Structural and Electronic Properties of Thiophene-Based Supramolecular Architectures: Influence of the Underlying Metal Surfaces
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
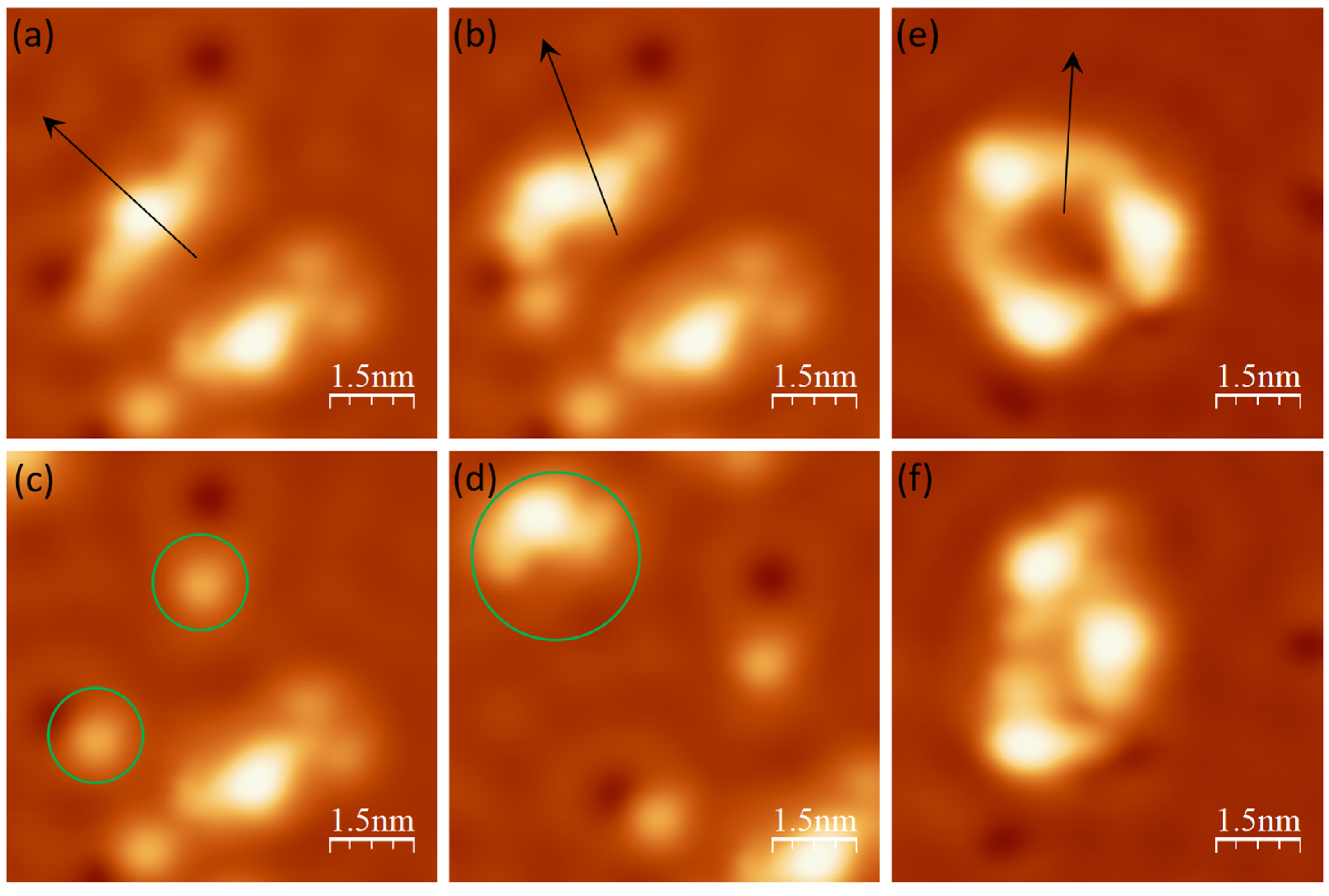
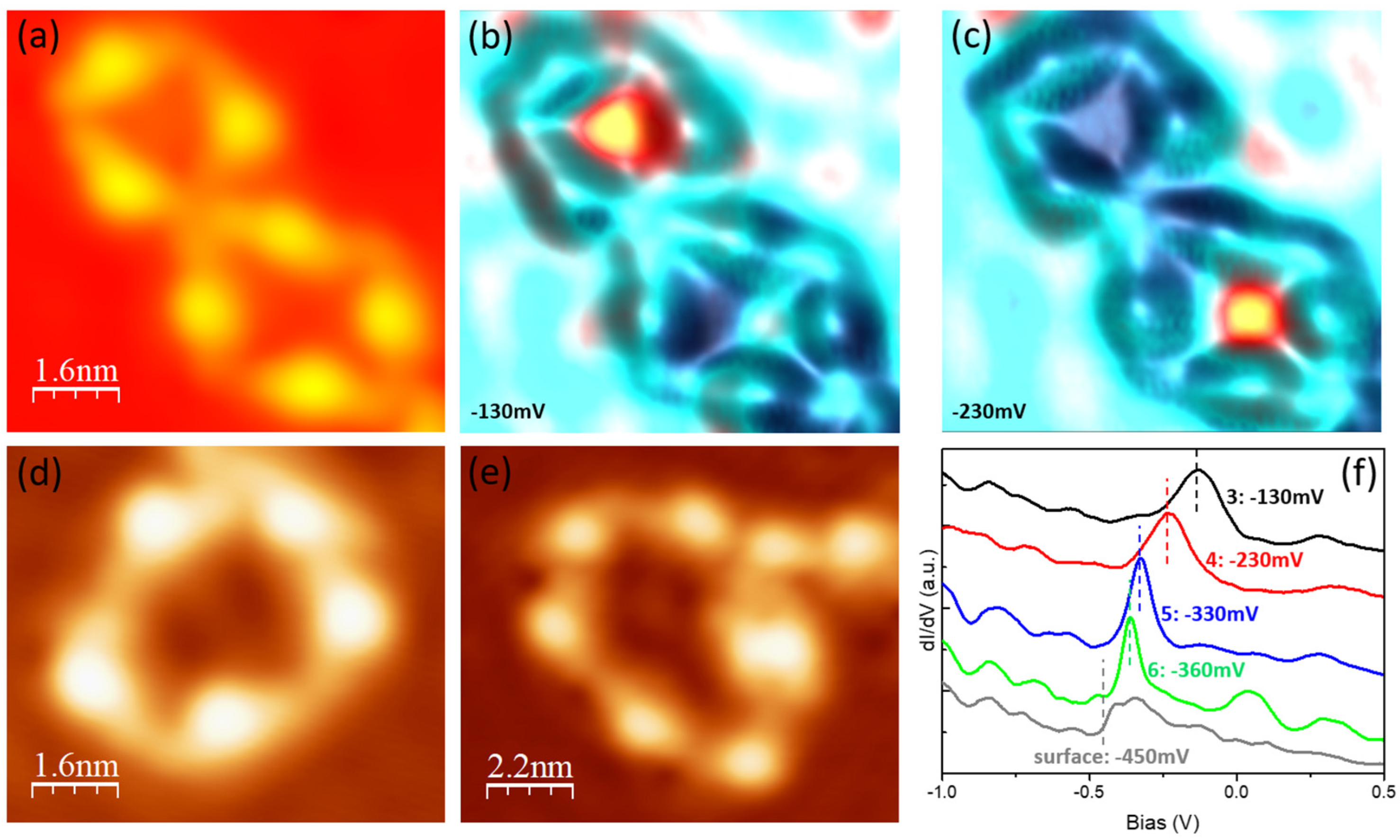
3.1. Assembled Structures and Electronic Properties on Au(111)
3.2. Assembled Structures and Electronic Properties on Ag(111)
3.3. Assembled Structures and Electronic Properties of Cu(111)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, R.H.; Mark, A.R. Molecular Electronics. Phys. Today 2003, 56, 43–49. [Google Scholar]
- Xiang, D.; Wang, X.; Jia, C.; Lee, T.; Guo, X. Molecular-Scale Electronics: From Concept to Function. Chem. Rev. 2016, 116, 4318–4440. [Google Scholar] [CrossRef]
- Li, T.; Bandari, V.K.; Schmidt, O.G. Molecular Electronics: Creating and Bridging Molecular Junctions and Promoting Its Commercialization. Adv. Mater. 2023, 35, e2209088. [Google Scholar] [CrossRef]
- Xin, N.; Guan, J.; Zhou, C.; Chen, X.; Gu, C.; Li, Y.; Ratner, M.A.; Nitzan, A.; Stoddart, J.F.; Guo, X. Concepts in the design and engineering of single-molecule electronic devices. Nat. Rev. Phys. 2019, 1, 211–230. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhong, J.Q.; Lin, J.D.; Hu, W.P.; Wu, K.; Xu, G.Q.; Wee, A.T.S.; Chen, W. Towards single molecule switches. Chem. Soc. Rev. 2015, 44, 2998–3022. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Fereiro, J.A.; Bayat, A.; Pritam, A.; Zharnikov, M.; Mondal, P.C. Nanoscale molecular rectifiers. Nat. Rev. Chem. 2023, 7, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Miao, X. Surface-supported metal-organic frameworks with geometric topological diversity via scanning tunneling microscopy. Iscience 2024, 27, 109392. [Google Scholar] [CrossRef]
