Microfluidic Compartmentalization Platforms for Single Cell Analysis
Abstract
:1. Introduction
2. Microvalve
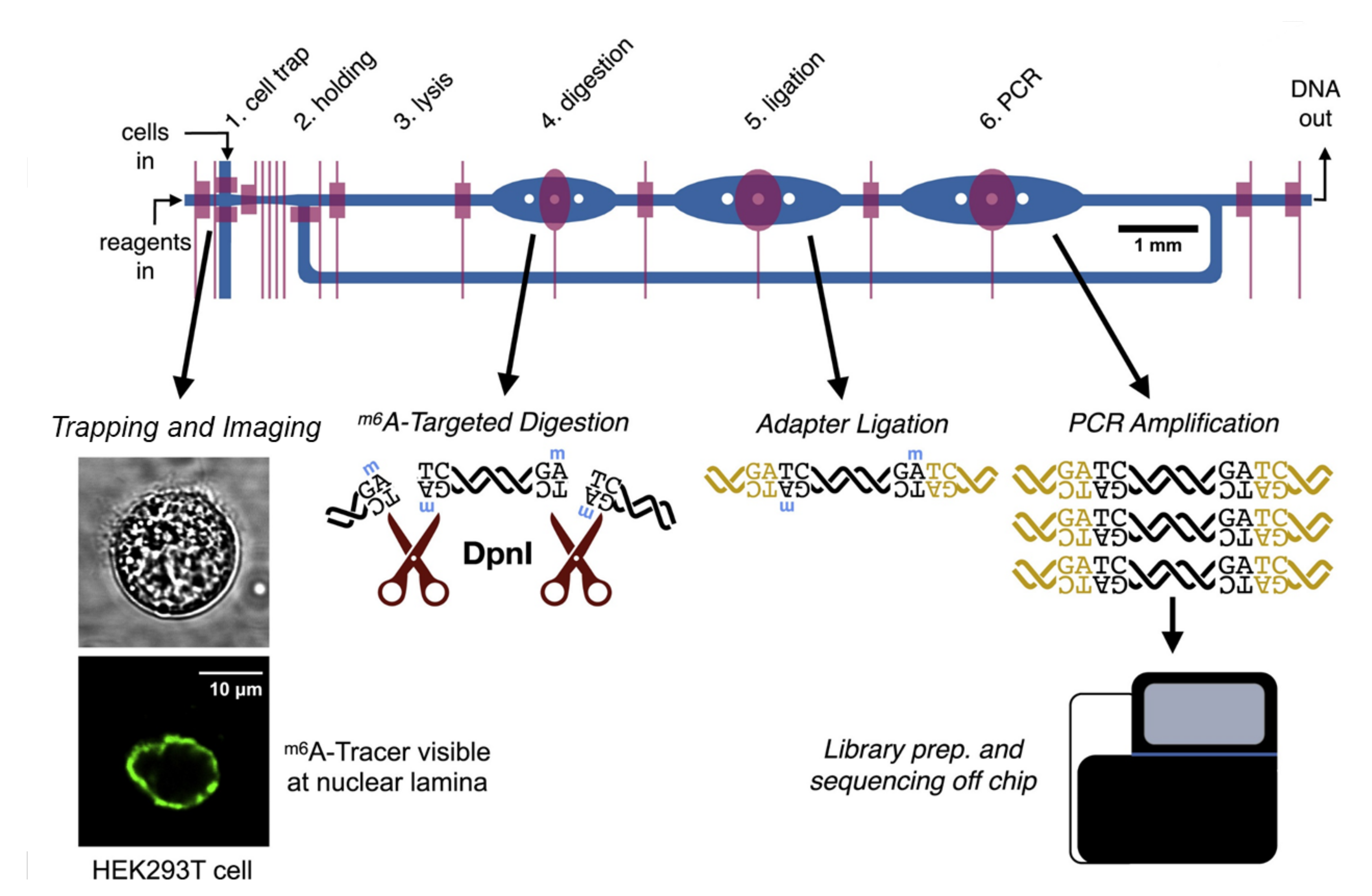
2.1. Genomics
2.2. Proteomics
3. Micro/Nano-Wells
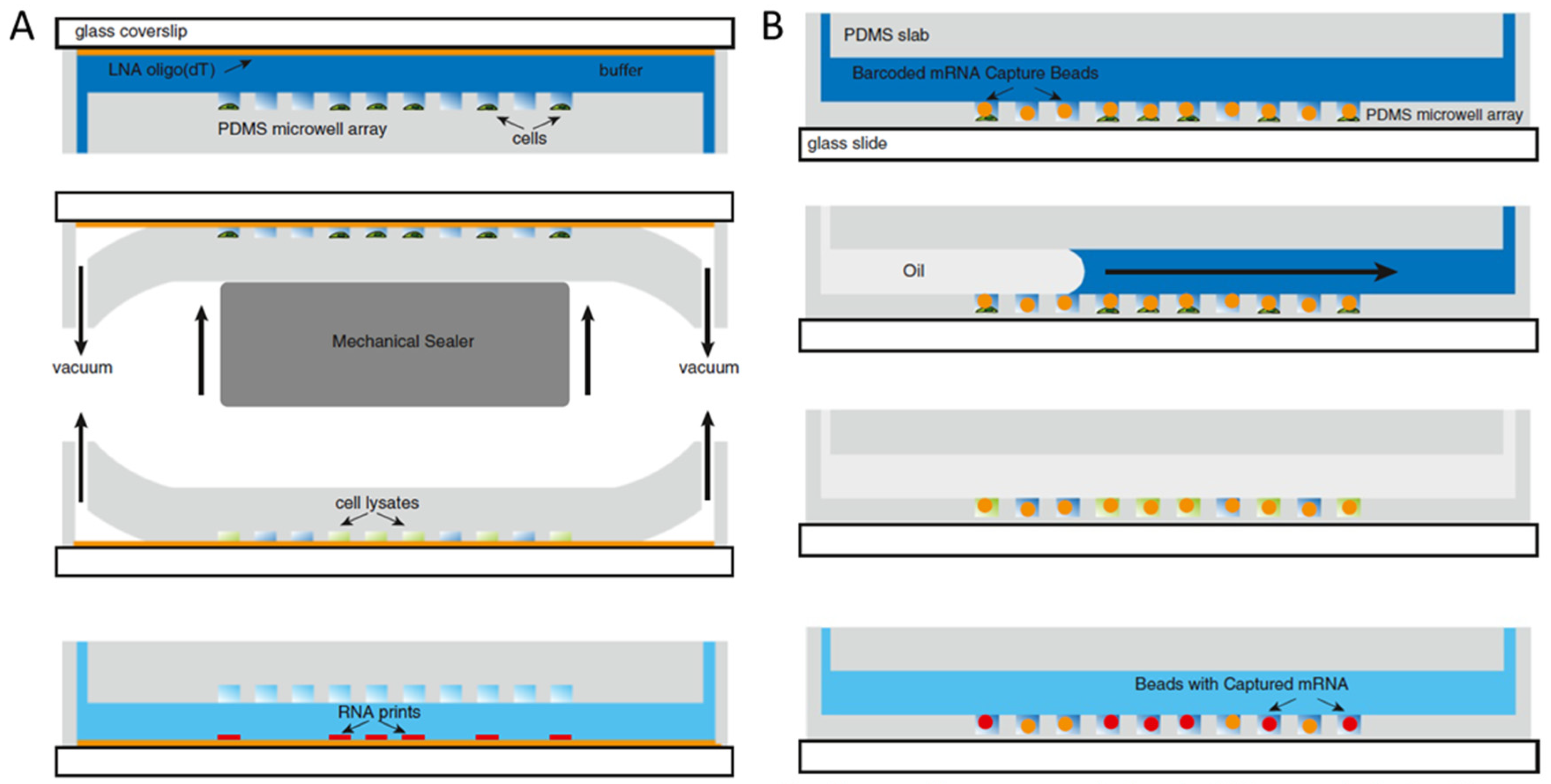
3.1. Genomics
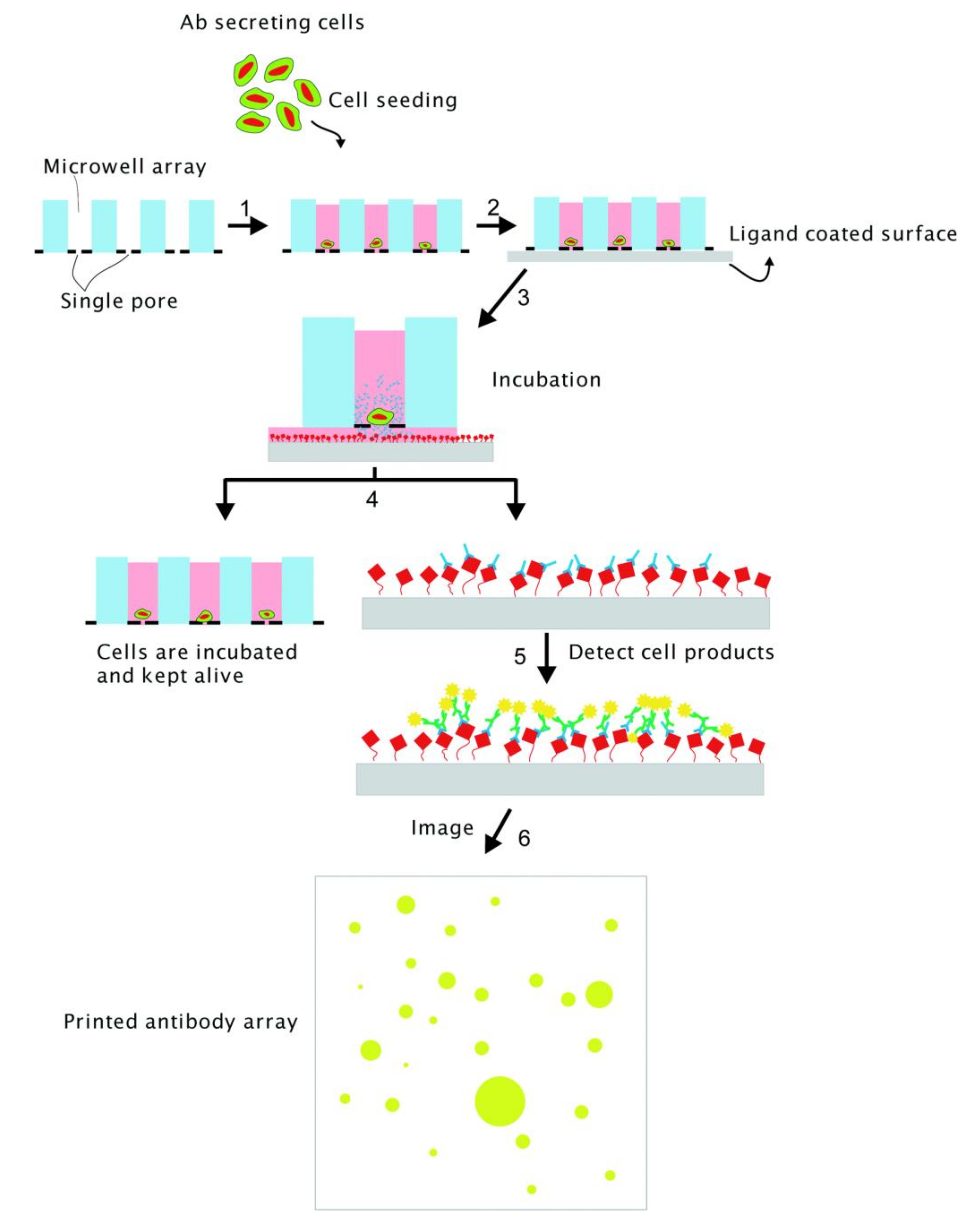
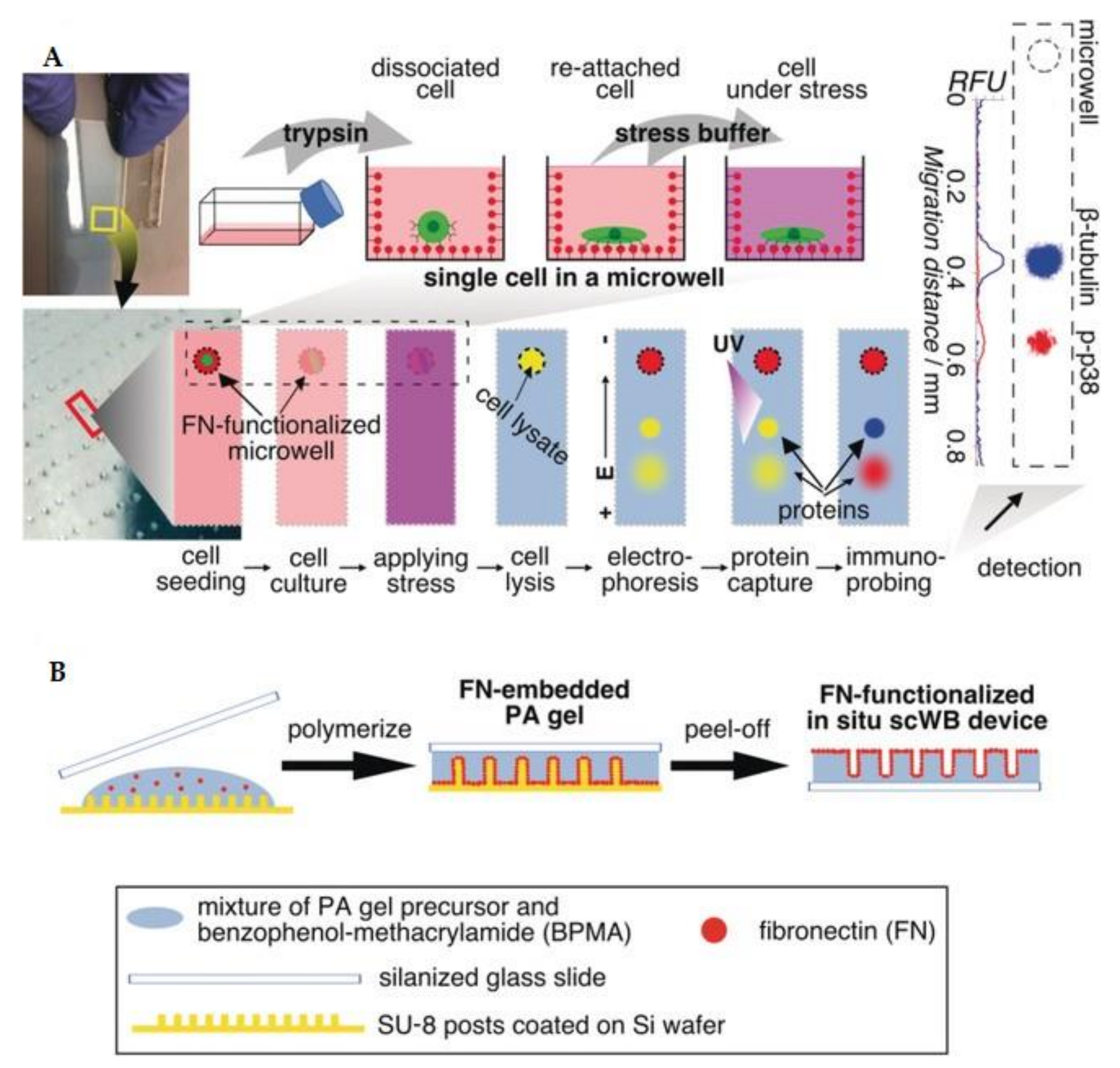
3.2. Proteomics
4. Droplet Microfluidics
4.1. Genomics
4.2. Proteomics
5. Conclusions and Outlooks
Funding
Informed Consent Statement
Conflicts of Interest
References
- Slack, M.D.; Martinez, E.D.; Wu, L.F.; Altschuler, S.J. Characterizing heterogeneous cellular responses to perturbations. Proc. Natl. Acad. Sci. USA 2008, 49, 19306–19311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frick, P.L.; Paudel, B.B.; Tyson, D.R.; Quaranta, V. Quantifying heterogeneity and dynamics of clonal fitness in response to perturbation. J. Cell. Physiol. 2015, 230, 1403–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spudich, J.L.; Koshland, D.E. Non-genetic individuality: Chance in the single cell. Nature 1976, 262, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Junker, J.P.; van Oudenaarden, A. Every Cell Is Special: Genome-wide Studies Add a New Dimension to Single-Cell Biology. Cell 2014, 157, 8–11. [Google Scholar] [CrossRef] [Green Version]
- Altschuler, S.J.; Wu, L.F. Cellular Heterogeneity: Do Differences Make a Difference? Cell 2010, 141, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Heath, J.R.; Ribas, A.; Mischel, P.S. Single-cell analysis tools for drug discovery and development. Nat. Rev. Drug Discov. 2016, 15, 204–216. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chen, S.; Kong, M.; Wang, Z.; Costa, K.D.; Li, R.A.; Sun, D. Enhanced cell sorting and manipulation with combined optical tweezer and microfluidic chip technologies. Lab Chip 2011, 11, 3656–3662. [Google Scholar] [CrossRef]
