Development and In-Depth Characterization of Bacteria Repellent and Bacteria Adhesive Antibody-Coated Surfaces Using Optical Waveguide Biosensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. OWLS Instrument and Related Protocols
2.2. Blocking Solutions in OWLS Experiments
2.3. Antibody Immobilization Strategies and Reagents in OWLS Experiments
2.4. Bacterial Culture
2.5. Bacteria-Specific Antibodies and Relevant Solutions
2.6. Testing the Binding Ability of Anti-E. coli Antibodies Using ELISA
3. Results and Discussion
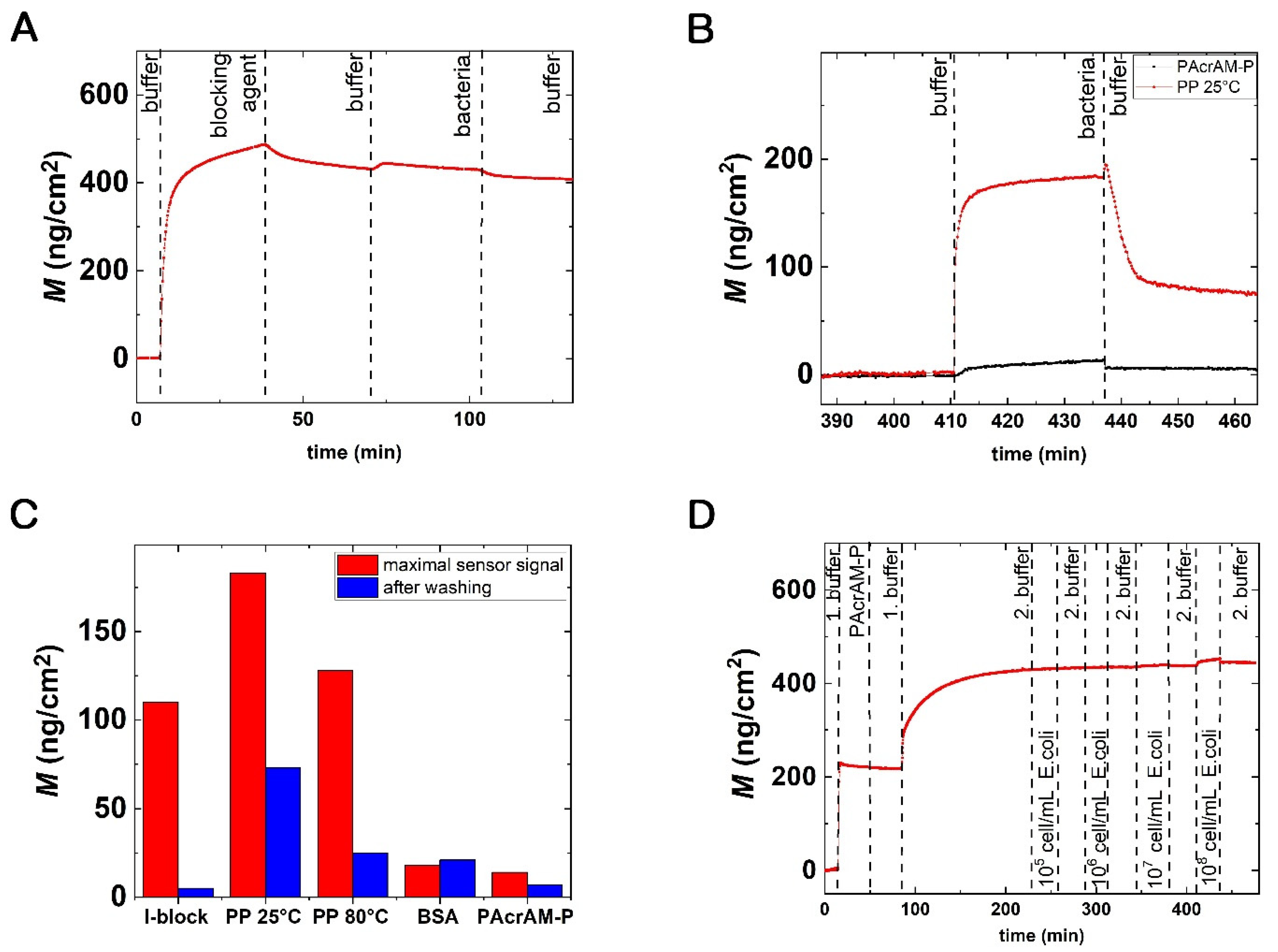
3.1. Bacteria Repellent Coatings Tested by In Situ OWLS Measurements
3.2. Binding Abilities of Different Antibodies Tested with ELISA
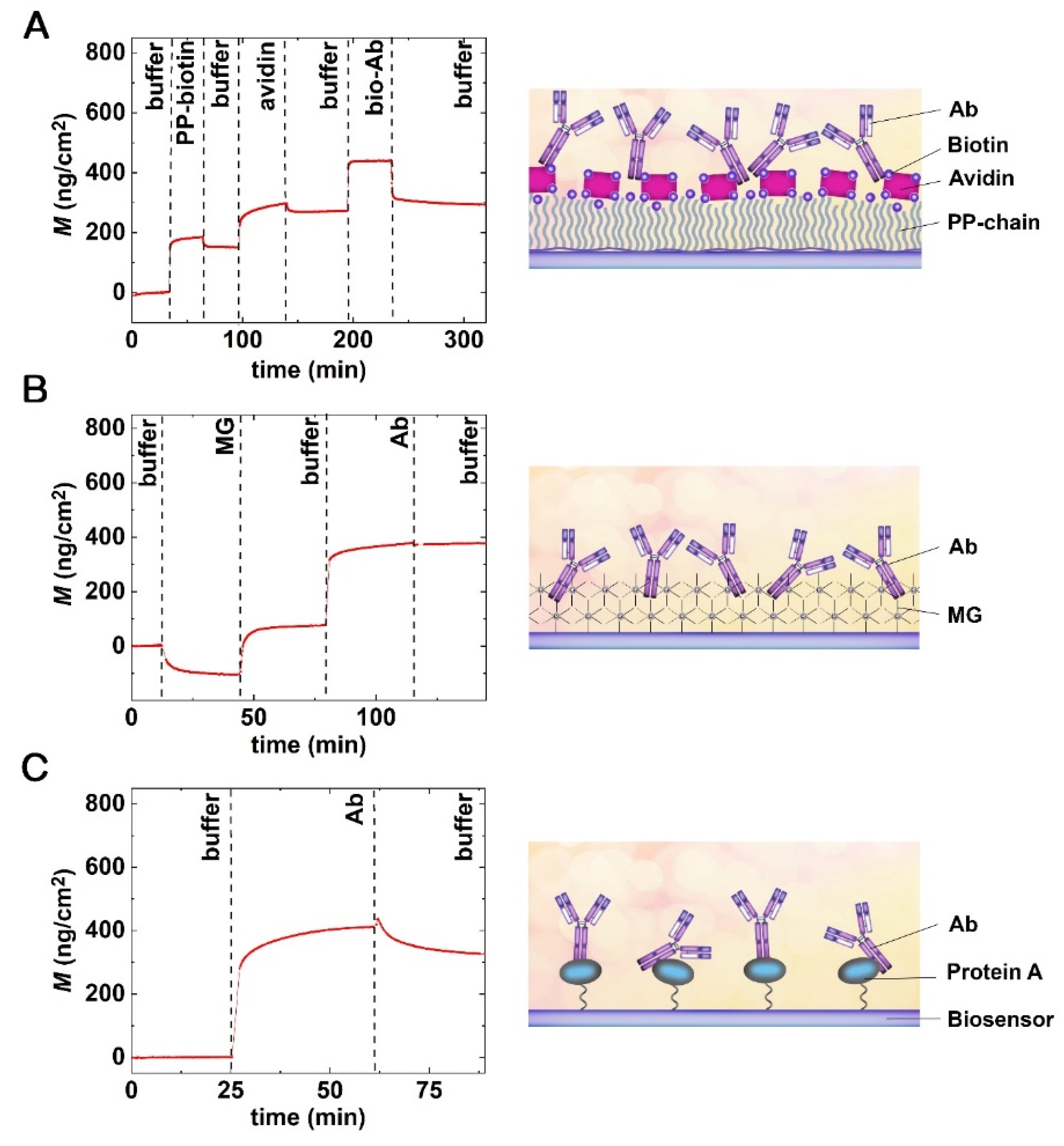
3.3. Various Strategies of Antibody Immobilization on OWLS Chip Surfaces
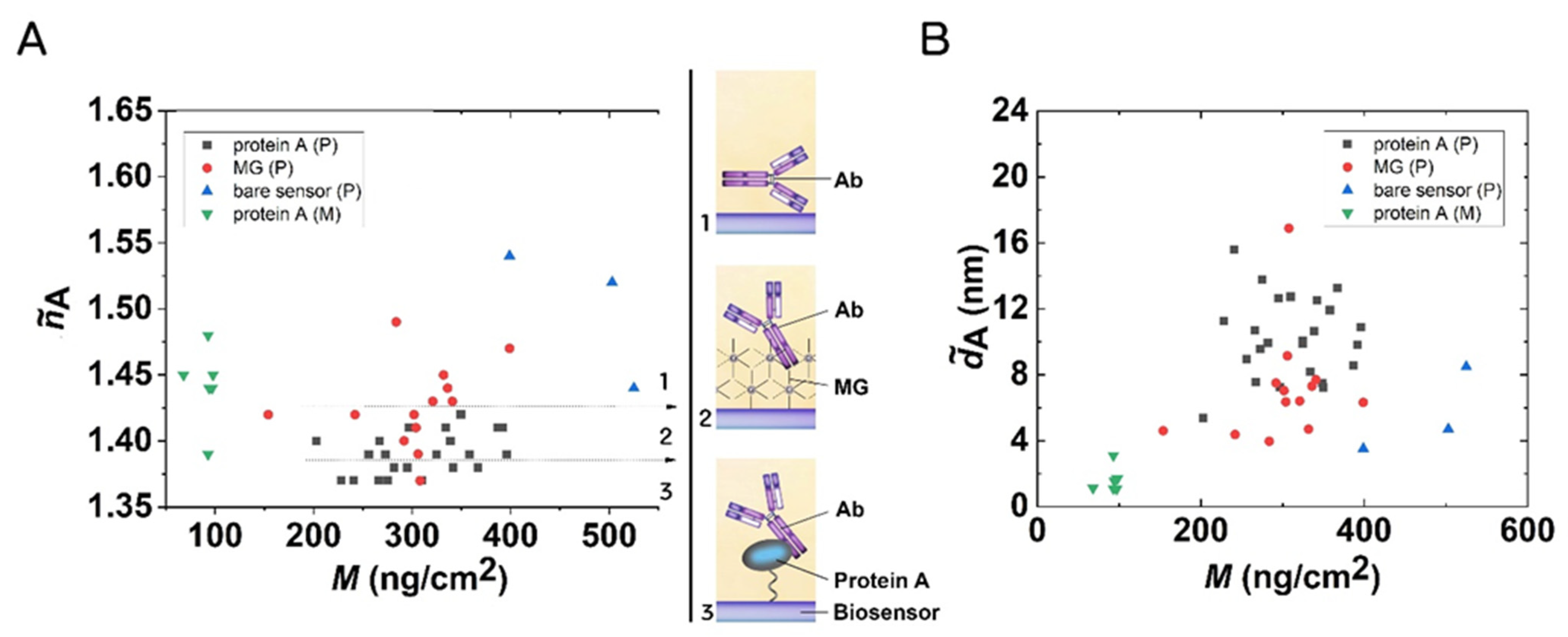
3.4. Surface Mass Densities and Structures of Antibody Layers
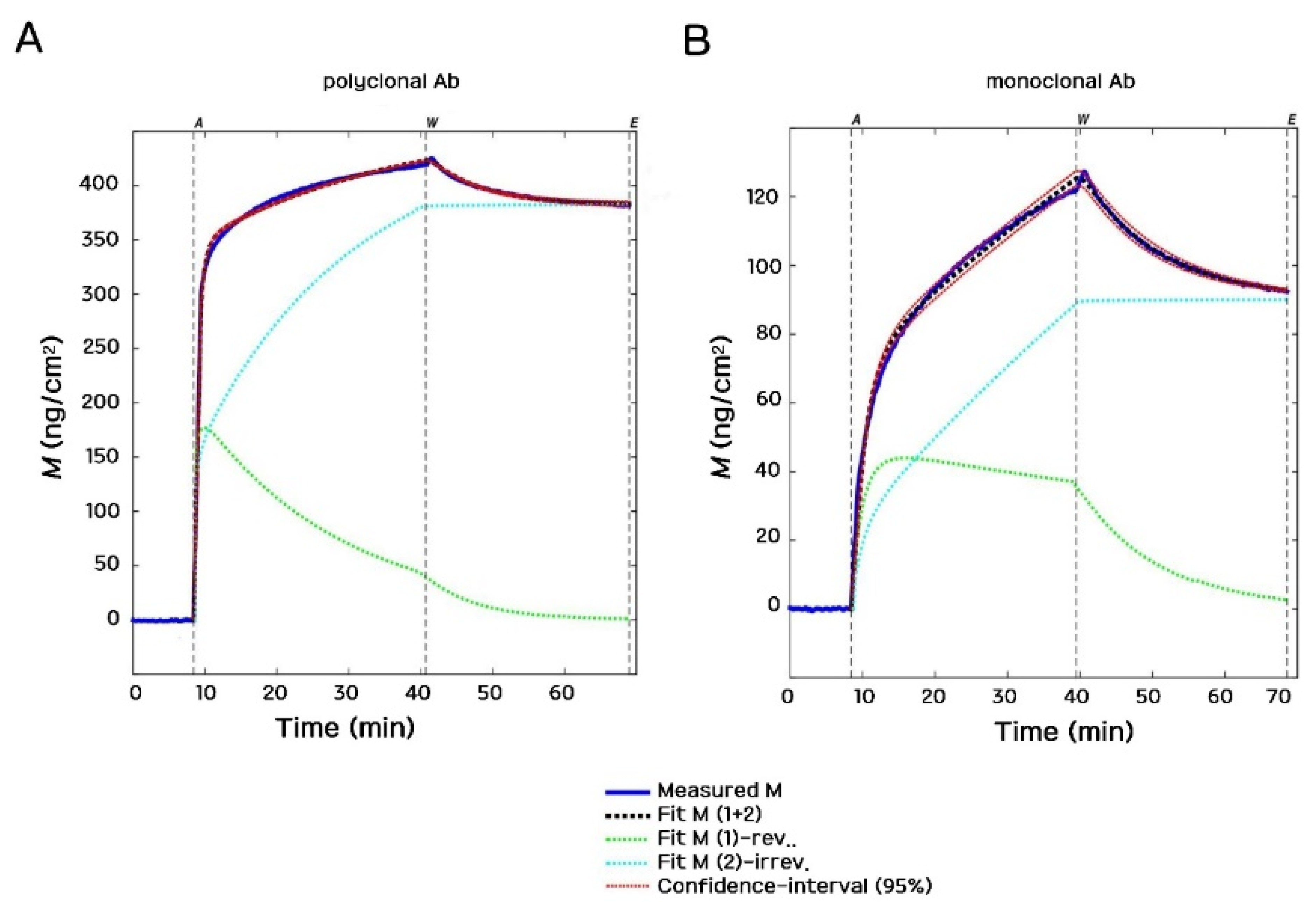
3.5. Kinetic Analysis of Antibody Deposition—Kinetic Fits of Real-Time Biosensor Data
3.6. Detection of Bacterial Adsorption on the Protein A-Based Antibody-Coated Surfaces with PAcrAM-P Blocking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vörös, J.; Ramsden, J.J.; Csúcs, G.; Szendrő, I.; De Paul, S.M.; Textor, M.; Spencer, N.D. Optical Grating Coupler Biosensors. Biomaterials 2002, 23, 3699–3710. [Google Scholar] [CrossRef]
- Tiefenthaler, K.; Lukosz, W. Sensitivity of Grating Couplers as Integrated-Optical Chemical Sensors. J. Opt. Soc. Am. B 1989, 6, 209–220. [Google Scholar] [CrossRef]
- Frutiger, A.; Tanno, A.; Hwu, S.; Tiefenauer, R.F.; Vörös, J.; Nakatsuka, N. Nonspecific Binding—Fundamental Concepts and Consequences for Biosensing Applications. Chem. Rev. 2021, 121, 8095–8160. [Google Scholar] [CrossRef]