- Piquero-Zulaica, I.; Lobo-Checa, J.; El-Fattah, Z.M.A.; Ortega, J.E.; Klappenberger, F.; Auwärter, W.; Barth, J.V. Engineering quantum states and electronic landscapes through surface molecular nanoarchitectures. Rev. Mod. Phys. 2022, 94, 045008. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Bu, D.; Cai, J.; Chen, H.; Chen, Q.; Chen, T.; Cheng, F.; Chi, L.; Dong, W.; et al. Recent progress on surface chemistry II: Property and characterization. Chin. Chem. Lett. 2024, 36, 110100. [Google Scholar] [CrossRef]
- Stone, I.; Starr, R.L.; Zang, Y.; Nuckolls, C.; Steigerwald, M.L.; Lambert, T.H.; Roy, X.; Venkataraman, L. A single-molecule blueprint for synthesis. Nat. Rev. Chem. 2021, 5, 695–710. [Google Scholar] [CrossRef]
- Hao, X.; Yang, H.; Niu, M.; Wang, T.; Ji, H.; Brumboiu, I.E.; Grazioli, C.; Guarnaccio, A.; Cossaro, A.; Li, Y.; et al. From Chains to Arrays: Substrate-Mediated Self-Assembly of Diboron Molecules. Nanomaterials 2024, 14, 1952. [Google Scholar] [CrossRef] [PubMed]
- Wegner, D.; Yamachika, R.; Wang, Y.; Brar, V.W.; Bartlett, B.M.; Long, J.R.; Crommie, M.F. single-molecule charge transfer and bonding at an organic/inorganic interface: Tetracyanoethylene on noble metals. Nano Lett. 2008, 8, 131–135. [Google Scholar] [CrossRef]
- Yuan, C.; Xue, N.; Zhang, Y.; Li, N.; Shen, Z.; Hou, S.; Wang, Y. Controlling Metal-Organic Structure by Tuning Molecular Size, Supported Substrate, and Type of Metal. J. Clust. Sci. 2020, 32, 327–330. [Google Scholar] [CrossRef]
- Piquero-Zulaica, I.; Hu, W.; Seitsonen, A.P.; Haag, F.; Küchle, J.; Allegretti, F.; Lyu, Y.; Chen, L.; Wu, K.; El-Fattah, Z.M.A.; et al. Unconventional Band Structure via Combined Molecular Orbital and Lattice Symmetries in a Surface-Confined Metallated Graphdiyne Sheet. Adv. Mater. 2024, 36, e2405178. [Google Scholar] [CrossRef]
