1. Introduction
Losing a hand is a tremendously physical trauma to any individual. Amputated individuals face a huge difficulty in performing daily activities independently [
1], which can also lead to unemployment and social isolation [
2]. According to statistics, only 66% of them can resume work after that [
3].
To restore the functionality of hands in daily life and in work place, wearing prostheses is one of the necessary options for amputees. There are three types of prosthetic hands: cosmetic hand, body-power hand and Myoelectric hand [
4,
5]. Among them, Myoelectric prosthesis hand is the most promising one, which allows an amputee to controls the robotic hand by reading his/her muscle actives using Surface Electromyography (sEMG) sensors on the residual forearm. The computer chip will read in muscle signals and convert signals into executable commands.
Interpretation on muscle signals is essential for the control of electric powered prosthetic hands, which requires machine learning algorithms to classify muscular electric signals into corresponding hand movement patterns. In most of the published papers, scientists use myoelectric signals recorded during firmly grasped periods for grasp classification, which yielded satisfactory classification outcomes [
6,
7,
8,
9,
10,
11]. For instance, the research done by Jiang et al. [
7] using 3 s firm grasp sEMG signals achieved approximately 85% accuracy for classifying 16 grasp gestures. However, the firmly grasped periods occur at the end of reaching and grasping, giving no time to control arm movement in a real-life environment [
12].
When including muscle activities recorded from entire grasp period, the classification accuracy decreased. In Cognolato et al.’s report [
13], the accuracy of the classification for 10 grasp gestures was approximately 63% to 82% by using the sEMG signals during the whole grasp period.
To solve this problem, developing a method to classify grasp pattern using sEMG data recorded in the earlier grasp period with a high accuracy is necessary. In this study, we investigate how grasp classification accuracy changes over the entire reaching and grasping process, and identify a period in early grasp phase that can achieve the best grasp classification outcome. We call this period as sweet period. Once the sweet period is identified, we can develop a better classification strategy that can be used in the real-time environment.
Specifically, we first apply and compare several processing methods for the feature extraction of the sEMG signals. Then, we conduct an experiment to find the sweet period that is suitable for early grasp classification with the best classification outcome. Finally, we will conduct another experiment to compare several common training and testing strategies to identify an effective strategy for better real-time grasp classification. We hypothesize that the muscle activities recorded in the early period of hand grasping can provide sufficient information to achieve the same or higher accuracy of grasp classification than other time periods with a reduced delay for prosthetic hand control.
2. Materials and Methods
2.1. Data Collection
The data used in this study were from an open-source dataset collected by Cognolato et al. [
13], where the sEMG data were recorded from 30 healthy subjects (27 male and 3 female), with an average age of 46.63 ± 15.11 years.
Twelve sEMG sensors were placed on the forearm of each subject, producing twelve columns of sEMG data, respectively. Due to the hardware problem, no myoelectric data were received from electrode number eight during the acquisition of subject S024. Therefore, the sEMG data for this subject were recorded from eleven electrodes instead of twelve [
13].
Ten grasp gestures were performed in this data collection which were selected based on the hand taxonomies [
14,
15,
16,
17] and grasp frequency in Activities of Daily Living [
18]. The participant performed each gesture for four repetitions, and in each repetition, the same gesture was performed three times using three different objects, respectively. A designated experimenter vocally guided the participant to perform which gestures and grasp which objects. The data were labelled according to the vocal instruction. The list of gestures and objects are shown in
Table 1.
In the data post-processing part, the abnormal samples were replaced with the precedent valid samples when filtering outliers [
13]. As there might be a delay between the participants’ response to the vocal instructions [
13], the sEMG activation time might not be matched perfectly with the stimulus time. Therefore, relabeling was performed to calibrate this difference using the method described by Kuzborskij et al. [
19].
2.2. Electromyography Feature Extraction and Selection
In the feature extraction process, we first determined the suitable window size for deriving features [
20]. As shown in
Table 2, several sizes of the overlapped window were tested, which are 50 ms, 100 ms, 200 ms, 500 ms, and 1000 ms. As the increase of the window size, the accuracy keeps increasing, which means that the more data we used to derive features, the better performance we could get. However, considering the capability of Myoelectric prosthesis in the real-life condition, a large window would delay the grasp action from the prosthetic hand. On the other hand, it can be seen that, when increasing the window size over 200 ms, the increase of the accuracy is less than 1%, which is a very small increase. Therefore, to keep the balance between accuracy and implementation speed, we chose 200 ms as the window length with the step of 50 ms, which is a 75% overlap between successive windows.
