Explore the Structural and Electronic Properties at the Organic/Organic Interfaces of Thiophene-Based Supramolecular Architectures
Abstract
1. Introduction
2. Methods
3. Results and Discussion
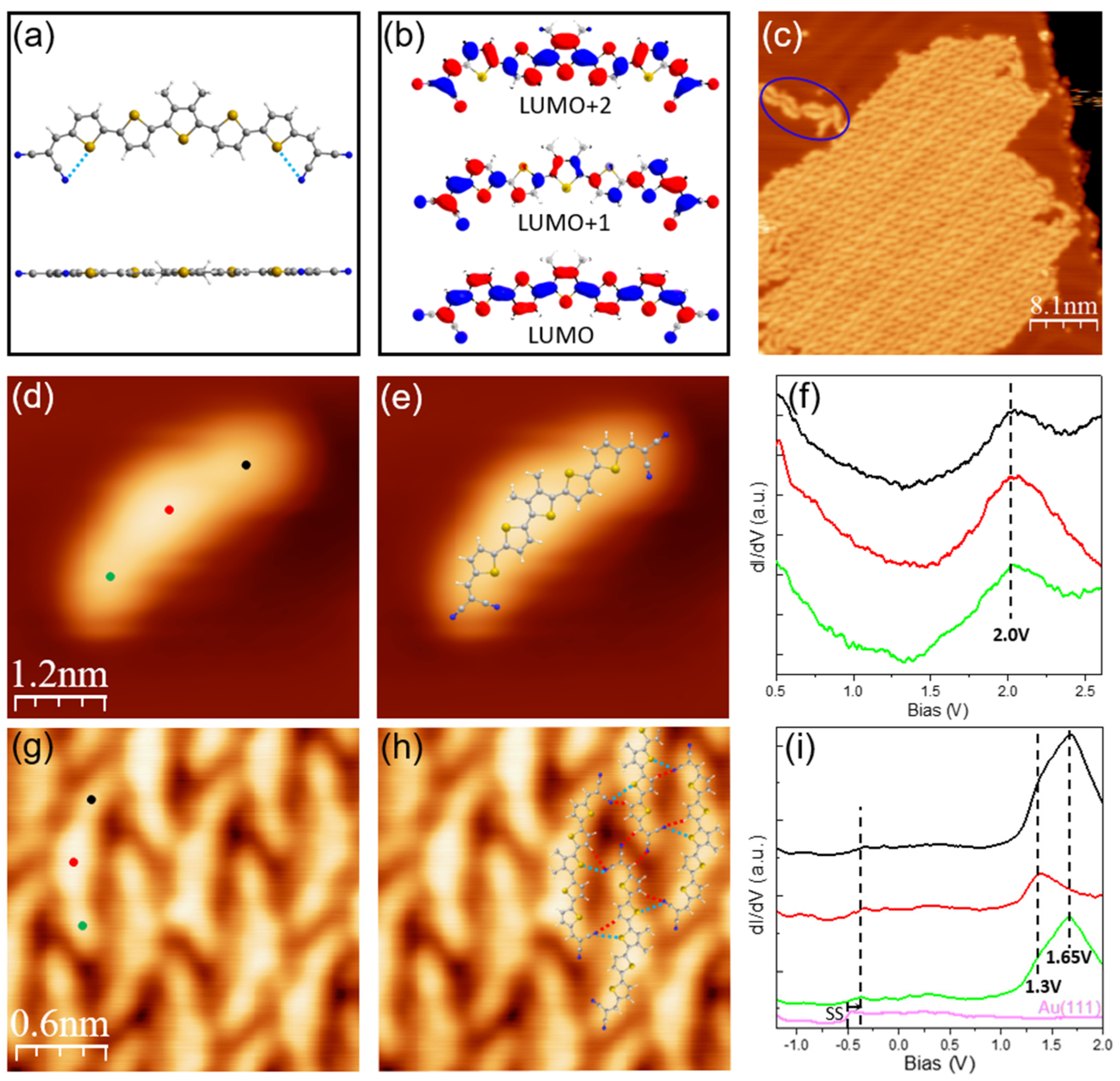
3.1. Structural and Electronic Properties of DCV5T-Me2: From Sub-Monolayer to Bilayer
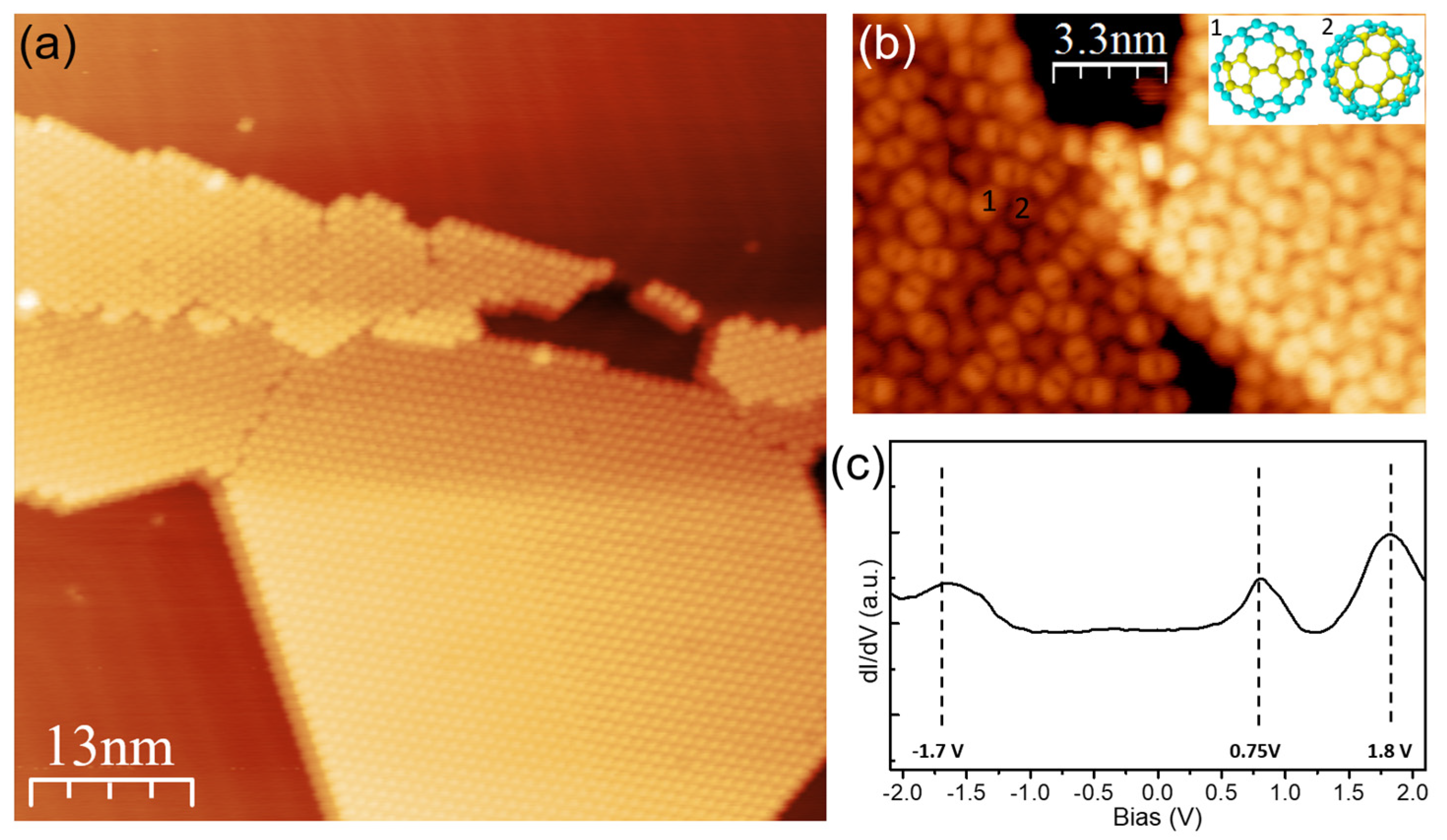
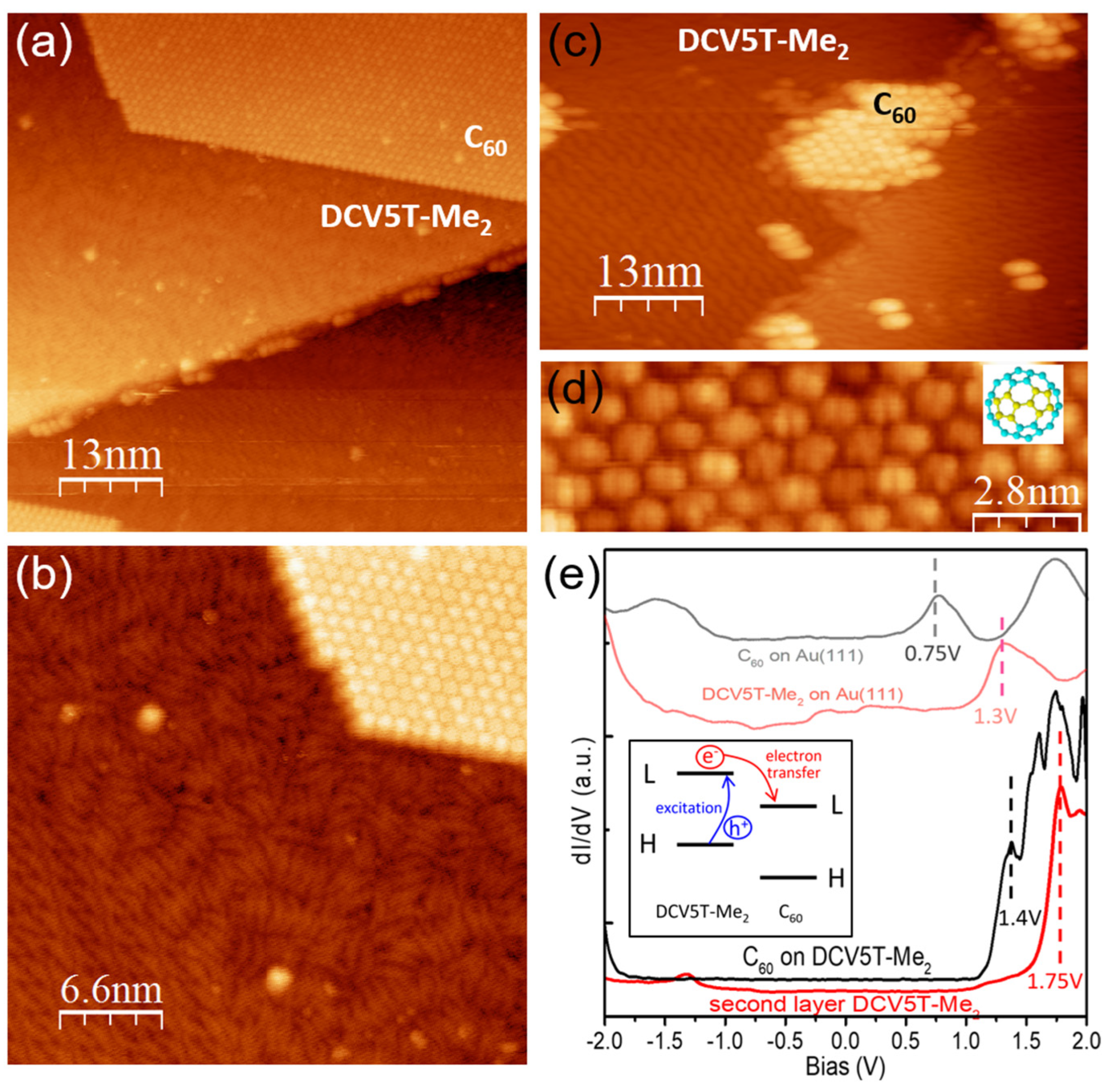
3.2. Structural and Electronic Properties at DCV5T-Me2/C60 Interface
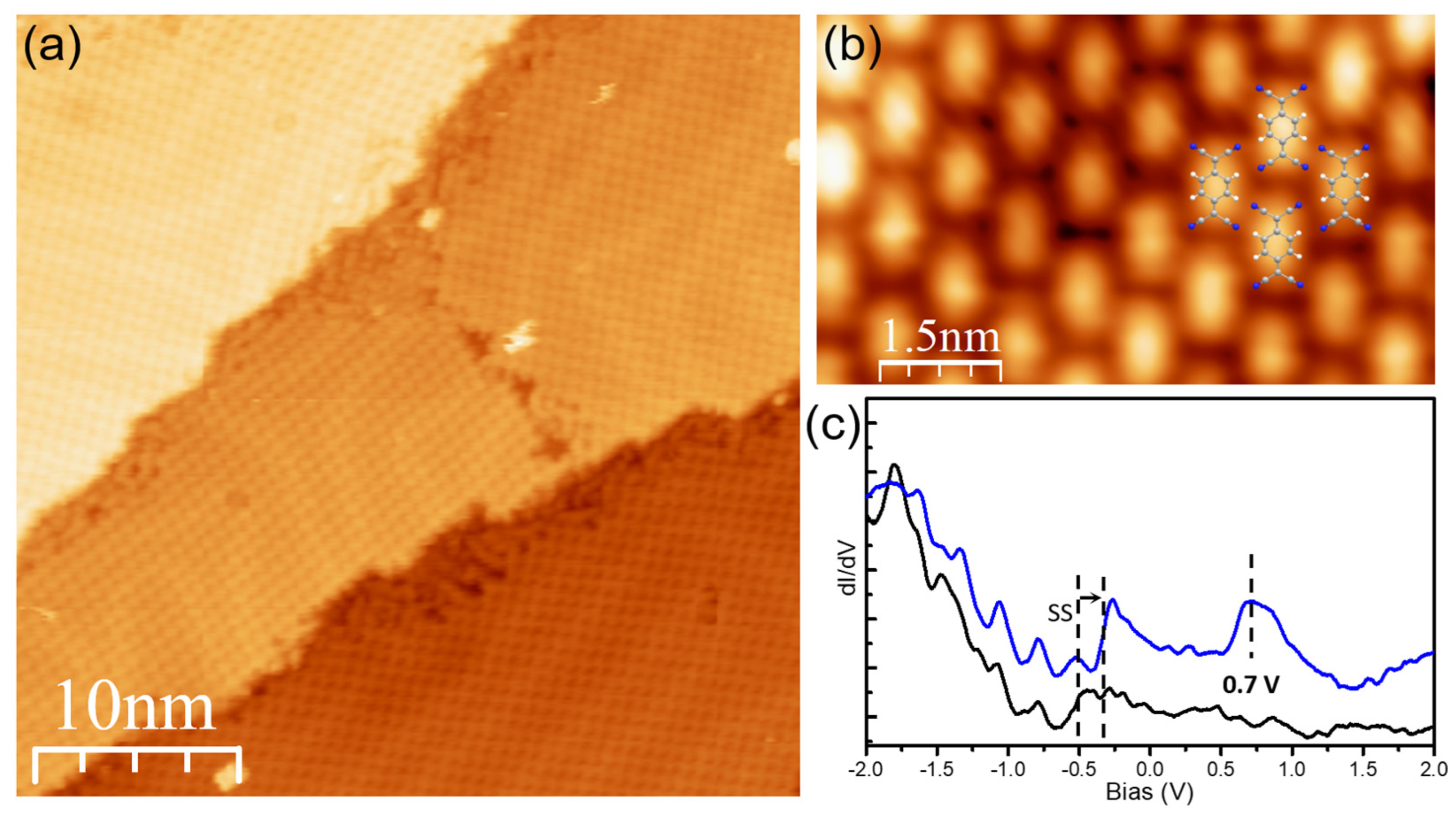
3.3. Structural and Electronic Properties at DCV5T-Me2/TCNQ Interface
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, R.; Fereiro, J.A.; Bayat, A.; Pritam, A.; Zharnikov, M.; Mondal, P.C. Nanoscale molecular rectifiers. Nat. Rev. Chem. 2023, 7, 106–122. [Google Scholar] [CrossRef]
- Heath, J.R.; Ratner, M.A. Molecular Electronics. Phys. Today 2003, 56, 43–49. [Google Scholar] [CrossRef]
- Li, T.; Bandari, V.K.; Schmidt, O.G. Molecular Electronics: Creating and Bridging Molecular Junctions and Promoting Its Commercialization. Adv. Mater. 2023, 35, e2209088. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Z.; Bu, D.; Cai, J.; Chen, H.; Chen, Q.; Chen, T.; Cheng, F.; Chi, L.; Dong, W.; et al. Recent progress on surface chemistry II: Property and characterization. Chin. Chem. Lett. 2024, 36, 110100. [Google Scholar] [CrossRef]
- Xiang, D.; Wang, X.; Jia, C.; Lee, T.; Guo, X. Molecular-Scale Electronics: From Concept to Function. Chem. Rev. 2016, 116, 4318–4440. [Google Scholar] [CrossRef]