- Li, S.-W.; Zhang, R.-X.; Kang, L.-X.; Li, D.-Y.; Xie, Y.-L.; Wang, C.-X.; Liu, P.-N. Steering Metal–Organic Network Structures through Conformations and Configurations on Surfaces. ACS Nano 2021, 15, 18014–18022. [Google Scholar] [CrossRef] [PubMed]
- Piquero-Zulaica, I.; Li, J.; El-Fattah, Z.M.A.; Solianyk, L.; Gallardo, I.; Monjas, L.; Hirsch, A.K.H.; Arnau, A.; Ortega, J.E.; Stöhr, M.; et al. Surface state tunable energy and mass renormalization from homothetic quantum dot arrays. Nanoscale 2019, 11, 23132–23138. [Google Scholar] [CrossRef]
- Klappenberger, F.; Kühne, D.; Krenner, W.; Silanes, I.; Arnau, A.; de Abajo, F.J.G.; Klyatskaya, S.; Ruben, M.; Barth, J.V. tunable quantum dot arrays formed from self-assembled metal-organic networks. Phys. Rev. Lett. 2011, 106, 026802. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Jiang, Z.; Wang, Y.; Hou, S. Electronic confining effects in Sierpiński triangle fractals. Phys. Rev. B 2018, 97, 115451. [Google Scholar] [CrossRef]
- Colazzo, L.; Mohammed, M.S.G.; Gallardo, A.; El-Fattah, Z.M.A.; Pomposo, J.A.; Jelínek, P.; de Oteyza, D.G. Controlling the stereospecific bonding motif of Au-thiolate links. Nanoscale 2019, 11, 15567–15575. [Google Scholar] [CrossRef]
- Yamachika, R.; Grobis, M.; Wachowiak, A.; Crommie, M.F. Controlled atomic doping of a single C60 molecule. Science 2004, 304, 281–284. [Google Scholar] [CrossRef]
- Wang, W.; Hong, Y.; Shi, X.; Minot, C.; Van Hove, M.A.; Tang, B.Z.; Lin, N. Inspecting Metal-Coordination-Induced Perturbation of Molecular Ligand Orbitals at a Submolecular Resolution. J. Phys. Chem. Lett. 2011, 1, 2295–2298. [Google Scholar] [CrossRef]