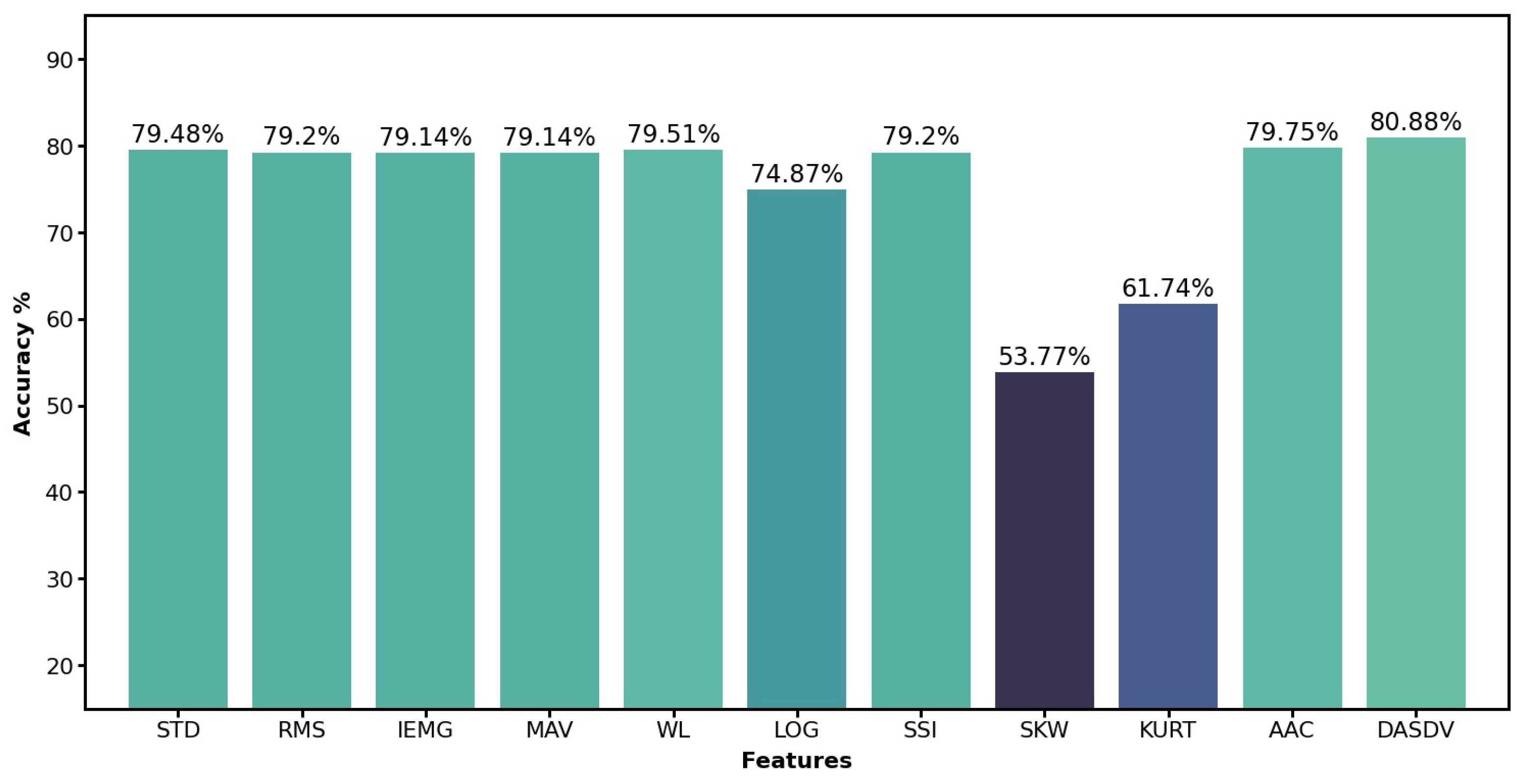
To assure the recognition accuracy by using proper features, we tested eleven commonly used features, which were Standard Deviation (STD), Root Mean Square (RMS), Integrated EMG (IEMG), Mean Absolute Value (MAV), Waveform Length (WL), Log Detector (LOG), Simple Square Integral (SSI), Skewness (SKW), Kurtosis (KURT), Average Amplitude Change (AAC) and Difference Absolute Standard Deviation Value (DASDV) [
21]. We dropped three lowest performance features, whichwere LOG, SKW and KURT and chose the rest eight with the highest accuracy as the final features for the following research. The performance of these features are shown in
Figure 1. After applying the eight features to the sEMG signals, the data set was converted from 12 columns to 96 columns. Due to the sensor hardware issue mentioned in the first subsection, the sEMG data of subject S024 was changed from 11 columns to 88 columns.
