17.5 kJ mol−1 |
| Heat of vaporization | 48 kJ mol−1 |
| Specific heat | 199–219 J/(kg.K) |
| Refractive index | 1.002495 at λ = 589 nm (vapor) 1.26 at 260 nm and 4.5 at 720 nm (thin film) |
| Chemical Parameter(s) | Details |
|---|---|
| Oxidation states | Telluride (Te2−) (−2), elemental (Te0) (0), tellurite (TeO32−) (+4), and tellurate (TeO42−) (+6). Tellurite (TeO32−) (+4) is the most common. |
| Common Te alloys | Bismuth telluride (Bi2Te3) with Se or Sb, mercury cadmium telluride (HgCdTe), cadmium zinc telluride (CdZnTe), etc. |
| Common Te minerals | Calaverite (AuTe2), sylvanite (AgAuTe4), krennerite (AuTe2), nagyagite [AuPb(Sb, Bi)Te2–3S6], and tellurobismuthite (Bi2Te3) |
| Main Te-oxides in crystalline, amorphous, and colloidal forms | Telluride (Te2−), tellurite (TeO32−), tellurate (TeO4−), tellurium dioxide (TeO2), and tellurium trioxide (TeO3) |
| Tellurium hydrides | Hydrogen telluride (H2Te), hydrogen-rich varieties such as H4Te, and H5Te2 |
| Tellurium acids | Tellurous acid (H2TeO3) and telluric acid [Te(OH)6] |
| Tellurium electron conifiguration | [Kr] 5s2 4d10 5p4 |
| Organo-tellurium compounds | Functional group –TeH is called tellurols and they are found in compounds such as dimethyl telluride (Te(CH3)2) and diphenyl ditelluride (C12H10Te2) |
| Isotopes of tellurium | Stable forms are 120Te, 124Te, 125Te, and 126Te |
| Common Te crystal morphologies | n-whiskers, f-whiskers, s-whiskers, etc. |
| Te Compound (s) | Molecular Formula | Molecular Weight | Synonyms |
|---|---|---|---|
| Tellurous acid | H2O3Te | 177.6 g/mol | Dihydrogen trioxotellurate |
| Tellurinic acid | H2O2Te | 161.6 g/mol | Hydridohydroxidooxidotellurium |
| Tellane | H2Te | 129.6 g/mol | Hydrogen telluride |
| Tellurium, mol. | Te2 | 255.2 g/mol | Di tellurium |
| Copper telluride | CuTe | 191.1 g/mol | Copper monotelluride |
| Copper tellurate | CuO4Te | 255.1 g/mol | Copper(2+) tellurium tetraoxide |
| Cuprous telluride | Cu2Te | 254.7 g/mol | Copper (I) telluride |
| Tellurium trioxide | TeO3 | 175.6 g/mol | Tellurium(VI) oxide |
| Tellurium dioxide | TeO2 | 159.6 g/mol | Tellurium oxide |
| Cadmium telluride | CdTe | 240.0 g/mol | Cadmium monotelluride |
| Cadmium tellurate | CdTeO4 | 304.0 g/mol | Cadmium tellurium tetraoxide |
| Bismuth telluride | Bi2Te3 | 800.8 g/mol | Bismuth selenide telluride |
| Mercury telluride | HgTe | 328.2 g/mol | Tellanylidenemercury |
| Dimethyl telluride | C2H6Te | 157.7 g/mol | Dimethyltellurium |
| Diphenyl ditelluride | C12H10Te2 | 409.4 g/mol | Ditelluride, diphenyl |
| Potassium tellurite | K2TeO3 | 253.8 g/mol | Dipotassium trioxotellurate |
| Potassium tellurate | K2TeO4 | 269.8 g/mol | Potassium tellurate(VI) |
| Sodium tellurite | Na2TeO3 | 221.6 g/mol | Disodium trioxotellurate |
| Sodium tellurate | Na2TeO4 | 237.6 g/mol | Disodium tetraoxotellurate |
| Tellurium hexafluoride | F6Te | 241.6 g/mol | Tellurium(VI) fluoride |
| Phenol, 4,4′-tellurobis- | C12H10O2Te | 313.8 g/mol | bis(4-hydroxyphenyl)telluride |