- Wang, W.; Shi, X.; Wang, S.; Liu, J.; Van Hove, M.A.; Liu, P.N.; Zhang, R.-Q.; Lin, N. Cooperative Modulation of Electronic Structures of Aromatic Molecules Coupled to Multiple Metal Contacts. Phys. Rev. Lett. 2013, 110, 046802. [Google Scholar] [CrossRef]
- Henningsen, N.; Rurali, R.; Limbach, C.; Drost, R.; Pascual, J.I.; Franke, K.J. Site-Dependent Coordination Bonding in Self-Assembled Metal-Organic Networks. J. Phys. Chem. Lett. 2010, 2, 55–61. [Google Scholar] [CrossRef]
- Albrecht, F.; Neu, M.; Quest, C.; Swart, I.; Repp, J. Formation and characterization of a molecule–metal–molecule bridge in real space. J. Am. Chem. Soc. 2013, 135, 9200–9203. [Google Scholar] [CrossRef] [PubMed]
- Nazin, G.V.; Qiu, X.H.; Ho, W. Visualization and spectroscopy of a metal-molecule-metal bridge. Science 2003, 302, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, X.; Lin, C.; Zhang, R.Q.; Minot, C.; Van Hove, M.A.; Hong, Y.; Tang, B.Z.; Lin, N. Manipulating Localized Molecular Orbitals by Single-Atom Contacts. Phys. Rev. Lett. 2010, 105, 126801. [Google Scholar] [CrossRef]
- Yang, Z.; Lotze, C.; Corso, M.; Baum, S.; Franke, K.J.; Pascual, J.I. Direct Imaging of the Induced-Fit Effect in Molecular Self-Assembly. Small 2019, 15, e1804713. [Google Scholar] [CrossRef]
- Fitzner, R.; Reinold, E.; Mishra, A.; Mena-Osteritz, E.; Ziehlke, H.; Körner, C.; Leo, K.; Riede, M.; Weil, M.; Tsaryova, O.; et al. Dicyanovinyl–Substituted Oligothiophenes: Structure-Property Relationships and Application in Vacuum-Processed Small Molecule Organic Solar Cells. Adv. Funct. Mater. 2011, 21, 897–910. [Google Scholar] [CrossRef]
- Haid, S.; Mishra, A.; Uhrich, C.; Pfeiffer, M.; Bäuerle, P. Dicyanovinylene-Substituted Selenophene–Thiophene Co-oligomers for Small-Molecule Organic Solar Cells. Chem. Mater. 2011, 23, 4435–4444. [Google Scholar] [CrossRef]