- Aerts, J.T.; Louis, K.R.; Crandall, S.R.; Govindaiah, G.; Cox, C.L.; Sweedler, J.V. Patch clamp electrophysiology and capillary electrophoresis-mass spectrometry metabolomics for single cell characterization. Anal. Chem. 2014, 86, 3203–3208. [Google Scholar] [CrossRef]
- Tavakoli, H.; Zhou, W.; Ma, L.; Perez, S.; Ibarra, A.; Xu, F.; Zhan, S.; Li, X. Recent advances in microfluidic platforms for single-cell analysis in cancer biology, diagnosis and therapy. TrAC Trends Anal. Chem. 2019, 117, 13–26. [Google Scholar] [CrossRef]
- Davey, H.M.; Kell, D.B. Flow cytometry and cell sorting of heterogeneous microbial populations: The importance of single-cell analyses. Microbiol. Rev. 1996, 60, 641–696. [Google Scholar] [CrossRef]
- Adan, A.; Alizada, G.; Kiraz, Y.; Baran, Y.; Nalbant, A. Flow cytometry: Basic principles and applications. Crit. Rev. Biotechnol. 2016, 37, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Nebe-Von-Caron, G. Functional single-cell analyses: Flow cytometry and cell sorting of microbial populations and communities. FEMS Microbiol. Rev. 2010, 34, 554–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Brouchon, J.; Calvo-Calle, J.M.; Xia, J.; Sun, L.; Zhang, X.; Clayton, K.L.; Ye, F.; Weitz, D.A.; Heyman, J.A. Droplet encapsulation improves accuracy of immune cell cytokine capture assays. Lab Chip 2020, 20, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Briones, J.; Espulgar, W.; Koyama, S.; Takamatsu, H.; Tamiya, E.; Saito, M. A design and optimization of a high throughput valve based microfluidic device for single cell compartmentalization and analysis. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Marshall, D. Microfluidics for single cell analysis. Curr. Opin. Biotechnol. 2012, 23, 110–119. [Google Scholar] [CrossRef]
- Luo, T.; Fan, L.; Zhu, R.; Sun, D. Microfluidic Single-Cell Manipulation and Analysis: Methods and Applications. Micromachines 2019, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.; Lee, L.P. Disposable integrated microfluidics with self-aligned planar microlenses. Sens. Actuators B Chem. 2004, 99, 615–622. [Google Scholar] [CrossRef]
- Guo, M.T.; Rotem, A.; Heyman, J.A.; Weitz, D.A. Droplet microfluidics for high-throughput biological assays. Lab Chip 2012, 12, 2146–2155. [Google Scholar] [CrossRef]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef]
- Jammes, F.C.; Maerkl, S.J. How single-cell immunology is benefiting from microfluidic technologies. Microsystems Nanoeng. 2020, 6, 1–14. [Google Scholar] [CrossRef]
- Yu, Z.; Jin, J.; Shui, L.; Chen, H.; Zhu, Y. Recent advances in microdroplet techniques for single-cell protein analysis. TrAC Trends Anal. Chem. 2021, 143, 116411. [Google Scholar] [CrossRef]
- Gao, D.; Jin, F.; Zhou, M.; Jiang, Y. Recent advances in single cell manipulation and biochemical analysis on microfluidics. Analyst 2019, 144, 766–781. [Google Scholar] [CrossRef]
- Unger, M.A.; Chou, H.-P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic Microfabricated Valves and Pumps by Multilayer Soft Lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Jia, F.; Zhang, W.; Shi, F.; Fang, Z.; Zhao, H.; Hu, Z.; Wei, Z. Device for whole genome sequencing single circulating tumor cells from whole blood. Lab Chip 2019, 19, 3168–3178. [Google Scholar] [CrossRef]