2.3. Classification Models
Gradient boosting decision tree, such as XGBoost [
22] and Light Gradient Boosting Machine (LightGBM) [
23], is a popular machine learning algorithm used by a large amount of data scientists recently, which can achieve a high performance by using decision trees as weak learners and assembling them to come up with one strong learner. Considering the high feature dimensions and large data size, we chose LightGBM as the classifier which runs faster while maintaining a high level of accuracy by utilizing two novel techniques called Gradient-Based One-Side Sampling (GOSS) and Exclusive Feature Bunding (EFB) [
23]. In the experiment of Ke et al. (2017), LightGBM can accelerate the training process up to twenty times than XGBoost.
We tuned the hyperparameters by using the training set of all the subjects and obtained the best results as follows: the learning rate is 0.1; no limit was set for the maximum depth; the number of estimators is 100; the number of leaves is 31; the remaining parameters are set to the default values.
2.4. Phase-Based Grasp Analysis
Normally, a typical reaching and grasping process can be divided into three phases [
24,
25]:
The Reaching Phase: starts from the hand lifting off, and ends by touching the object. During this phase, the hand is accelerated to a peak velocity and then is decelerated and brought to touch the target object. The hand usually opens to be configured to the target grasp gesture (pre-shape) [
26].
The Early Grasping Phase: begins at the moment when the hand initially contacts the object, and gradually closes the fingers until the hand starts to firmly grasp the object.
The Firm Grasping Phase: the target object is firmly grasped and hand shape is maintained relatively steady.
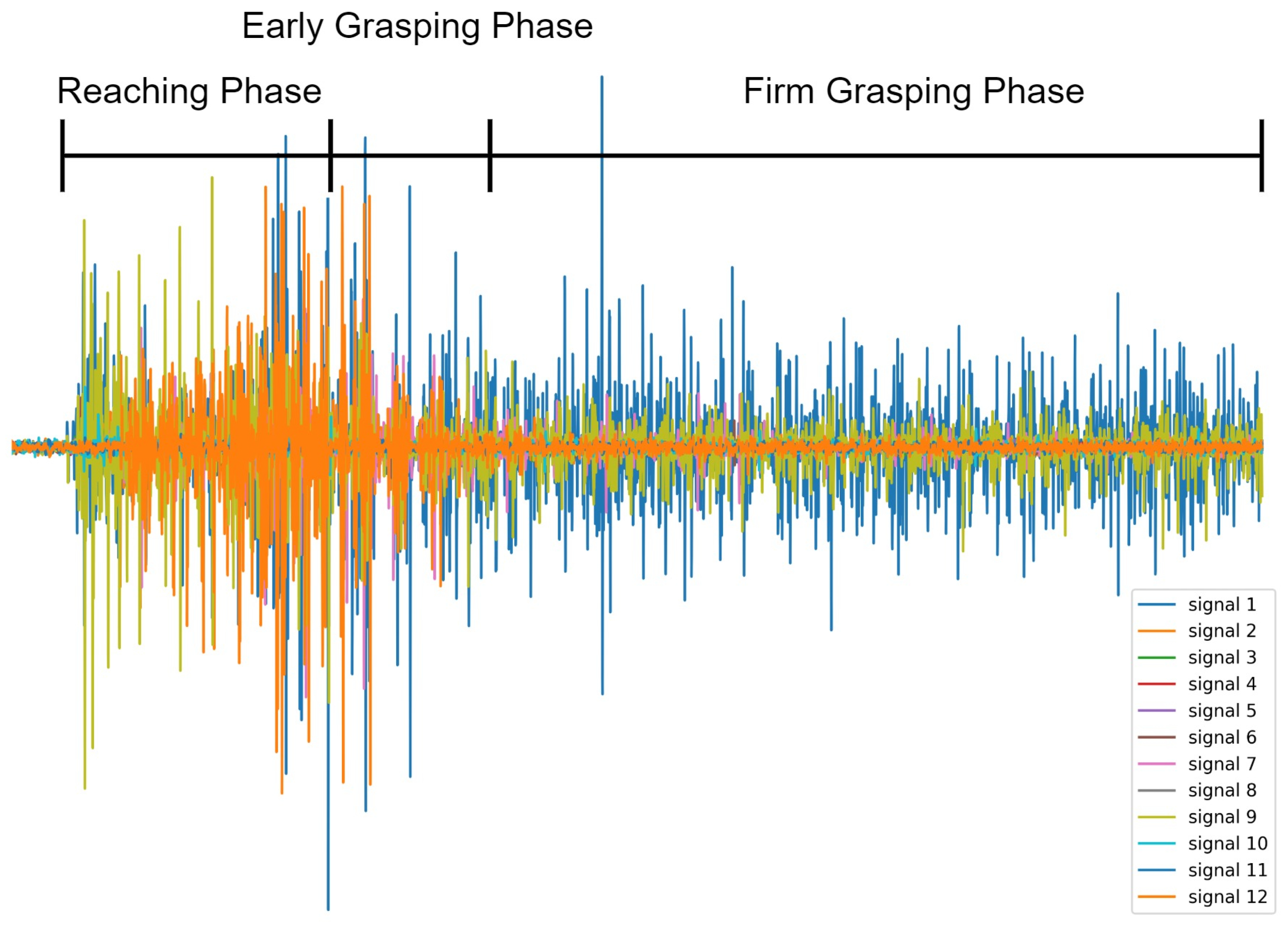
We segmented the Reaching, Early grasping, and Firm Grasping phases of each grasp gesture from each subject by observing corresponding videos frame by frame and calculated the average duration of each phase from all the observations. The judgment criteria for entering an Early Grasping Phase was the moment that the hand started to touch the target object, the judgement criteria for entering a Firm Grasping Phase was the moment that the target grasp gesture was completely formed and the hand started to keep relatively steady. According to the segmentation, Early Grasping Phase and Firm Grasping Phase started averagely 1020 ms and 1604 ms from the beginning of Reaching Phase, respectively. An example of grasp phases overlaid with sEMG signals during a full grasp trail is shown in