- Rigo, S.; Cai, C.; Gunkel-Grabole, G.; Maurizi, L.; Zhang, X.; Xu, J.; Palivan, C.G. Nanoscience-Based Strategies to Engineer Antimicrobial Surfaces. Adv. Sci. 2018, 5, 1700892. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Brenes-Acuña, M.; Castro-Rojas, A.; Cordero-Salmerón, R.; Lopretti-Correa, M.; Vega-Baudrit, J.B. Biosensors for the Detection of Bacterial and Viral Clinical Pathogens. Sensors 2020, 20, 6926. [Google Scholar] [CrossRef]
- D’Agata, R.; Bellassai, N.; Jungbluth, V.; Spoto, G. Recent Advances in Antifouling Materials for Surface Plasmon Resonance Biosensing in Clinical Diagnostics and Food Safety. Polymers 2021, 13, 1929. [Google Scholar] [CrossRef] [PubMed]
- Achinas, S.; Charalampogiannis, N.; Euverink, G.J.W. A Brief Recap of Microbial Adhesion and Biofilms. Appl. Sci. 2019, 9, 2801. [Google Scholar] [CrossRef] [Green Version]
- Venkateswaran, S.; Gwynne, P.J.; Wu, M.; Hardman, A.; Lilienkampf, A.; Pernagallo, S.; Blakely, G.; Swann, D.G.; Bradley, M.; Gallagher, M.P. High-Throughput Identification of Bacteria Repellent Polymers for Medical Devices. J. Vis. Exp. 2016, 117, e54382. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.K.; Yegin, Y.; Yang, F.; Zhang, M.; Li, J.; Huang, S.; Verkhoturov, S.V.; Schweikert, E.A.; Perez-Lewis, K.; Scholar, E.A.; et al. The Influence of Surface Chemistry on the Kinetics and Thermodynamics of Bacterial Adhesion. Sci. Rep. 2018, 8, 17247. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Lachance, B.; Sunahara, G.; Luong, J.H.T. An In-Depth Analysis of Electric Cell-Substrate Impedance Sensing to Study the Attachment and Spreading of Mammalian Cells. Anal. Chem. 2002, 74, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Janshoff, A.; Kunze, A.; Michaelis, S.; Heitmann, V.; Reiss, B.; Wegener, J. Cell Adhesion Monitoring Using Substrate-Integrated Sensors. J. Adhes. Sci. Technol. 2010, 24, 2079–2104. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical Review on Biofilm Methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [Green Version]