- Schuster, B.; Junkin, M.; Kashaf, S.S.; Romero-Calvo, I.; Kirby, K.; Matthews, J.; Weber, C.R.; Rzhetsky, A.; White, K.P.; Tay, S. Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Kellogg, R.A.; Gomez-Sjoberg, R.; A Leyrat, A.; Tay, S. High-throughput microfluidic single-cell analysis pipeline for studies of signaling dynamics. Nat. Protoc. 2014, 9, 1713–1726. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Olsen, T.; Zhu, J.; Tao, J.; Ponnaiya, B.; Amundson, S.A.; Brenner, D.J.; Lin, Q. A bead-based microfluidic approach to integrated single-cell gene expression analysis by quantitative RT-PCR. RSC Adv. 2015, 5, 4886–4893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakadan, S.M.; Shalek, S.M.P.A.K.; Weitz, D.A. Scaling by shrinking: Empowering single-cell ‘omics’ with microfluidic devices. Nat. Rev. Genet. 2017, 18, 345–361. [Google Scholar] [CrossRef]
- Shalek, A.K.; Satija, R.; Shuga, J.; Trombetta, J.J.; Gennert, D.; Lu, D.; Chen, P.; Gertner, R.S.; Gaublomme, J.T.; Yosef, N.; et al. Single-cell RNA-seq reveals dynamic paracrine control of cellular variation. Nature 2014, 510, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Szulwach, K.E.; Chen, P.; Wang, X.; Wang, J.; Weaver, L.S.; Gonzales, M.L.; Sun, G.; Unger, M.A.; Ramakrishnan, R. Single-Cell Genetic Analysis Using Automated Microfluidics to Resolve Somatic Mosaicism. PLoS ONE 2015, 10, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Frederick, J.; Wang, H.; Hui, S.; Backman, V.; Ji, Z. Spike-in normalization for single-cell RNA-seq reveals dynamic global transcriptional activity mediating anticancer drug response. NAR Genom. Bioinform. 2021, 3, 1–13. [Google Scholar] [CrossRef]