- Mishra, A.; Uhrich, C.; Reinold, E.; Pfeiffer, M.; Bäuerle, P. Synthesis and Characterization of Acceptor-Substituted Oligothiophenes for Solar Cell Applications. Adv. Energy Mater. 2011, 1, 265–273. [Google Scholar] [CrossRef]
- Mishra, A.; Bäuerle, P. Small molecule organic semiconductors on the move: Promises for future solar energy technology. Angew. Chem. Int. Ed. 2012, 51, 2020–2067. [Google Scholar] [CrossRef]
- Schrader, M.; Fitzner, R.; Hein, M.; Elschner, C.; Baumeier, B.; Leo, K.; Riede, M.; Bäuerle, P.; Andrienko, D. Comparative study of microscopic charge dynamics in crystalline acceptor-substituted oligothiophenes. J. Am. Chem. Soc. 2012, 134, 6052–6056. [Google Scholar] [CrossRef] [PubMed]
- Bogner, L.; Yang, Z.; Corso, M.; Fitzner, R.; Bäuerle, P.; Franke, K.J.; Pascual, J.I.; Tegeder, P. Electronic structure and excited state dynamics in a dicyanovinyl-substituted oligothiophene on Au(111). Phys. Chem. Chem. Phys. 2015, 17, 27118–27126. [Google Scholar] [CrossRef] [PubMed]
- Bogner, L.; Yang, Z.; Baum, S.; Corso, M.; Fitzner, R.; Bäuerle, P.; Franke, K.J.; Pascual, J.I.; Tegeder, P. Electronic States and Exciton Dynamics in Dicyanovinyl-Sexithiophene on Au(111). J. Phys. Chem. C 2016, 120, 27268–27275. [Google Scholar] [CrossRef]
- Yang, Z.; Lotze, C.; Franke, K.J.; Pascual, J.I. Metal–Organic Superlattices Induced by Long-Range Repulsive Interactions on a Metal Surface. J. Phys. Chem. C 2021, 125, 18494–18500. [Google Scholar] [CrossRef]