- Brynda, E.; Houska, M.; Brandenburg, A.; Wikerstål, A. Optical Biosensors for Real-Time Measurement of Analytes in Blood Plasma. Biosens. Bioelectron. 2002, 17, 665–675. [Google Scholar] [CrossRef]
- Brosinger, F.; Freimuth, H.; Lacher, M.; Ehrfeld, W.; Gedig, E.; Katerkamp, A.; Spener, F.; Cammann, K. A Label-Free Affinity Sensor with Compensation of Unspecific Protein Interaction by a Highly Sensitive Integrated Optical Mach-Zehnder Interferometer on Silicon. Sens. Actuators B Chem. 1997, 44, 350–355. [Google Scholar] [CrossRef]
- Steinitz, M. Quantitation of the Blocking Effect of Tween 20 and Bovine Serum Albumin in ELISA Microwells. Anal. Biochem. 2000, 282, 232–238. [Google Scholar] [CrossRef]
- Xuan, X.; Zhu, J.; Church, C. Particle Focusing in Microfluidic Devices. Microfluid. Nanofluid. 2010, 9, 1–16. [Google Scholar] [CrossRef]
- Longo, C.; Gambara, G.; Espina, V.; Luchini, A.; Patanarut, A.S.; Petricoin, E.F., III; Beretti, F. A Candidate Melanoma Biomarker in Serum. Exp. Dermatol. 2013, 20, 29–34. [Google Scholar] [CrossRef]
- Signore, M.; Reeder, K.A. Antibody Validation by Western Blotting. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 823, pp. 139–155. [Google Scholar] [CrossRef] [PubMed]
- Elbert, D.L.; Hubbell, J.A. Reduction of Fibrous Adhesion Formation by a Copolymer Possessing an Affinity for Anionic Surfaces. J. Biomed. Mater. Res. 1998, 42, 55–65. [Google Scholar] [CrossRef]
- Saldarriaga Fernández, I.C.; van der Mei, H.C.; Lochhead, M.J.; Grainger, D.W.; Busscher, H.J. The Inhibition of the Adhesion of Clinically Isolated Bacterial Strains on Multi-Component Cross-Linked Poly(Ethylene Glycol)-Based Polymer Coatings. Biomaterials 2007, 28, 4105–4112. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Kenausis, G.L.; Vörös, J.; Elbert, D.L.; Huang, N.; Hofer, R.; Ruiz-Taylor, L.; Textor, M.; Hubbell, J.A.; Spencer, N.D. Poly(L-Lysine)-g-Poly(Ethylene Glycol) Layers on Metal Oxide Surfaces: Attachment Mechanism and Effects of Polymer Architecture on Resistance to Protein Adsorption. J. Phys. Chem. B 2000, 104, 3298–3309. [Google Scholar] [CrossRef]
