Lateral Flow Immunoassay Coupled with Copper Enhancement for Rapid and Sensitive SARS-CoV-2 Nucleocapsid Protein Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Preparation of GCNPs
2.3. Synthesis of GCNP-Antibody I Detection Probes
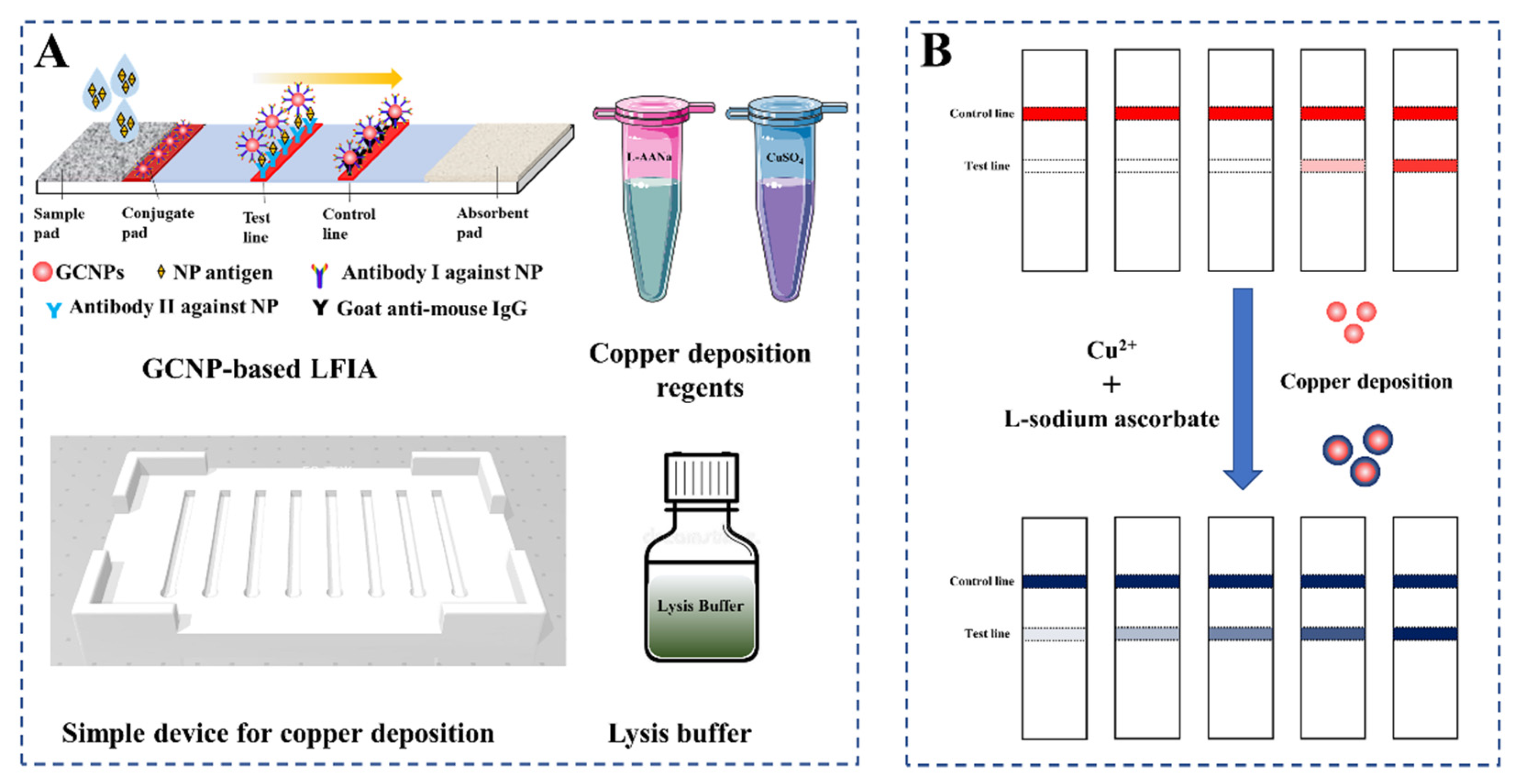
2.4. Preparation of LFIA Test Strips
2.5. Copper Deposition-Induced Signal Amplification on the LFIA Strip
3. Results and Discussion
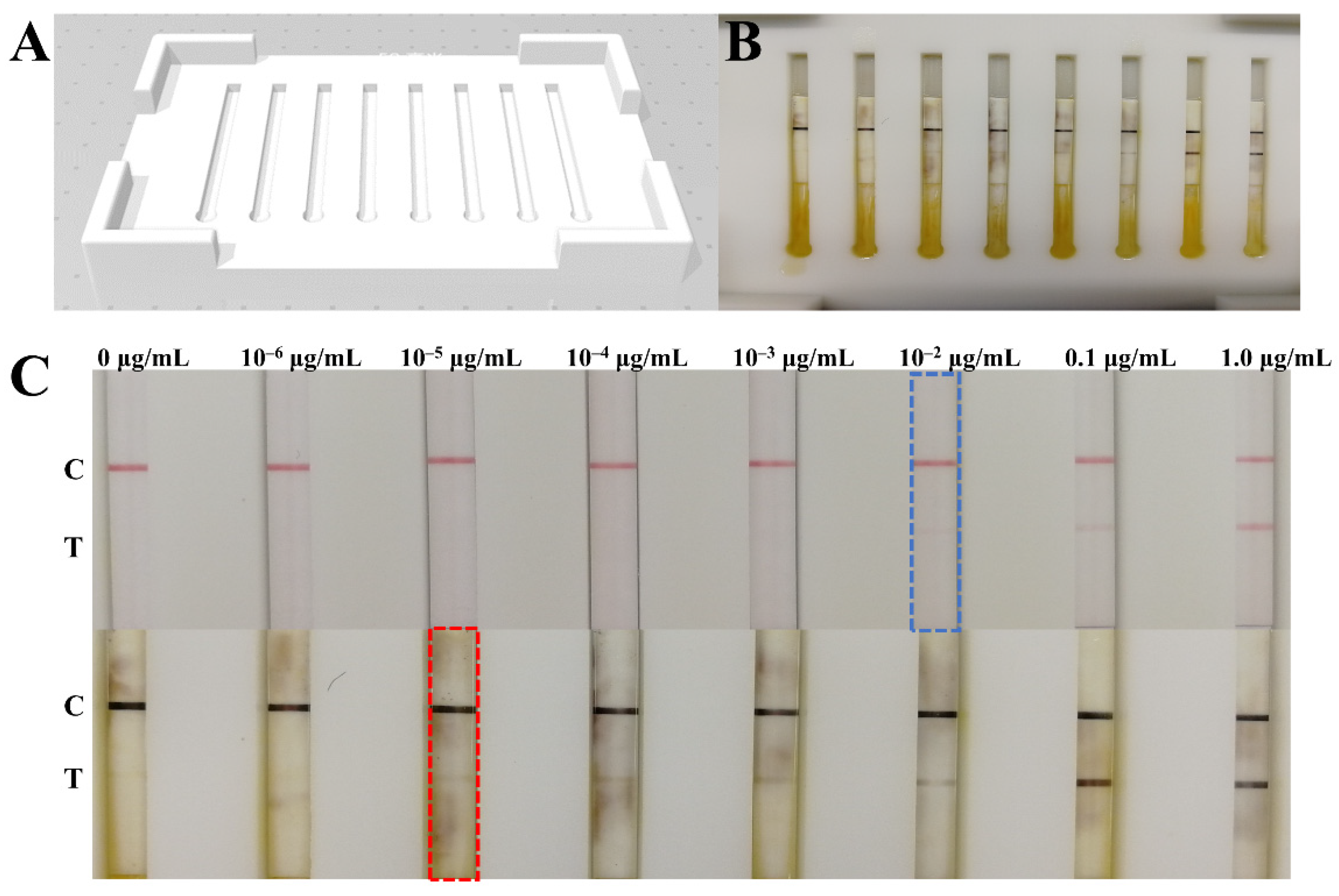
3.1. Validation of GCNP-Mediated Copper Deposition
3.2. Optimization of the Parameters
3.3. Signal Amplification for NP Antigen Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, W.Z.; Li, J.; He, X.Y.; Zhang, C.Q.; Li, C.R.; Li, Y.; Cheng, S.H.; Zhang, P.A. The diagnostic value of joint detection of serum IgM and IgG antibodies to 2019-nCoV in 2019-nCoV infection. Chin. J. Lab. Med. 2020, 43, E01–E06. [Google Scholar]
- Ong, D.S.Y.; de Man, S.J.; Lindeboom, F.A.; Koeleman, J.G.M. Comparison of diagnostic accuracies of rapid serological tests and ELISA to molecular diagnostics in patients with suspected COVID-19 presenting to the hospital. Clin. Microbiol. Infect. 2020, 26, 1094.e7–1094.e10. [Google Scholar] [CrossRef]
- Wang, C.W.; Yang, X.S.; Gu, B.; Liu, H.F.; Zhou, Z.H.; Shi, L.L.; Cheng, X.D.; Wang, S.Q. Sensitive and Simultaneous Detection of SARS-CoV-2-Specific IgM/IgG Using Lateral Flow Immunoassay Based on Dual-Mode Quantum Dot Nanobeads. Anal. Chem. 2020, 92, 15542–15549. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Di, B.; Hao, W.; Gao, Y.; Wang, M.; Qiu, L.; Wen, K.; Zhou, D.; Wu, X.; Lu, E. Monoclonal antibody-based antigen capture enzyme-linked immunosorbent assay reveals high sensitivity of the nucleocapsid protein in acute-phase sera of severe acute respiratory syndrome patients. Clin. Diagn. Lab. Immunol. 2005, 12, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, X.; Hao, W.; Wang, Y.; Di, B.; Yin, K.; Xu, Y.-C.; Feng, C.-S.; Wan, Z.-Y.; Cheng, V.C.C.; Yuen, K.-Y. Nucleocapsid protein as early diagnostic marker for SARS. Emerg. Infect. Dis. 2004, 10, 1947–1949. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Zhang, Y.Z. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazares, L.H.; Chaerkady, R.; Weng, S.H.S.; Boo, C.C.; Cimbro, R.; Hsu, H.