- Xin, N.; Guan, J.X.; Zhou, C.G.; Chen, X.J.N.; Gu, C.H.; Li, Y.; Ratner, M.A.; Nitzan, A.; Stoddart, J.F.; Guo, X.F. Concepts in the design and engineering of single-molecule electronic devices. Nat. Rev. Phys. 2019, 1, 211–230. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhong, J.Q.; Lin, J.D.; Hu, W.P.; Wu, K.; Xu, G.Q.; Wee, A.T.S.; Chen, W. Towards single molecule switches. Chem. Soc. Rev. 2015, 44, 2998–3022. [Google Scholar] [CrossRef]
- Stone, I.; Starr, R.L.; Zang, Y.P.; Nuckolls, C.; Steigerwald, M.L.; Lambert, T.H.; Roy, X.; Venkataraman, L. A single-molecule blueprint for synthesis. Nat. Rev. Chem. 2021, 5, 695–710. [Google Scholar] [CrossRef]
- Albrecht, F.; Neu, M.; Quest, C.; Swart, I.; Repp, J. Formation and characterization of a molecule-metal-molecule bridge in real space. J. Am. Chem. Soc. 2013, 135, 9200–9203. [Google Scholar] [CrossRef]
- Björk, J.; Matena, M.; Dyer, M.S.; Enache, M.; Lobo-Checa, J.; Gade, L.H.; Jung, T.A.; Stöhr, M.; Persson, M. STM fingerprint of molecule-adatom interactions in a self-assembled metal-organic surface coordination network on Cu(111). Phys. Chem. Chem. Phys. 2010, 12, 8815–8821. [Google Scholar] [CrossRef]
- Calzolari, A.; Ruini, A.; Catellani, A. Anchor group versus conjugation: Toward the gap-state engineering of functionalized ZnO(1010) surface for optoelectronic applications. J. Am. Chem. Soc. 2011, 133, 5893–5899. [Google Scholar] [CrossRef]
- Colazzo, L.; Mohammed, M.S.G.; Gallardo, A.; Abd El-Fattah, Z.M.; Pomposo, J.A.; Jelinek, P.; de Oteyza, D.G. Controlling the stereospecific bonding motif of Au-thiolate links. Nanoscale 2019, 11, 15567–15575. [Google Scholar] [CrossRef]