- Hayward, R.J.; Marsh, J.W.; Humphrys, M.S.; Huston, W.M.; Myers, G.S.A. Early Transcriptional Landscapes of Chlamydia trachomatis-Infected Epithelial Cells at Single Cell Resolution. Front. Cell. Infect. Microbiol. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gaublomme, J.T.; Yosef, N.; Lee, Y.; Gertner, R.S.; Yang, L.; Wu, C.; Pandolfi, P.P.; Mak, T.; Satija, R.; Shalek, A.K.; et al. Single-Cell Genomics Unveils Critical Regulators of Th17 Cell Pathogenicity. Cell 2015, 163, 1400–1412. [Google Scholar] [CrossRef] [Green Version]
- Buenrostro, J.D.; Wu, B.; Litzenburger, U.M.; Ruff, D.; Gonzales, M.L.; Snyder, M.P.; Chang, H.Y.; Greenleaf, W.J. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature 2015, 523, 486–490. [Google Scholar] [CrossRef]
- Sun, H.; Olsen, T.; Zhu, J.; Tao, J.; Ponnaiya, B.; Amundson, S.A.; Brenner, D.J.; Lin, Q. A microfluidic approach to parallelized transcriptional profiling of single cells. Microfluid. Nanofluidics 2015, 19, 1429–1440. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Sun, H. A Microchip for Integrated Single-Cell Gene Expression Profiling and Genotoxicity Detection. Sensors 2016, 16, 1489. [Google Scholar] [CrossRef] [Green Version]
- Sun, H. A multi-layer microchip for high-throughput single-cell gene expression profiling. Anal. Biochem. 2016, 508, 1–8. [Google Scholar] [CrossRef]
- Shang, J.; Welch, D.; Buonanno, M.; Ponnaiya, B.; Garty, G.; Olsen, T.; Amundson, S.A.; Lin, Q. An Integrated Preprocessing Approach for Exploring Single-Cell Gene Expression in Rare Cells. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, H.; Huang, Y.; Shu, W.; Feng, H.; Cai, J.; Zhong, J.F.; Chen, Y. Improving single-cell transcriptome sequencing efficiency with a microfluidic phase-switch device. Analyst 2019, 144, 7185–7191. [Google Scholar] [CrossRef] [PubMed]