Figure 2.
3. Experiments and Results
We conducted two experiments, the first one aimed to analyse the grasp classification accuracy during the three grasping phases and find out the best position and length of sweet period, another was to find out the best training strategy.
3.1. Data Processing
The grasp trials performed by the participants lasted approximately 4.5–5 s [
13]. We removed the data after 4.5 s to align all the trials the same length. Because the overlapped window step is 50 ms and the grasp period length is 4.5 s, 90 pieces of data were reminded for each trial.
In this study, each participant performed one grasp gesture four times (repetitions) which allowed us to split the sEMG data by repetitions to validate testing results. For all the cases in this study, we used three repetitions (75%) for training and one repetition (25%) for testing with leave-one-repetition-out cross-validation, which used one repetition data for testing and the rest three repetitions for training the model, and repeated this process four times to cover all repetitions for testing. To increase the reliability of the sEMG data set, there were three objects being grasped in each repetition with the same gesture as mentioned in the data collection section. In other words, there were 324,000 data samples (90 samples/grasp × 10 grasp gestures × 4 repetitions × 3 objects × 30 subjects) in the data set.
3.2. Phases and Sweet Period Analysis
Figure 3 shows the mean changes of testing accuracy of grasp classification during all the three phases. Each data point is averaged across all 900 trials from 30 participants.
From
Figure 3 we can see that the accuracy increases from 42% to 84% during the Reaching phase and then becomes stable at the start of Early Grasping phase at around the time of 1000 ms, fluctuating between 84% and 87% during the rest of the grasp period. The mean accuracy further increases to relatively stable at around the time of 1250 ms, where we then define the location of the sweet period.