- Li, S.W.; Zhang, R.X.; Kang, L.X.; Li, D.Y.; Xie, Y.L.; Wang, C.X.; Liu, P.N. Steering Metal-Organic Network Structures through Conformations and Configurations on Surfaces. ACS Nano 2021, 15, 18014–18022. [Google Scholar] [CrossRef] [PubMed]
- Piquero-Zulaica, I.; Lobo-Checa, J.; Abd El-Fattah, Z.M.; Ortega, J.E.; Klappenberger, F.; Auwärter, W.; Barth, J.V. Engineering quantum states and electronic landscapes through surface molecular nanoarchitectures. Rev. Mod. Phys. 2022, 94, 045008. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Miao, X.R. Surface-supported metal-organic frameworks with geometric topological diversity via scanning tunneling microscopy. Iscience 2024, 27, 109392. [Google Scholar] [CrossRef] [PubMed]
- Fitzner, R.; Reinold, E.; Mishra, A.; Mena-Osteritz, E.; Ziehlke, H.; Körner, C.; Leo, K.; Riede, M.; Weil, M.; Tsaryova, O.; et al. Dicyanovinyl-Substituted Oligothiophenes: Structure-Property Relationships and Application in Vacuum-Processed Small-Molecule Organic Solar Cells. Adv. Funct. Mater. 2011, 21, 897–910. [Google Scholar] [CrossRef]
- Haid, S.; Mishra, A.; Uhrich, C.; Pfeiffer, M.; Bäuerle, P. Dicyanovinylene-Substituted Selenophene-Thiophene Co-oligomers for Small-Molecule Organic Solar Cells. Chem. Mater. 2011, 23, 4435–4444. [Google Scholar] [CrossRef]
- Mishra, A.; Bauerle, P. Small molecule organic semiconductors on the move: Promises for future solar energy technology. Angew. Chem. Int. Ed. Engl. 2012, 51, 2020–2067. [Google Scholar] [CrossRef]