- Altemose, N.; Maslan, A.; Rios-Martinez, C.; Lai, A.; White, J.A.; Streets, A. μDamID: A Microfluidic Approach for Joint Imaging and Sequencing of Protein-DNA Interactions in Single Cells. Cell Syst. 2020, 11, 354–366.e9. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.N.; Gupta, A.; Zalavadia, M.D.; Streets, A. μCB-seq: Microfluidic cell barcoding and sequencing for high-resolution imaging and sequencing of single cells. Lab Chip 2020, 20, 3899–3913. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, J.A.; Aliaghaei, M.; Nguyen, Q.H.; Kessenbrock, K.; Haun, J.B. Microfluidic platform accelerates tissue processing into single cells for molecular analysis and primary culture models. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Finck, A.; Fan, R. Single-Cell Omics Analyses Enabled by Microchip Technologies. Annu. Rev. Biomed. Eng. 2019, 21, 365–393. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Qin, L.; Wei, W.; Geng, F.; Fan, R.; Shin, Y.S.; Guo, D.; Hood, L.; Mischel, P.S.; Heath, J.R. Single-cell proteomic chip for profiling intracellular signaling pathways in single tumor cells. Proc. Natl. Acad. Sci. USA 2012, 109, 419–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blazek, M.; Santisteban, T.S.; Zengerle, R.; Meier, M. Analysis of fast protein phosphorylation kinetics in single cells on a microfluidic chip. Lab Chip 2015, 15, 726–734. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Li, R.; Jia, F.; Hu, Z.; Li, Q.; Wei, Z. A microfluidic chip for screening high-producing hybridomas at single cell level. Lab Chip 2020, 20, 4043–4051. [Google Scholar] [CrossRef]