- Tauhardt, L.; Kempe, K.; Gottschaldt, M.; Schubert, U.S. Poly(2-Oxazoline) Functionalized Surfaces: From Modification to Application. Chem. Soc. Rev. 2013, 42, 7998–8011. [Google Scholar] [CrossRef]
- Weydert, S.; Zürcher, S.; Tanner, S.; Zhang, N.; Ritter, R.; Peter, T.; Aebersold, M.J.; Thompson-Steckel, G.; Forró, C.; Rottmar, M.; et al. Easy to Apply Polyoxazoline-Based Coating for Precise and Long-Term Control of Neural Patterns. Langmuir 2017, 33, 8594–8605. [Google Scholar] [CrossRef]
- Welch, N.G.; Scoble, J.A.; Muir, B.W.; Pigram, P.J. Orientation and Characterization of Immobilized Antibodies for Improved Immunoassays (Review). Biointerphases 2017, 12, 02D301. [Google Scholar] [CrossRef] [Green Version]
- Cass, T.; Ligler, F.S. Immobilized Biomolecules in Analysis: A Practical Approach; (The Practical Approach Series); Oxford University Press: Oxford, UK, 1999; pp. 1–240. [Google Scholar]
- Hermanson, G. Bioconjugate Techniques; Academic Press: Cambridge, MA, USA, 2013; pp. 1–1200. [Google Scholar]
- Trilling, A.K.; Beekwilder, J.; Zuilhof, H. Antibody Orientation on Biosensor Surfaces: A Minireview. Analyst 2013, 138, 1619–1627. [Google Scholar] [CrossRef] [Green Version]
- Zourob, M.; Elwary, S.; Turner, A.P.F. Principles of Bacterial Detection: Biosensors, Recognition; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–1002. [Google Scholar]
- Bocková, M.; Slabý, J.; Špringer, T.; Homola, J. Advances in Surface Plasmon Resonance Imaging and Microscopy and Their Biological Applications. Annu. Rev. Anal. Chem. 2019, 12, 151–176. [Google Scholar] [CrossRef]
- Wang, Y.; Knoll, W.; Dostalek, J. Bacterial Pathogen Surface Plasmon Resonance Biosensor Advanced by Long Range Surface Plasmons and Magnetic Nanoparticle Assays. Anal. Chem. 2012, 84, 8345–8350. [Google Scholar] [CrossRef] [PubMed]