- Mishra, A.; Uhrich, C.; Reinold, E.; Pfeiffer, M.; Bäuerle, P. Synthesis and Characterization of Acceptor-Substituted Oligothiophenes for Solar Cell Applications. Adv. Energy Mater. 2011, 1, 265–273. [Google Scholar] [CrossRef]
- Schrader, M.; Fitzner, R.; Hein, M.; Elschner, C.; Baumeier, B.; Leo, K.; Riede, M.; Bauerle, P.; Andrienko, D. Comparative study of microscopic charge dynamics in crystalline acceptor-substituted oligothiophenes. J. Am. Chem. Soc. 2012, 134, 6052–6056. [Google Scholar] [CrossRef]
- Yang, Z.; Corso, M.; Robles, R.; Lotze, C.; Fitzner, R.; Mena-Osteritz, E.; Bauerle, P.; Franke, K.J.; Pascual, J.I. Orbital redistribution in molecular nanostructures mediated by metal-organic bonds. ACS Nano 2014, 8, 10715–10722. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.C.; Lotze, C.; Corso, M.; Baum, S.; Franke, K.J.; Pascual, J.I. Direct Imaging of the Induced-Fit Effect in Molecular Self-Assembly. Small 2019, 15, 1804713. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.C.; Lotze, C.; Franke, K.J.; Pascual, J.I. Metal-Organic Superlattices Induced by Long-Range Repulsive Interactions on a Metal Surface. J. Phys. Chem. C 2021, 125, 18494–18500. [Google Scholar] [CrossRef]
- Xia, S.Z.; Xu, X.F.; Tian, Y.; Zhong, J.Q.; Yang, Z.C. Protected electronic structures of unoccupied molecular orbitals in metal-organic contacts. Appl. Surf. Sci. 2025, 688, 162367. [Google Scholar] [CrossRef]