| Diphenyl ditelluride | C12H10Te2 | 409.4 g/mol | Phenyl ditelluride |
| Synthesis Type | Produced by | Nanparticle Features | Main Purpose of the Study | Refs. |
|---|---|---|---|---|
| Biosynthesis | Streptomyces graminisoli. | Crystal shape (12–25 nm) | Antibacterial activity; minimum inhibitory concentration was 50 μg mL−1 | [11] |
| Biogenic method | Penicillium chrysogenum PTCC 5031 | 50.16 nm | Exploited biomolecules and enzymes secreted from P. chrysogenum at room temperature | [42] |
| Biosynthesis of nano-Te rods | Gayadomonas sp. TNPM15 | 15–23 nm | Acted against phytopathogenic fungi by disruption of integrity and membrane permeability of fungal spores | [18] |
| Biogenic Te-NPs | Bacterial marine isolates | Smaller than 100 nm | Antimicrobial activity | [43] |
| Biosynthesized Te-NPs | Lysinibacillus sp. EBL303. | Rod-shaped (22–148 nm) | Bioremediation of tellurite and phenol at polluted sites | [44] |
| Tellurium nano-rods | Shewanella baltica | From 8–75 nm | Reduced methylene blue through photo-catalytic and anti-biofilm activity | [45] |
| Biogenetic nano-Te particles | Mortierella sp. AB1 | From 100–500 nm | Antibacterial against Escherichia coli, Shigella dysenteriae, Salmonella typhimurium, and Enterobacter sakazakii | [46] |
| Biogenic Te-NPs. | Aspergillus welwitschiae | Spherical shape (60.80 nm) | Antibacterial activity against Staphylococcus aureus and E. coli | [47] |
| Biosynthesis of Te-NPs | Biomolecules of gallic acid | 19.74 nm | Multifunctional agents and biomedical applications | [48] |
| Green synthesis of Te-NPs | Allium sativum extract | 350 nm | Evaluation of the cytoprotective and antioxidant activities of Co-Te-NPs | [49] |
| Nano-Te Structures | Common Form | Method of Synthesis | Additional Details |
|---|---|---|---|
| Zero-dimensional Te nano-structures | NPs in a spherical morphology | Green and chemical synthesis or laser ablation in liquids | Sizes of produced Te-NPs depend on solvents used |
| One-dimensional (1D) Te nano-structures | Nano-wires (Te-NWs) | Microwave-assisted synthesis, hydrothermal methods, and vapor–solid method | Te-NWs are controlled by the temperature of reaction, substrate, and growth time |
| Nano-tubes (Te-NTs) | Physical vapor deposition | Te-NTs are controlled by substrate and deposition temperature | |
| Nano-ribbons (Te-NRs) | Hydrothermal and vapor deposition methods | Te-NRs are controlled by pH, temperature, and reaction time | |
| Tellurium nano-rods | Hydrothermal methods | Surfactants can control diameters and lengths of nano-Te rods | |
| Tellurium belt-shaped structures (Te-NPs) | Thermal evaporation and deposition methods | Temperature and ambient atmosphere control NPs | |
| Two-dimensional (2D) nano-Te structure | 2D tellurene lesser layers | Physical vapor deposition and liquid-phase evolution | Temperature and thermodynamics control NPs |
| Three-dimensional (3D) nano-Te structure | Flower-like 3D Te nano-structures | Solvothermal method, dissolution, and recrystallization | Type of solvents (e.g., water, amide, or alcohol) and temperatures control NPs |