- Bouguelia, S.; Roupioz, Y.; Slimani, S.; Mondani, L.; Casabona, M.G.; Durmort, C.; Vernet, T.; Calemczuk, R.; Livache, T. On-Chip Microbial Culture for the Specific Detection of Very Low Levels of Bacteria. Lab Chip 2013, 13, 4024–4032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Feijter, J.A.; Benjamins, J.; Veer, F.A. Ellipsometry as a Tool to Study the Adsorption Behavior of Synthetic and Biopolymers at the Air–Water Interface. Biopolymers 1978, 17, 1759–1772. [Google Scholar] [CrossRef]
- Orgovan, N.; Patko, D.; Hos, C.; Kurunczi, S.; Szabó, B.; Ramsden, J.J.; Horvath, R. Sample Handling in Surface Sensitive Chemical and Biological Sensing: A Practical Review of Basic Fluidics and Analyte Transport. Adv. Colloid Interface Sci. 2014, 211, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Duanis-Assaf, D.; Kenan, E.; Sionov, R.; Steinberg, D.; Shemesh, M. Proteolytic Activity of Bacillus Subtilis upon κ-Casein Undermines Its “Caries-Safe” Effect. Microorganisms 2020, 8, 221. [Google Scholar] [CrossRef] [Green Version]
- Bhat, M.Y.; Dar, T.A.; Singh, L.R. Casein Proteins: Structural and Functional Aspects. In Milk Proteins–From Structure to Biological Properties and Health Aspects; InTech: Rijeka, Croatia, 2016; pp. 3–18. [Google Scholar] [CrossRef] [Green Version]
- Barnes, L.-M.; Lo, M.R.; Adams, M.R.; Chamberlain, A.H.L. Effect of Milk Proteins on Adhesion of Bacteria to Stainless Steel Surfaces. Appl. Environ. Microbiol. 1999, 65, 4543–4548. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Li, S.; Park, M.-K.; Zhang, Z.; Cheng, Z.; Petrenko, V.A.; Chin, B.A. Blocking Agent Optimization for Nonspecific Binding on Phage Based Magnetoelastic Biosensors. J. Electrochem. Soc. 2012, 159, B818–B823. [Google Scholar] [CrossRef]