To find the optimal length of the sweet period, we designed different sliding windows with sizes of 300 ms, 400 ms, 500 ms, 600 ms, 700 ms, 800 ms, 900 ms and 1000 ms. The sliding window moved along with the time with step 50 ms, and in each move, it calculated and recorded the mean accuracy. We analyzed the records from the sliding window, and the results are given in
Figure 4.
From
Figure 4, we can see that the mean accuracy increases with the increase of window length significantly during the Reaching phase and beginning of the Early Grasping phase (at about 1100 ms) but not significantly afterward. For instance, although the window length of 1000 ms can reach the highest accuracy of 86.3%, it takes a much longer time than the length of 300 ms with an accuracy of 85.5%. Therefore, the length of the sweet period is set as 300 ms, and the position is set from 1100 ms to 1400 ms, which makes it entirely located in the Early Grasping phase as the blue region shown in
Figure 3.
3.3. Comparison Experiment
In the comparison experiment, we tested six strategies using different training and testing data, as shown in
Table 3.
In cases 1–3, we used all the three grasp phases as training data and reduced the testing data size, from all three phases to only the firm grasping phase, then to the sweet period. The purpose of performing these three comparisons was to study which phase/period was the better choice for testing data when using all grasp phases as training data. Besides, to figure out which phase played a better role model training, we studied another five cases. For cases 4–5, we used Firm Grasping Phase for training and reduced the testing data size. In cases 6–7, we used a combined Phases for training and sweet period for testing. In case 8, we used the data in the sweet period for both training and testing. It is worth mentioning again, the cross-validation method used for all the cases was leave-one-repetition-out cross-validation which used one repetition data for testing and the rest three repetitions for training the model, and repeated this process four times to cover all repetitions for testing, such that all testing data was excluded from training the model. For example, in one testing repetition of case 8, the data from the sweet period of three repetitions were used for training the model and the rest one for testing. The results are presented in
Table 3.
As shown in the
Table 3, we get the highest accuracy of 85.50% when we train with the all grasp phases and test with the only sweet period. Besides, from case 1 to 3, we find that if we keep the training data unchanged, the accuracy increases as the decrease of testing data size.
4. Discussion
Our hypothesis is supported by the results that there is a sweet period located in the Early Grasping Phase where sEMG signals can be used to achieve a similar or higher accuracy and lower delay of grasp classification than other time windows, which would help to improve the performance of robotic hand implementation in the real-life applications. This is important as the classifier can get the data much faster instead of waiting the muscle getting into the Firm Grasping Phase.
We found that during the Reaching Phase, the mean accuracy of this phase is only about 63%. This is because, in this period, the subjects moved their hands to reach the object and start to perform the grasp gesture, keeping the muscle status changing. Therefore, the sEMG signals in this period fluctuate very much, making it difficult in decoding the sEMG signals, see
Figure 2.
When getting into the Early Grasping phase, the accuracy reaches approximately 85%, which is as high as that in the Firm Grasping phase. The possible reason for this is the hand has already fully formed into the target gesture during the Early Grasping phase. Although this formed gesture is slightly different to the final target gesture, it can provide sufficient information for the classification. Therefore, the accuracy reaches to a high level at the start of the Early Grasping phase. After the subject firmly grasps the object (getting into the Firm Grasping phase), the accuracy keeps stable at around 85% because the sEMG signals started to be stable, which also make the classification performance stable.
Notice that the sEMG signal is more active in the Reaching and Early Grasping phases with high amplitude of the sEMG waveform as shown in
Figure 2. This is because the hand starts to perform the corresponding grasping gestures related activities such as hand aperture, where the sEMG signals from the forearm are usually active with higher amplitude than other phases [
26], although the hand has not grasped to the object during the Reaching Phase. In contrast, starting from the mid-Early Grasping phase to the whole Firm Grasping Phase, the muscle status keeps relatively unchanged, which makes the amplitude sEMG signal slightly lower than that in the reaching and grasping phase; this is also why better grasp classification performance was achieved during the Early Grasping phase and the Firm Grasping Phase where the sEMG signal patterns are relatively similar.