- Yang, Z.; Corso, M.; Robles, R.; Lotze, C.; Fitzner, R.; Mena-Osteritz, E.; Bäuerle, P.; Franke, K.J.; Pascual, J.I. Orbital redistribution in molecular nanostructures mediated by metal-organic bonds. ACS Nano 2014, 8, 10715–10722. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodriguez, J.M.; Colchero, J.; Gomez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 13705. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, T.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision C.02; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Torrente, I.F.; Franke, K.J.; Pascual, J.I. Structure and electronic configuration of tetracyanoquinodimethane layers on a Au(111) surface. Int. J. Mass Spectrom. 2008, 277, 269–273. [Google Scholar] [CrossRef]
- Gonzalez-Lakunza, N.; Cañas-Ventura, M.E.; Ruffieux, P.; Rieger, R.; Müllen, K.; Fasel, R.; Arnau, A. Hydrogen-Bonding Fingerprints in Electronic States of Two-Dimensional Supramolecular Assemblies. Chemphyschem 2009, 10, 2943–2946. [Google Scholar] [CrossRef]
- Kröger, J.; Jensen, H.; Berndt, R.; Rurali, R.; Lorente, N. Molecular orbital shift of perylenetetracarboxylic-dianhydride on gold. Chem. Phys. Lett. 2007, 438, 249–253. [Google Scholar] [CrossRef]
- Riss, A.; Wickenburg, S.; Gorman, P.; Tan, L.Z.; Tsai, H.-Z.; de Oteyza, D.G.; Chen, Y.-C.; Bradley, A.J.; Ugeda, M.M.; Etkin, G.; et al. Local Electronic and Chemical Structure of Oligo-acetylene Derivatives Formed Through Radical Cyclizations at a Surface. Nano Lett. 2014, 14, 2251–2255. [Google Scholar] [CrossRef] [PubMed]
- Skriver, H.L.; Rosengaard, N.M. Surface energy and work function of elemental metals. Phys. Rev. B 1992, 46, 7157–7168. [Google Scholar] [CrossRef] [PubMed]
- Repp, J.; Meyer, G.; Stojković, S.M.; Gourdon, A.; Joachim, C. Molecules on insulating films: Scanning-tunneling microscopy imaging of individual molecular orbitals. Phys. Rev. Lett. 2005, 94, 026803. [Google Scholar] [CrossRef] [PubMed]