- Horcas, I.; Fernandez, R.; Gomez-Rodriguez, J.M.; Colchero, J.; Gomez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision C.02; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Bogner, L.; Yang, Z.; Corso, M.; Fitzner, R.; Bäuerle, P.; Franke, K.J.; Pascual, J.I.; Tegeder, P. Electronic structure and excited state dynamics in a dicyanovinyl-substituted oligothiophene on Au(111). Phys. Chem. Chem. Phys. 2015, 17, 27118–27126. [Google Scholar] [CrossRef]
- Torrente, I.F.; Franke, K.J.; Pascual, J.I. Structure and electronic configuration of tetracyanoquinodimethane layers on a Au(111) surface. Int. J. Mass. Spectrom. 2008, 277, 269–273. [Google Scholar] [CrossRef]
- Gonzalez-Lakunza, N.; Cañas-Ventura, M.E.; Ruffieux, P.; Rieger, R.; Müllen, K.; Fasel, R.; Arnau, A. Hydrogen-Bonding Fingerprints in Electronic States of Two-Dimensional Supramolecular Assemblies. Chemphyschem 2009, 10, 2943–2946. [Google Scholar] [CrossRef]
- Kröger, J.; Jensen, H.; Berndt, R.; Rurali, R.; Lorente, N. Molecular orbital shift of perylenetetracarboxylic-dianhydride on gold. Chem. Phys. Lett. 2007, 438, 249–253. [Google Scholar] [CrossRef]
- Piquero-Zulaica, I.; Li, J.; Abd El-Fattah, Z.M.; Solianyk, L.; Gallardo, I.; Monjas, L.; Hirsch, A.K.H.; Arnau, A.; Ortega, J.E.; Stohr, M.; et al. Surface state tunable energy and mass renormalization from homothetic quantum dot arrays. Nanoscale 2019, 11, 23132–23138. [Google Scholar] [CrossRef]
- Klappenberger, F.; Kuhne, D.; Krenner, W.; Silanes, I.; Arnau, A.; Garcia de Abajo, F.J.; Klyatskaya, S.; Ruben, M.; Barth, J.V. Tunable quantum dot arrays formed from self-assembled metal-organic networks. Phys. Rev. Lett. 2011, 106, 026802. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Jiang, Z.; Wang, Y.; Hou, S. Electronic confining effects in Sierpiński triangle fractals. Phys. Rev. B 2018, 97, 115451. [Google Scholar] [CrossRef]