-E.; Rajan, S.; Dall’Acqua, W.; Clarke, L.; Ren, K.; et al. Development of a Parallel Reaction Monitoring Mass Spectrometry Assay for the Detection of SARS-CoV2 Spike Glycoprotein and Nucleoprotein. Anal. Chem. 2020, 92, 13813–13821. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Zourob, M. Development of a Low-Cost Cotton-Tipped Electrochemical Immunosensor for the Detection of SARS-CoV-2. Anal. Chem. 2021, 93, 1826–1833. [Google Scholar] [CrossRef]
- Eissa, S.; Alhadrami, H.A.; Al-Mozaini, M.; Hassan, A.M.; Zourob, M. Voltammetric-based immunosensor for the detection of SARS-CoV-2 nucleocapsid antigen. Microchim. Acta 2021, 188, 199. [Google Scholar] [CrossRef]
- Grant, B.D.; Anderson, C.E.; Williford, J.R.; Alonzo, L.F.; Glukhova, V.A.; Boyle, D.S.; Weigl, B.H.; Nichols, K.P. SARS-CoV-2 Coronavirus Nucleocapsid Antigen-Detecting Half-Strip Lateral Flow Assay Toward the Development of Point of Care Tests Using Commercially Available Reagents. Anal. Chem. 2020, 92, 11305–11309. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Sui, Z.W.; Huang, Z.H.; Xie, J.; Wen, K.; Zhang, Y.Z.; Huang, W.F.; Mi, W.; Peng, K.; Dai, X.H.; et al. Point-of-care test system for detection of immunoglobulin-G and -M against nucleocapsid protein and spike glycoprotein of SARS-CoV-2. Sens. Actuators B Chem. 2021, 331, 129415. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, J.H.; Mi, J.K.; Sun, C.P.; Kim, S.I. Development of a SARS-CoV-2-specific biosensor for antigen detection using scFv-Fc fusion proteins. Biosens. Bioelectron. 2020, 175, 112868. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Wen, K.; Zhang, J.; Chen, J.; Han, C.; Chen, Y.W.; Wang, S.F.; Deng, G.H.; Zhou, H.W.; Wu, Y.Z. Accuracy of a nucleocapsid protein antigen rapid test in the diagnosis of SARS-CoV-2 infection. Clin. Microbiol. Infect. 2021, 27, 289.e1–289.e4. [Google Scholar] [CrossRef]
- Corman, V.M.; Haage, V.C.; Bleicker, T.; Schmidt, M.L.; Mühlemann, B.; Zuchowski, M.; Jo, W.K.; Tscheak, P.; Möncke-Buchner, E.; Müller, M.A.; et al. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: A single-centre laboratory evaluation study. Lancet Microbe 2021, 2, e311–e319. [Google Scholar] [CrossRef]