| Chiral-shaped Te nano-structures | Chiral nano-materials (NMs) | Using chiral biomolecules as initiators | Chiral NMs are controlled by different synthetic conditions |
| Te-Alloys | Molecular Formula | Main Findings | Refs. |
|---|---|---|---|
| Alloys from sodium, yttrium, sulfur, and tellurium | NaYS2(1−x)Te2x alloys (with x = 0, 0.33, 0.67, and 1) | These alloys are potential light energy converters and considered attractive for photovoltaic applications | [64] |
| Alloys of tellurium fluorides | Te-F binary system | Stable Te-fluorides (TeF4, TeF6, and TeF8,) support strong d–p covalent interactions in the Te-F system at high pressure | [65] |
| Iridium–tellurium alloy | IrTe | Can promote adsorption of N2 and lower the Gibbs free energy for electrocatalytic N2 reduction reactions | [66] |
| Bismuth–telluride alloy | Bi2Te3 | This alloy has good performance in thermoelectric materials near room temperature | [67] |
| Cesium–tellurium–titanium alloy | Cs2Te1−xTixI6 | This alloy possesses large absorption coefficients in the visible light region as stable, eco-friendly and high-efficiency light absorbers used in optoelectronic applications | [68] |
| Rubidium–tin–tellurium alloy | Rb2Sn1−xTexI6 | Promising alloy using the Sn–Te mixture as a potential substitute for lead in photovoltaic materials | [69] |
| Tellurium-embedded carbon nano-fibers | Te@C-NF electrode | Poly-tellurides and K2Te-embedded carbon nano-fibers are high-rate and long-life electrodes for high-energy-storage materials | [70] |
| Potassium–tellurium battery system | K-Te | Converting Te to K2Te3 and ultimately to K5Te3 in a carbonate electrolyte-based K-Te battery system to promote and develop high-energy-density K-S/Se/Te batteries | [71] |
| Potassium–tellurium battery system | K-Te | Utilizes biochar from mangosteen shell in a hierarchical porous host to Te during K+ storage in K-Te battery | [72] |
| Amorphous selenium (a-Se)–tellurium alloy | Se-Te alloys | Improving quantum efficiency and conversion efficiencies for a-Se1−xTex (x = 0, 0.03, 0.05, 0.08) devices as a function of applied field, along with different band gaps in Se-Te alloys | [73] |
| Comparison Item | Bulk-Tellurium | Nano-Tellurium |
|---|---|---|
| Main common forms | Soluble oxyanionic forms | Natural and engineered nano-particles (NPs) |
| Abundance | 1–5 ppb in Earth’s crust 0.008–0.03 ppm in soil 15 ppb in seawater Around 0.27 ppm in plants | >4 ppm in the regolith depending on weathering of Te-ores >100 ppm in hotspots |
| Essentiality | Non-essential | Not confirmed yet |
| Exposure pathway(s) | Food (ingestion) followed by inhalation and then dermal. | Bioavailability of Te-NPs through dermal absorption, ingestion, or inhalation. |
| Foodstuff exposure and human daily intake | Dairy products, meat, and cereals; in general, there is <1 mg Te kg−1 in food and humans should not exceed an intake of >0.1 mg of Te day−1 | Depends on natural or engineered NPs and their properties |