Using all three grasp phases for training the model and only using sweet period for controlling is found to be the best strategy for Myoelectric prosthetic hand application in real-life condition, not only because the sweet period during Early Grasping phase is suitable for prosthesis control as discussed before, but also this strategy can also increase the recognition accuracy compared to other strategies. The possible reason of higher accuracy achieved by this strategy could be more variation data was included in the model training. From case 3 and 6 in the
Table 3, we can see that if we remove the Firm Grasping Phase from training set, the accuracy decreases from 85.5% to 81.01%. This means that the Firm Grasping Phase is essential for training data because it may contain the information about the final target gesture. From case 3 and 7, we find that if we remove Reaching Phase from training set, the accuracy decreases from 85.5% to 82.51%. This means that Reaching Phase is also important for training data because it is the progress in which the gesture is formed.
For case 5, the accuracy is only 60.80% when only using the Firm Grasping Phase for training because this period lost much information about gesture formation in Reaching and Early Grasping Phases. For case 8, the accuracy reaches 74.99% only using sweet period for training because this training data also lost the part of information about the gesture in the Reaching Phase and the Firm Grasping Phase. However, using all phases data for training and the sweet period data for testing achieved the best accuracy, which can be the common practice in real-life situations where training a model is not time-sensitive.
5. Conclusions
In order to reduce the delay of myoprosthetic hand control in the real-life situation while maintaining a high recognition accuracy, we investigated the grasp classification performance during three grasping phases to identify the sweet period. We found that the sweet period located between 1.1 s and 1.4 s from the start of the hand grasping which happens in the Early Grasping phase before the hand is firmly grasped.
Furthermore, we found using sEMG from all three grasping phases (Reaching, Early Grasping, and Firm Grasping phases) for grasp classification model training achieved the best accuracy. Together with the identified sweet period for controlling, the grasp classification accuracy and the response speed of prosthetic hand can be balanced to achieve high performance.
Author Contributions
Conceptualization, S.W., X.J.; methodology, S.W., J.Z., X.J.; software, S.W.; validation, S.W., J.Z., B.Z., X.J.; formal analysis, S.W., J.Z., X.J.; investigation, S.W., J.Z.; resources, S.W., X.J.; data curation, S.W.; writing—original draft preparation, S.W., X.J.; writing—review and editing, J.Z., X.J., B.Z., S.W.; visualization, S.W.; supervision, X.J., B.Z.; project administration, X.J., B.Z,; funding acquisition, X.J., B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant number RGPIN-2020-05525.
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to the public available dataset we used has already approved.
Informed Consent Statement
Not applicable.
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest.
References
- Niedernhuber, M.; Barone, D.G.; Lenggenhager, B. Prostheses as extensions of the body: Progress and challenges. Neurosci. Biobehav. Rev. 2018, 92, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.H.; Yang, H.S.; Yang, H.E.; Lee, S.Y.; Kwon, J.W.; Yun, B.D.; Choi, J.Y.; Kim, S.N.; Jeong, H.W. A Survey on Activities of Daily Living and Occupations of Upper Extremity Amputees. Ann. Rehabil. Med. 2011, 35, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.; Marinček, Č. Return to work after lower limb amputation. Disabil. Rehabil. 2007, 29, 1323–1329. [Google Scholar] [CrossRef]
- Maat, B.; Smit, G.; Plettenburg, D.; Breedveld, P. Passive prosthetic hands and tools: A literature review. Prosthetics Orthot. Int. 2018, 42, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Castellini, C.; Smagt, P. Surface EMG in advanced hand prosthetics. Biol. Cybern. 2009, 100, 35–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Li, C.; Li, G.; Jiang, G.; Jiang, D.; Liu, H.; Zheng, Z.; Shu, W. Gesture recognition based on kinect and sEMG signal fusion. Mob. Netw. Appl. 2018, 23, 797–805. [Google Scholar] [CrossRef]