- Seiya, Y.; Yuko, S.T.; Michiko, K.; Osamu, A.; Yoshihiro, K. Comparison of Rapid Antigen Tests for COVID-19. Viruses 2020, 12, 1420. [Google Scholar]
- Wang, C.W.; Cheng, X.D.; Liu, L.Y.; Zhang, X.C.; Yang, X.S.; Zheng, S.; Rong, Z.; Wang, S.Q. Ultrasensitive and Simultaneous Detection of Two Specific SARS-CoV-2 Antigens in Human Specimens Using Direct/Enrichment Dual-Mode Fluorescence Lateral Flow Immunoassay. ACS Appl. Mater. Interfaces 2021, 13, 40342–40353. [Google Scholar] [CrossRef]
- Wang, C.W.; Yang, X.S.; Zheng, S.; Cheng, X.D.; Xiao, R.; Li, Q.J.; Wang, W.Q.; Liu, X.X.; Wang, S.Q. Development of an ultrasensitive fluorescent immunochromatographic assay based on multilayer quantum dot nanobead for simultaneous detection of SARS-CoV-2 antigen and influenza A virus. Sens. Actuators B Chem. 2021, 345, 130372. [Google Scholar] [CrossRef]
- Liu, D.; Ju, C.H.; Han, C.; Shi, R.; Chen, X.H.; Duan, D.M.; Yan, J.H.; Yan, X.Y. Nanozyme chemiluminescence paper test for rapid and sensitive detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 173, 112817. [Google Scholar] [CrossRef]
- Shan, S.; Lai, W.H.; Xiong, Y.H.; Wei, H.; Xu, H.Y. Novel strategies to enhance lateral flow immunoassay sensitivity for detecting foodborne pathogens. J. Agric. Food Chem. 2015, 63, 745–753. [Google Scholar] [CrossRef]
- Tian, M.L.; Lei, L.L.; Xie, W.Y.; Yang, Q.M.; Li, C.M.; Liu, Y.S. Copper deposition-induced efficient signal amplification for ultrasensitive lateral flow immunoassay. Sens. Actuators B Chem. 2019, 282, 96–103. [Google Scholar] [CrossRef]
- Liu, Y.S.; Zhang, Z.Y.; Yu, J.; Xie, J.; Li, C.M. A concentration dependent multicolor conversion strategy for ultrasensitive colorimetric immunoassay with the naked eye. Anal. Chim. Acta 2017, 963, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Xie, J.; Zhang, Z.Y.; Lu, Z.S. An ultrasensitive colorimetric strategy for protein O-GlcNAcylation detection via copper deposition-enabled nonenzymatic signal amplification. RSC Adv. 2016, 6, 89484–89491. [Google Scholar] [CrossRef]
- Berg, E.A.; Fishman, J.B. Labeling Antibodies Using Colloidal Gold. Cold Spring Harb. Protoc. 2020, 4, 099333. [Google Scholar] [CrossRef]