- Riquelme, M.V.; Zhao, H.; Srinivasaraghavan, V.; Pruden, A.; Vikesland, P.; Agah, M. Optimizing Blocking of Nonspecific Bacterial Attachment to Impedimetric Biosensors. Sens. Bio-Sensing Res. 2016, 8, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Fu, L. Development of Phage/Antibody Immobilized Magnetostrictive Biosensors. Ph.D. Thesis, Auburn University, Auburn, AL, USA, 2010. [Google Scholar]
- Ogaki, R.; Andersen, O.Z.; Jensen, G.V.; Kolind, K.; Kraft, D.C.E.; Pedersen, J.S.; Foss, M. Temperature-Induced Ultradense PEG Polyelectrolyte Surface Grafting Provides Effective Long-Term Bioresistance against Mammalian Cells, Serum, and Whole Blood. Biomacromolecules 2012, 13, 3668–3677. [Google Scholar] [CrossRef]
- Green, M.N. Avidin. Adv. Protein Chem. 1975, 29, 85–133. [Google Scholar] [CrossRef] [PubMed]
- Horvath, R.; Ramsden, J.J. Quasi-Isotropic Analysis of Anisotropic Thin Films on Optical Waveguides. Langmuir 2007, 23, 9330–9334. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, N.; Patko, D.; Orgovan, N.; Kurunczi, S.; Ramsden, J.J.; Vonderviszt, F.; Horvath, R. Optical Anisotropy of Flagellin Layers: In Situ and Label-Free Measurement of Adsorbed Protein Orientation Using OWLS. Anal. Chem. 2013, 85, 5382–5389. [Google Scholar] [CrossRef]
- Saftics, A.; Kurunczi, S.; Peter, B.; Szekacs, I.; Ramsden, J.J.; Horvath, R. Data Evaluation for Surface-Sensitive Label-Free Methods to Obtain Real-Time Kinetic and Structural Information of Thin Films: A Practical Review with Related Software Packages. Adv. Colloid Interface Sci. 2021, 294, 102431–102433. [Google Scholar] [CrossRef]
- Schaaf, P.; Talbot, J. Surface Exclusion Effects in Adsorption Processes. J. Chem. Phys. 1989, 91, 4401–4409. [Google Scholar] [CrossRef]
- Cruz, J.; Graells, T.; Walldén, M.; Hjort, K. Inertial Focusing with Sub-Micron Resolution for Separation of Bacteria. Lab Chip 2019, 19, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Di Carlo, D.; Irimia, D.; Tompkins, R.G.; Toner, M. Continuous Inertial Focusing, Ordering, and Separation of Particles in Microchannels. Proc. Natl. Acad. Sci. USA 2007, 104, 18892–18897. [Google Scholar] [CrossRef] [Green Version]