- Stroscio, J.A.; Eigler, D.M. Atomic and molecular manipulation with the scanning tunneling microscope. Science 1991, 254, 1319–1326. [Google Scholar] [CrossRef]
- Manoharan, H.C.; Lutz, C.P.; Eigler, D.M. Quantum mirages formed by coherent projection of electronic structure. Nature 2000, 403, 512–515. [Google Scholar] [CrossRef]
- Crommie, M.F.; Lutz, C.P.; Eigler, D.M. Confinement of electrons to quantum corrals on a metal surface. Science 1993, 262, 218–220. [Google Scholar] [CrossRef]
- Xia, S.; Xu, X.; Tian, Y.; Zhong, J.; Yang, Z. Protected electronic structures of unoccupied molecular orbitals in metal-organic contacts. Appl. Surf. Sci. 2025, 688, 162367. [Google Scholar] [CrossRef]
- Walch, H.; Dienstmaier, J.; Eder, G.; Gutzler, R.; Schlögl, S.; Sirtl, T.; Das, K.; Schmittel, M.; Lackinger, M. Extended Two-Dimensional Metal–Organic Frameworks Based on Thiolate–Copper Coordination Bonds. J. Am. Chem. Soc. 2011, 133, 7909–7915. [Google Scholar] [CrossRef]
- Vitali, L.; Levita, G.; Ohmann, R.; Comisso, A.; De Vita, A.; Kern, K. Portrait of the potential barrier at metal–organic nanocontacts. Nat. Mater. 2010, 9, 320–323. [Google Scholar] [CrossRef]
- Kyriakou, G.; Boucher, M.B.; Jewell, A.D.; Lewis, E.A.; Lawton, T.J.; Baber, A.E.; Tierney, H.L.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Isolated Metal Atom Geometries as a Strategy for Selective Heterogeneous Hydrogenations. Science 2012, 335, 1209–1212. [Google Scholar] [CrossRef]
- Leo, G.; Letizia, S.; André, G.; Christian, J.; Rieder, K.-H.; Joachim, C. Scattering of surface state electrons at large organic molecules. Phys. Rev. Lett. 2004, 93, 056103. [Google Scholar]
- Crommie, M.F.; Lutz, C.P.; Eigler, D.M. Imaging standing waves in a two-dimensional electron gas. Nature 1993, 363, 524–527. [Google Scholar] [CrossRef]