- Yang, Z. Structural and Electronic Properties of Thiophene-based Supramolecular Architectures on Metal Surfaces. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2014. [Google Scholar]
- Fartash, A. Dielectric properties of orientationally ordered/disordered C60(111) films. Phys. Rev. B 1996, 54, 17215–17222. [Google Scholar] [CrossRef] [PubMed]
- Schull, G.; Berndt, R. Orientationally ordered (7 × 7) superstructure of C60 on AU(111). Phys. Rev. Lett. 2007, 99, 226105. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.F.; Yang, J.; Wang, H.; Zeng, C.; Li, Q.; Wang, B.; Hou, J.G.; Zhu, Q.; Chen, D.M. Low-temperature orientationally ordered structures of two-dimensional C60. J. Am. Chem. Soc. 2003, 125, 169–172. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Huang, H.; Chen, L.; Wee, A.T. Orientationally ordered C60 on p-sexiphenyl nanostripes on Ag111. ACS Nano 2008, 2, 693–698. [Google Scholar] [CrossRef]
- Franke, K.J.; Schulze, G.; Henningsen, N.; Fernandez-Torrente, I.; Pascual, J.I.; Zarwell, S.; Ruck-Braun, K.; Cobian, M.; Lorente, N. Reducing the molecule-substrate coupling in C60-based nanostructures by molecular interactions. Phys. Rev. Lett. 2008, 100, 036807. [Google Scholar] [CrossRef]
- Fernandez-Torrente, I.; Kreikemeyer-Lorenzo, D.; Strozecka, A.; Franke, K.J.; Pascual, J.I. Gating the charge state of single molecules by local electric fields. Phys. Rev. Lett. 2012, 108, 036801. [Google Scholar] [CrossRef]