- Ramachandraiah, H.; Ardabili, S.; Faridi, A.M.; Gantelius, J.; Kowalewski, J.M.; Mårtensson, G.; Russom, A. Dean Flow-Coupled Inertial Focusing in Curved Channels. Biomicrofluidics 2014, 8, 034117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kašpárková, V.; Halabalová, V.; Šimek, L.; Růžička, J.; Janča, J. Separation of Bacteria in Temperature Gradient: Micro-Thermal Focusing Field-Flow Fractionation. J. Biochem. Biophys. Methods 2007, 70, 685–687. [Google Scholar] [CrossRef]
- Longton, R.W.; Cole, J.S.; Quinn, P.F. Isoelectric Focusing of Bacteria: Species Location within an Isoelectric Focusing Column by Surface Charge. Arch. Oral Biol. 1975, 20, 103–106. [Google Scholar] [CrossRef]
- Kenyon, S.M.; Meighan, M.M.; Hayes, M.A. Recent Developments in Electrophoretic Separations on Microfluidic Devices. Electrophoresis 2011, 32, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Hunt, T.P.; Mayers, B.T.; Alsberg, E.; Whitesides, G.M.; Westervelt, R.M.; Ingber, D.E. Combined Microfluidic-Micromagnetic Separation of Living Cells in Continuous Flow. Biomed. Microdevices 2006, 8, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.D.; Kim, U.; Soh, H.T. Multitarget Magnetic Activated Cell Sorter. Proc. Natl. Acad. Sci. USA 2008, 105, 18165–18170. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.R.; Cox, E.C.; Austin, R.H.; Sturm, J.C. Continuous Particle Separation Through Deterministic Lateral Displacement. Science 2004, 304, 987–990. [Google Scholar] [CrossRef]
- Shi, J.; Ahmed, D.; Mao, X.; Lin, S.-C.S.; Lawit, A.; Huang, T.J. Acoustic Tweezers: Patterning Cells and Microparticles Using Standing Surface Acoustic Waves (SSAW). Lab Chip 2009, 9, 2890–2895. [Google Scholar] [CrossRef] [PubMed]