- Jiang, X.; Merhi, L.K.; Xiao, Z.G.; Menon, C. Exploration of Force Myography and surface Electromyography in hand gesture classification. Med. Eng. Phys. 2017, 41, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Merhi, L.K.; Menon, C. Force exertion affects grasp classification using force myography. IEEE Trans.-Hum.-Mach. Syst. 2017, 48, 219–226. [Google Scholar] [CrossRef]
- Asfour, M.; Menon, C.; Jiang, X. A Machine Learning Processing Pipeline for Reliable Hand Gesture Classification of FMG Signals with Stochastic Variance. Sensors 2021, 21, 1504. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Zhao, Z.Y.; Yang, J.H.; Lantz, V.; Wang, K.Q. Multiple hand gesture recognition based on surface EMG signal. In Proceedings of the 2007 1st IEEE International Conference on Bioinformatics and Biomedical Engineering, Wuhan, China, 6–8 July 2007; pp. 506–509. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Zhao, Z.Y.; Yang, J.H.; Lantz, V.; Wang, K.Q. Hand gesture recognition research based on surface EMG sensors and 2D-accelerometers. In Proceedings of the 2007 11th IEEE International Symposium on Wearable Computers, Boston, MA, USA, 11–13 October 2007; pp. 11–14. [Google Scholar] [CrossRef]
- Ummar, A.F.; Nisheena, V.I. The Upper Limb Invariant Myoelectric Prosthetic Control: A Review. In Proceedings of the 2020 International Conference on Futuristic Technologies in Control Systems Renewable Energy (ICFCR), Malappuram, India, 23–24 September 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Cognolato, M.; Gijsberts, A.; Gregori, V.; Saetta, G.; Giacomino, K.; Hager, A.G.M.; Gigli, A.; Faccio, D.; Tiengo, C.; Bassetto, F.; et al. Gaze, visual, myoelectric, and inertial data of grasps for intelligent prosthetics. Sci. Data 2020, 7, 43. [Google Scholar] [CrossRef]
- Cutkosky, M. On grasp choice, grasp models, and the design of hands for manufacturing tasks. IEEE Trans. Robot. Autom. 1989, 5, 269–279. [Google Scholar] [CrossRef]
- Sebelius, F.C.; Rosén, B.N.; Lundborg, G.N. Refined Myoelectric Control in Below-Elbow Amputees Using Artificial Neural Networks and a Data Glove. J. Hand Surg. 2005, 30, 780–789. [Google Scholar] [CrossRef]
- Crawford, B.; Miller, K.; Shenoy, P.; Rao, R. Real-time classification of electromyographic signals for robotic control. AAAI 2005, 5, 523–528. [Google Scholar]
- Feix, T.; Romero, J.; Schmiedmayer, H.B.; Dollar, A.M.; Kragic, D. The GRASP Taxonomy of Human Grasp Types. IEEE Trans.-Hum.-Mach. Syst. 2016, 46, 66–77. [Google Scholar] [CrossRef]
- Bullock, I.M.; Zheng, J.Z.; De La Rosa, S.; Guertler, C.; Dollar, A.M. Grasp Frequency and Usage in Daily Household and Machine Shop Tasks. IEEE Trans. Haptics 2013, 6, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Kuzborskij, I.; Gijsberts, A.; Caputo, B. On the challenge of classifying 52 hand movements from surface electromyography. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 4931–4937. [Google Scholar] [CrossRef] [Green Version]
- Englehart, K.; Hudgins, B. A robust, real-time control scheme for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 2003, 50, 848–854. [Google Scholar] [CrossRef]
- Phinyomark, A.; Phukpattaranont, P.; Limsakul, C. Feature reduction and selection for EMG signal classification. Expert Syst. Appl. 2012, 39, 7420–7431. [Google Scholar] [CrossRef]
- Friedman, J.; Hastie, T.; Tibshirani, R. The Elements of Statistical Learning; Springer Series in Statistics New York; Springer: New York, NY, USA, 2001; Volume 1. [Google Scholar]
- Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.Y. LightGBM: A Highly Efficient Gradient Boosting Decision Tree. Adv. Neural Inf. Process. Syst. 2017, 30, 3146–3154. [Google Scholar]
- Mason, C.R.; Gomez, J.E.; Ebner, T.J. Primary motor cortex neuronal discharge during reach-to-grasp: Controlling the hand as a unit. Arch. Ital. Biol. 2002, 140, 229–236. [Google Scholar] [CrossRef]
- Supuk, T.; Bajd, T.; Kurillo, G. Assessment of reach-to-grasp trajectories toward stationary objects. Clin. Biomech. 2011, 26, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Batzianoulis, I.; Krausz, N.E.; Simon, A.M.; Hargrove, L.; Billard, A. Decoding the grasping intention from electromyography during reaching motions. J. Neuroeng. Rehabil. 2018, 15, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).