| Main sources of exposure | Mainly Cd-Te in solar panels and from copper mining refineries | Cd-Te-quantum dots (QDs) and other nano-alloys of tellurium |
| Main applications | Te can be used as an alloy for Peltier devices, phase change optical magnetic disks, and solar panels | Alloys of Te with selenium, cadmium, zinc, and other metals can be used to produce NMs such as QDs |
| Mobility in the environment | Tellurium is a mobile element in the environment (mainly mining) | Te-NPs may transport similarly to other natural nano-materials like Au-NPs |
| Suggestied mechanisms to enhance human health | Boosts antioxidant defenses, acts as pro-oxidants, generates ROS, and induces apoptosis | Exerts antioxidant, lipid-lowering, and free radical scavenging activities; can be used as antitumor and chemopreventive agents |
| Toxicity (established) | Low concentrations of Te species are toxic | Elemental (Te0) is non-toxic to organisms |
| Toxicity (exposure dose) | TeIVO32-(aq) toxic to microbes at about ~1 mg L−1 (4 μM) | Bio-Te-NPs caused toxicity to Pseudomonas pseudoalcaligenes in mice at 6 mg kg−1 |
| Occupational exposure limits | Threshold limit value (TLV): 0.1 mg m−3 as 8 h total weighted average (TWA) | Not yet known |
| Toxicity (sources) | Highly toxic forms: tellurite, IV (TeO32−) and tellurate, VI (TeO66−) | Chemical Te-NPs are generally more toxic than biological or green forms |
| Toxicity (forms) | Organo-Te compounds are generally less toxic compared to mineral forms | Generally, the common toxic nano-form is Cd-Te QDs depending on size of NPs |
| Median lethal dose (LD50)—oral | K2TeO3 caused complete toxicity at 12.5 mg kg−1 in mice | Biogenic nano-Te rods had acute toxicity at 60 mg kg−1 in mice |
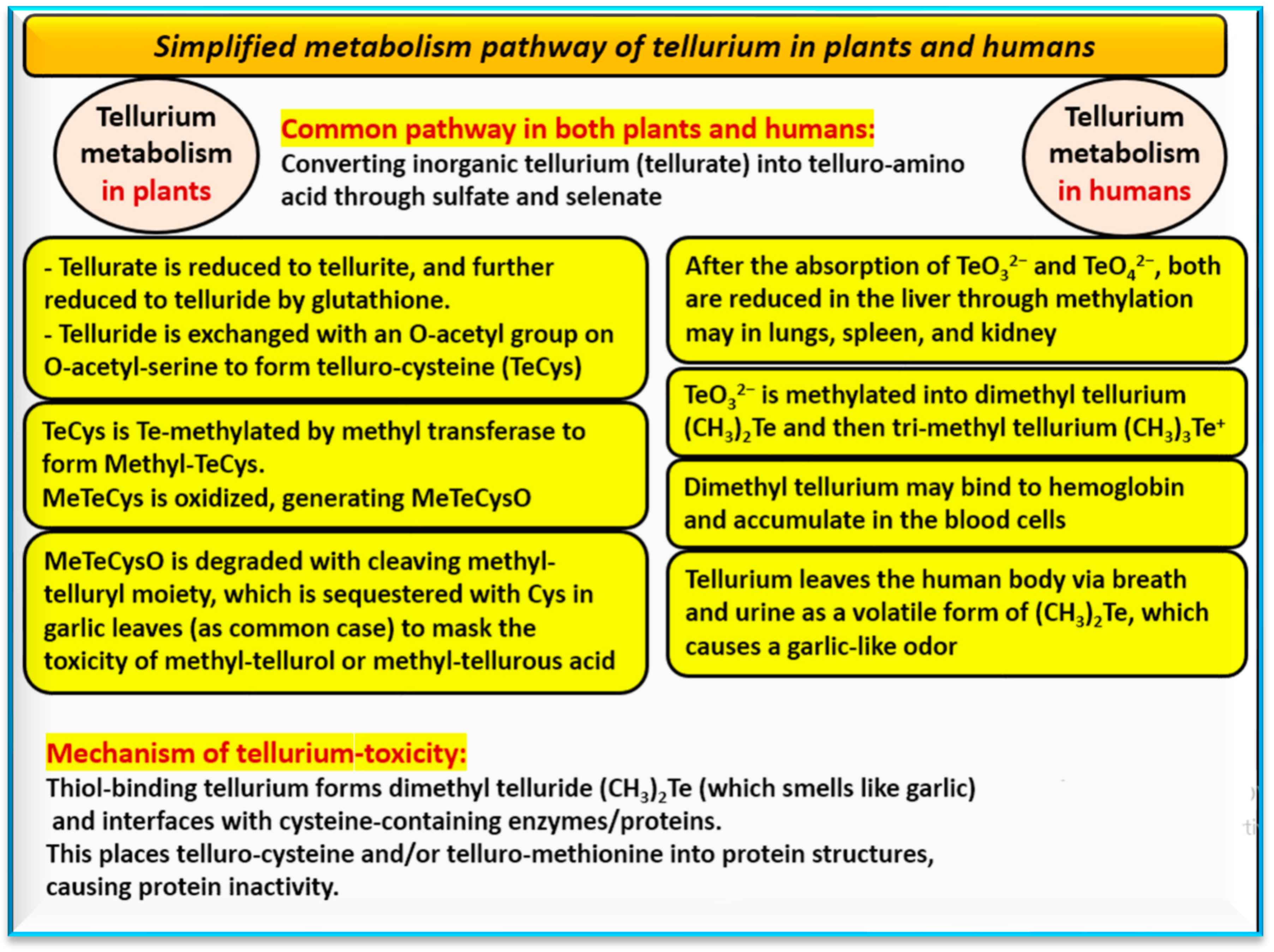
| Metabolic pathway | Reduces TeO32− and TeO42− in the liver, methylates to (CH3)2Te and (CH3)3Te)+, binds to hemoglobin, accumulates in the blood cells in humans | In general, bio-Te-NPs are insoluble in plants, depending on type of nano-tellurium (e.g., TeO2-NPs, TeO2-NP–acetic acid, and TeO2-NP–gallic acid) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sári, D.; Ferroudj, A.; Semsey, D.; El-Ramady, H.; Brevik, E.C.; Prokisch, J. Tellurium and Nano-Tellurium: Medicine or Poison? Nanomaterials 2024, 14, 670. https://doi.org/10.3390/nano14080670
Sári D, Ferroudj A, Semsey D, El-Ramady H, Brevik EC, Prokisch J. Tellurium and Nano-Tellurium: Medicine or Poison? Nanomaterials. 2024; 14(8):670. https://doi.org/10.3390/nano14080670
Chicago/Turabian StyleSári, Daniella, Aya Ferroudj, Dávid Semsey, Hassan El-Ramady, Eric C. Brevik, and József Prokisch. 2024. "Tellurium and Nano-Tellurium: Medicine or Poison?" Nanomaterials 14, no. 8: 670. https://doi.org/10.3390/nano14080670
APA StyleSári, D., Ferroudj, A., Semsey, D., El-Ramady, H., Brevik, E. C., & Prokisch, J. (2024). Tellurium and Nano-Tellurium: Medicine or Poison? Nanomaterials, 14(8), 670. https://doi.org/10.3390/nano14080670