- Zourob, M.; Hawkes, J.J.; Coakley, W.T.; Brown, B.J.T.; Fielden, P.R.; McDonnell, M.B.; Goddard, N.J. Optical Leaky Waveguide Sensor for Detection of Bacteria with Ultrasound Attractor Force. Anal. Chem. 2005, 77, 6163–6168. [Google Scholar] [CrossRef] [PubMed]
- Antfolk, M.; Muller, P.B.; Augustsson, P.; Bruus, H.; Laurell, T. Focusing of Sub-Micrometer Particles and Bacteria Enabled by Two-Dimensional Acoustophoresis. Lab Chip 2014, 14, 2791–2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farkas, E.; Tarr, R.; Gerecsei, T.; Saftics, A.; Kovács, K.D.; Stercz, B.; Domokos, J.; Peter, B.; Kurunczi, S.; Szekacs, I.; et al. Development and In-Depth Characterization of Bacteria Repellent and Bacteria Adhesive Antibody-Coated Surfaces Using Optical Waveguide Biosensing. Biosensors 2022, 12, 56. https://doi.org/10.3390/bios12020056
Farkas E, Tarr R, Gerecsei T, Saftics A, Kovács KD, Stercz B, Domokos J, Peter B, Kurunczi S, Szekacs I, et al. Development and In-Depth Characterization of Bacteria Repellent and Bacteria Adhesive Antibody-Coated Surfaces Using Optical Waveguide Biosensing. Biosensors. 2022; 12(2):56. https://doi.org/10.3390/bios12020056
Chicago/Turabian StyleFarkas, Eniko, Robert Tarr, Tamás Gerecsei, Andras Saftics, Kinga Dóra Kovács, Balazs Stercz, Judit Domokos, Beatrix Peter, Sandor Kurunczi, Inna Szekacs, and et al. 2022. "Development and In-Depth Characterization of Bacteria Repellent and Bacteria Adhesive Antibody-Coated Surfaces Using Optical Waveguide Biosensing" Biosensors 12, no. 2: 56. https://doi.org/10.3390/bios12020056
APA StyleFarkas, E., Tarr, R., Gerecsei, T., Saftics, A., Kovács, K. D., Stercz, B., Domokos, J., Peter, B., Kurunczi, S., Szekacs, I., Bonyár, A., Bányai, A., Fürjes, P., Ruszkai-Szaniszló, S., Varga, M., Szabó, B., Ostorházi, E., Szabó, D., & Horvath, R. (2022). Development and In-Depth Characterization of Bacteria Repellent and Bacteria Adhesive Antibody-Coated Surfaces Using Optical Waveguide Biosensing. Biosensors, 12(2), 56. https://doi.org/10.3390/bios12020056