- Seufert, K.; Auwärter, W.; de Abajo, F.J.G.; Ecija, D.; Vijayaraghavan, S.; Joshi, S.; Barth, J.V. Controlled Interaction of Surface Quantum-Well Electronic States. Nano Lett. 2013, 13, 6130–6135. [Google Scholar] [CrossRef] [PubMed]
- Pennec, Y.; Auwärter, W.; Schiffrin, A.; Weber-Bargioni, A.; Riemann, A.; Barth, J.V. Supramolecular gratings for tuneable confinement of electrons on metal surfaces. Nat. Nanotechnol. 2007, 2, 99–103. [Google Scholar] [CrossRef]
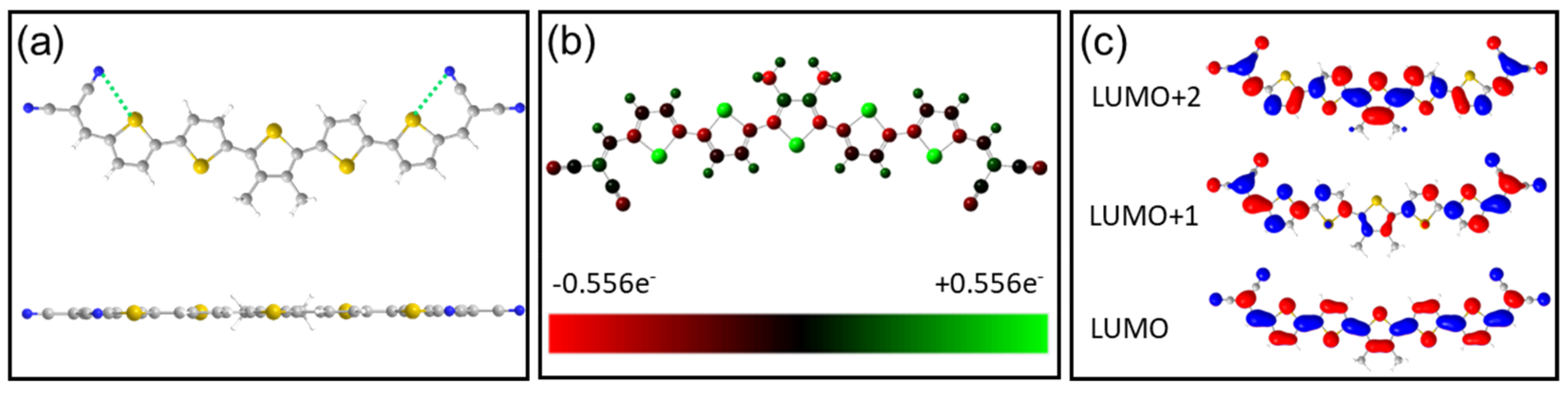
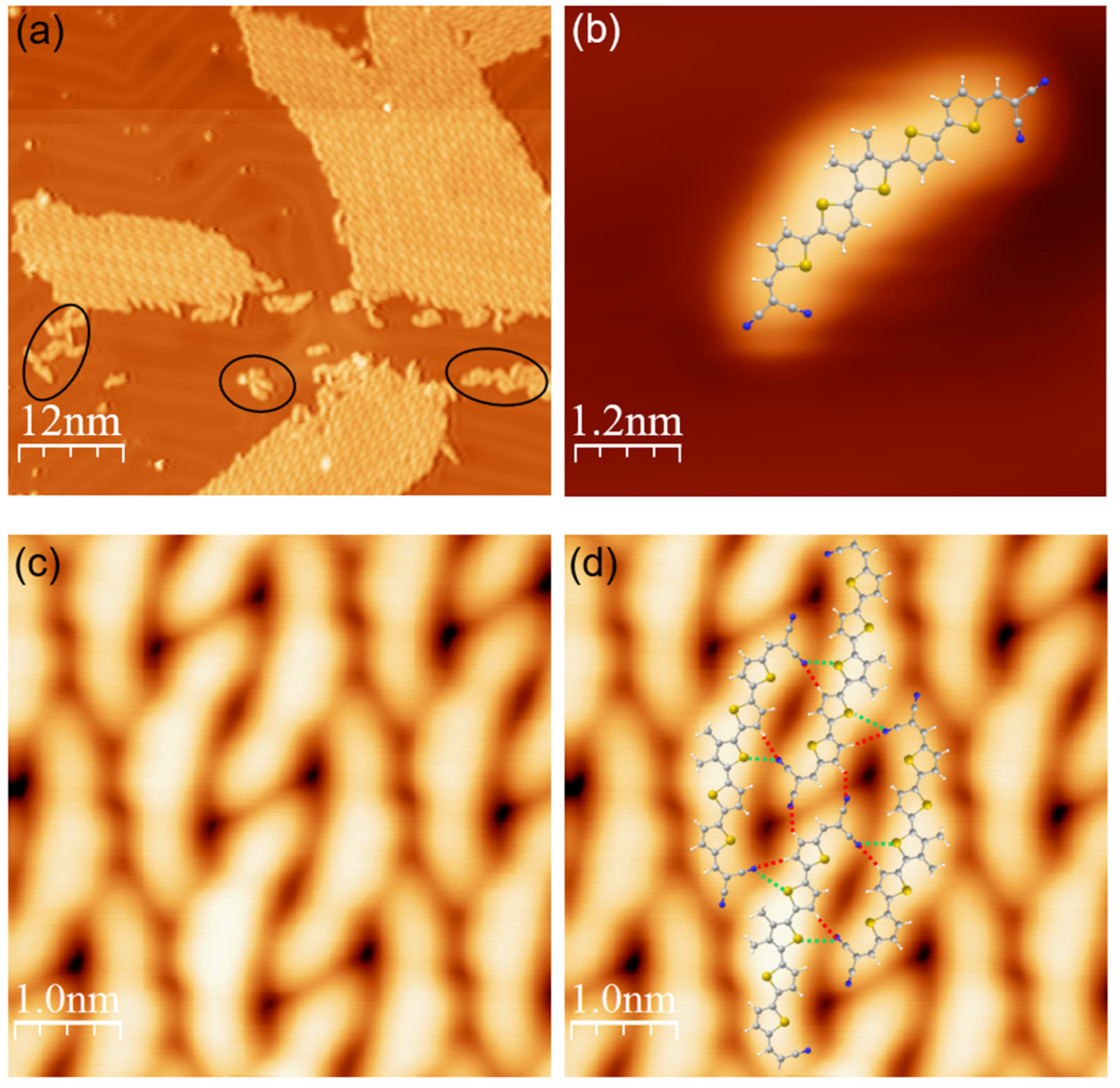
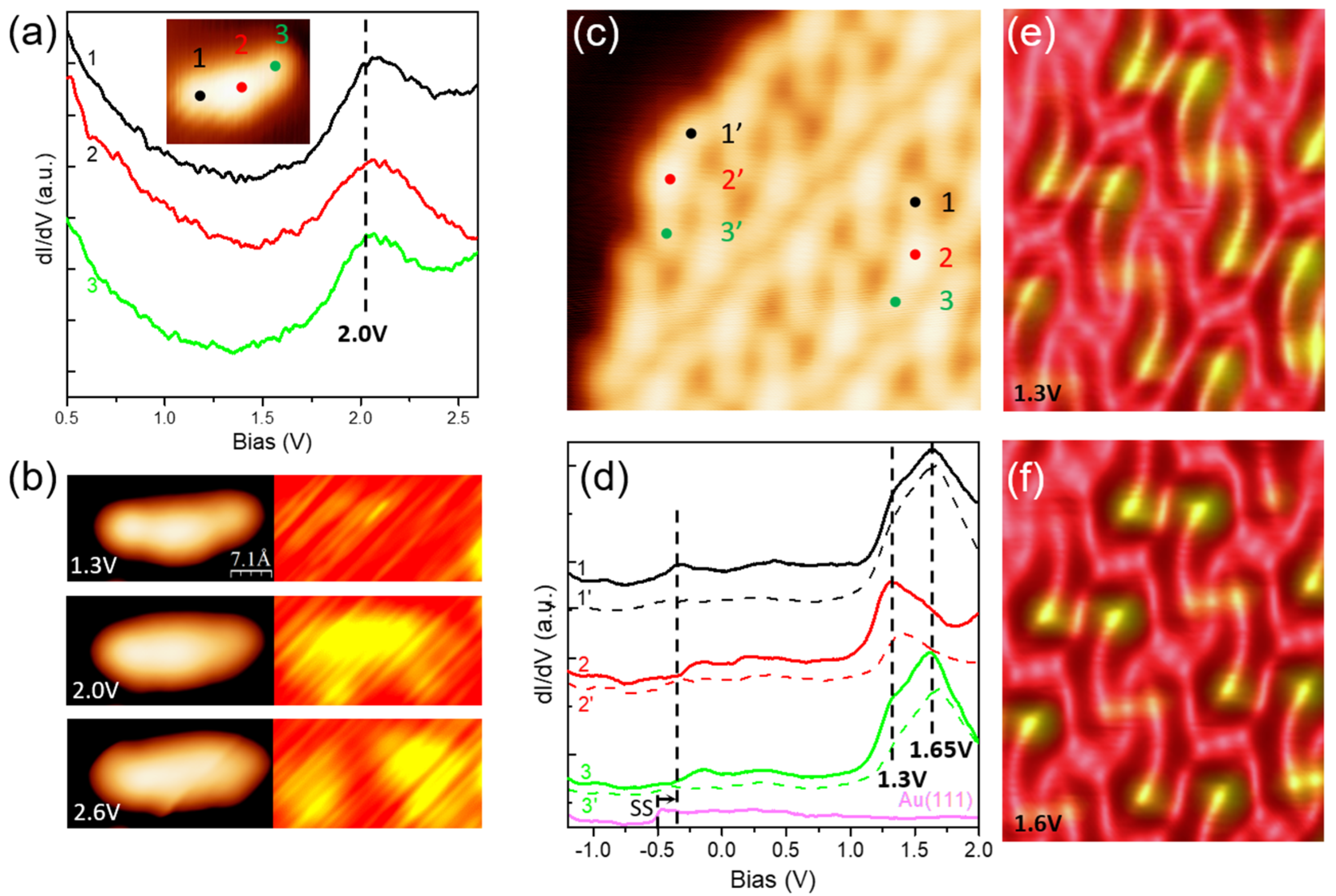


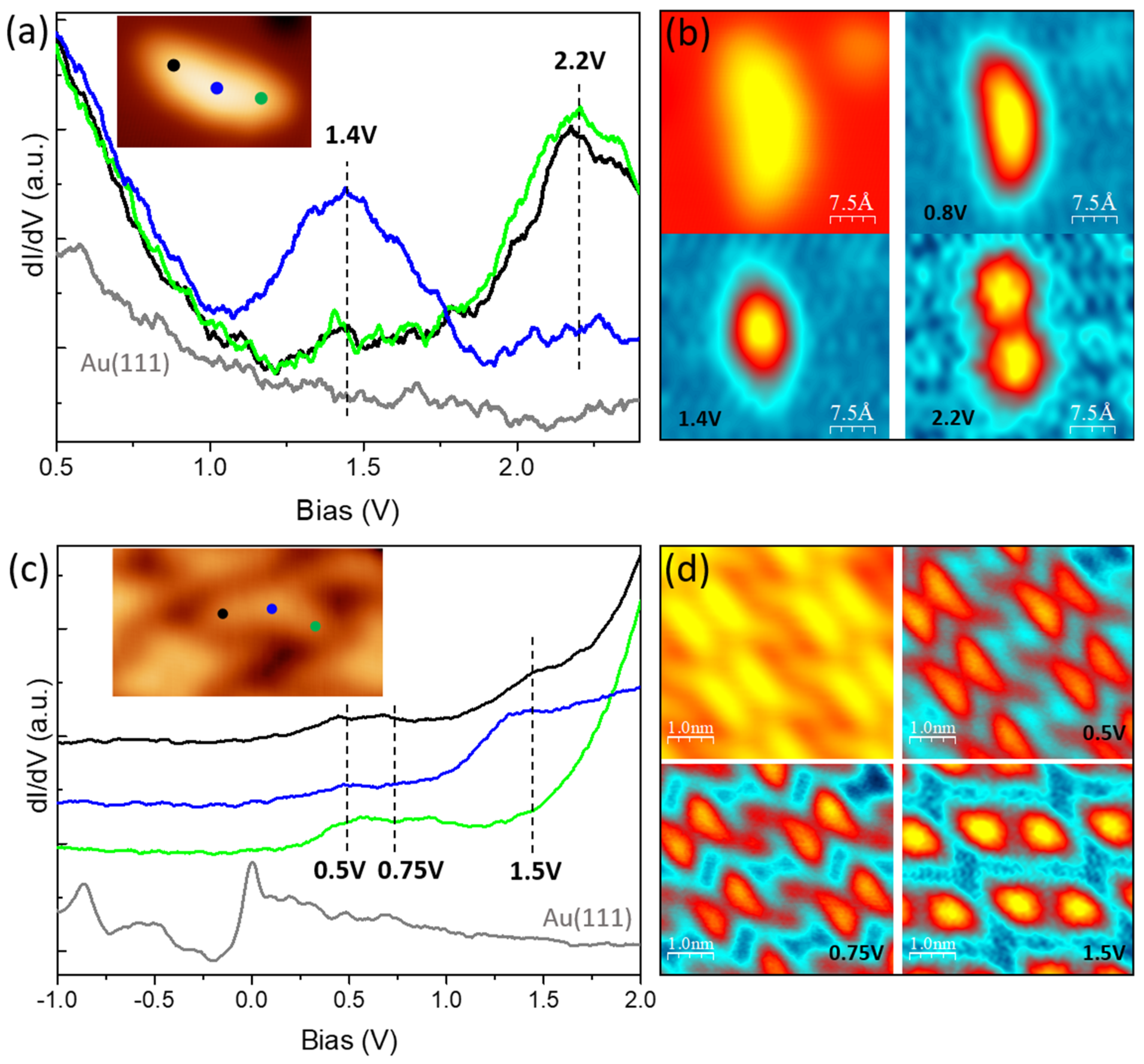


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, L.; Tian, Y.; Lu, H.; Xia, S.; Xu, X.; Yang, Z. Structural and Electronic Properties of Thiophene-Based Supramolecular Architectures: Influence of the Underlying Metal Surfaces. Nanomaterials 2025, 15, 572. https://doi.org/10.3390/nano15080572
Kang L, Tian Y, Lu H, Xia S, Xu X, Yang Z. Structural and Electronic Properties of Thiophene-Based Supramolecular Architectures: Influence of the Underlying Metal Surfaces. Nanomaterials. 2025; 15(8):572. https://doi.org/10.3390/nano15080572
Chicago/Turabian StyleKang, Lixia, Yao Tian, Hui Lu, Shunze Xia, Xianfei Xu, and Zechao Yang. 2025. "Structural and Electronic Properties of Thiophene-Based Supramolecular Architectures: Influence of the Underlying Metal Surfaces" Nanomaterials 15, no. 8: 572. https://doi.org/10.3390/nano15080572
APA StyleKang, L., Tian, Y., Lu, H., Xia, S., Xu, X., & Yang, Z. (2025). Structural and Electronic Properties of Thiophene-Based Supramolecular Architectures: Influence of the Underlying Metal Surfaces. Nanomaterials, 15(8), 572. https://doi.org/10.3390/nano15080572