- Pascal, M.; Nathalie, D.V.; Delphine, M.; Christian, J.; Ali, M.; Lize, C.; Sigi, V.W.; Vanessa, M.; Pierrette, M.; Karolien, S.; et al. Development and Potential Usefulness of the COVID-19 Ag Respi-Strip Diagnostic Assay in a Pandemic Context. Front. Med. 2020, 7, 225. [Google Scholar]



| Method | Sensitivity | Time | Reference |
|---|---|---|---|
| Colloidal gold nanoparticle based LFIA | 250 pg/mL | 15 min | Mertens et al. [26] |
| Half-strip lateral flow assay | 650 pg/mL | about 20 min | BD Grant et al. [12] |
| Cellulose nanobead-based LFIA | 20 ng/mL | 20 min | Kim et al. [14] |
| Fluorescent immunochromatographic assay | / | 10 min | Diao et al. [15] |
| Fluorescent immunochromatographic assay based on multilayer quantum dot nanobeads | 5.0 pg/mL | 15 min | Wang et al. [18] |
| Dual-Mode fluorescence lateral flow immunoassay | 0.5 pg/mL | 10 min | Wang et al. [19] |
| Cotton-tipped electrochemical immunosensor | 0.8 pg/mL | about 20 min | Shimaa Eissa and Mohammed Zourob [9,10] |
| Parallel reaction monitoring mass spectrometry assay | 2 × 105 viral particles/mL | about 3 h | Cazares et al. [9] |
| Colloidal gold nanoparticles based LFIA with copper deposition | 10 pg/mL | Within 20 min | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, T.; Jiao, X.; Liang, Z.; Zhao, H.; Zhao, Y.; Xie, J.; Jiang, Y.; Yu, X.; Fang, X.; Dai, X. Lateral Flow Immunoassay Coupled with Copper Enhancement for Rapid and Sensitive SARS-CoV-2 Nucleocapsid Protein Detection. Biosensors 2022, 12, 13. https://doi.org/10.3390/bios12010013
Peng T, Jiao X, Liang Z, Zhao H, Zhao Y, Xie J, Jiang Y, Yu X, Fang X, Dai X. Lateral Flow Immunoassay Coupled with Copper Enhancement for Rapid and Sensitive SARS-CoV-2 Nucleocapsid Protein Detection. Biosensors. 2022; 12(1):13. https://doi.org/10.3390/bios12010013
Chicago/Turabian StylePeng, Tao, Xueshima Jiao, Zhanwei Liang, Hongwei Zhao, Yang Zhao, Jie Xie, You Jiang, Xiaoping Yu, Xiang Fang, and Xinhua Dai. 2022. "Lateral Flow Immunoassay Coupled with Copper Enhancement for Rapid and Sensitive SARS-CoV-2 Nucleocapsid Protein Detection" Biosensors 12, no. 1: 13. https://doi.org/10.3390/bios12010013