- Jackel, F.; Perera, U.G.; Iancu, V.; Braun, K.F.; Koch, N.; Rabe, J.P.; Hla, S.W. Investigating molecular charge transfer complexes with a low temperature scanning tunneling microscope. Phys. Rev. Lett. 2008, 100, 126102. [Google Scholar] [CrossRef]
- Fernandez-Torrente, I.; Franke, K.J.; Pascual, J.I. Vibrational Kondo effect in pure organic charge-transfer assemblies. Phys. Rev. Lett. 2008, 101, 217203. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, L.; Lu, H.; Xia, S.; Xu, X.; Tian, Y.; Yang, Z. Explore the Structural and Electronic Properties at the Organic/Organic Interfaces of Thiophene-Based Supramolecular Architectures. Nanomaterials 2025, 15, 601. https://doi.org/10.3390/nano15080601
Kang L, Lu H, Xia S, Xu X, Tian Y, Yang Z. Explore the Structural and Electronic Properties at the Organic/Organic Interfaces of Thiophene-Based Supramolecular Architectures. Nanomaterials. 2025; 15(8):601. https://doi.org/10.3390/nano15080601
Chicago/Turabian StyleKang, Lixia, Hui Lu, Shunze Xia, Xianfei Xu, Yao Tian, and Zechao Yang. 2025. "Explore the Structural and Electronic Properties at the Organic/Organic Interfaces of Thiophene-Based Supramolecular Architectures" Nanomaterials 15, no. 8: 601. https://doi.org/10.3390/nano15080601
APA StyleKang, L., Lu, H., Xia, S., Xu, X., Tian, Y., & Yang, Z. (2025). Explore the Structural and Electronic Properties at the Organic/Organic Interfaces of Thiophene-Based Supramolecular Architectures. Nanomaterials, 15(8), 601. https://doi.org/10.3390/nano15080601