- Wu, A.; Kawahara, T.L.; Rapicavoli, N.A.; Van Riggelen, J.; Shroff, E.H.; Xu, L.; Felsher, D.W.; Chang, H.Y.; Quake, S.R. High throughput automated chromatin immunoprecipitation as a platform for drug screening and antibody validation. Lab Chip 2012, 12, 2190–2198. [Google Scholar] [CrossRef] [Green Version]
- Singhal, A.; Haynes, C.A.; Hansen, C.L. Microfluidic Measurement of Antibody−Antigen Binding Kinetics from Low-Abundance Samples and Single Cells. Anal. Chem. 2010, 82, 8671–8679. [Google Scholar] [CrossRef]
- Lecault, V.; VanInsberghe, M.; Sekulovic, S.; Knapp, D.J.; Wohrer, S.; Bowden, W.; Viel, F.; McLaughlin, T.; Jarandehei, A.; Miller, M.; et al. High-throughput analysis of single hematopoietic stem cell proliferation in microfluidic cell culture arrays. Nat. Methods 2011, 8, 581–586. [Google Scholar] [CrossRef]
- Junkin, M.; Kaestli, A.J.; Cheng, Z.; Jordi, C.; Albayrak, C.; Hoffmann, A.; Tay, S. High-Content Quantification of Single-Cell Immune Dynamics. Cell Rep. 2016, 15, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Seah, Y.F.S.; Hu, H.; Merten, C.A. Microfluidic single-cell technology in immunology and antibody screening. Mol. Asp. Med. 2018, 59, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Harriman, W.D.; Collarini, E.J.; Sperinde, G.V.; Strandh, M.; Fatholahi, M.M.; Dutta, A.; Lee, Y.; Mettler, S.E.; Keyt, B.A.; Ellsworth, S.L.; et al. Antibody discovery via multiplexed single cell characterization. J. Immunol. Methods 2009, 341, 135–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, J.C.; Ronan, J.L.; Grotenbreg, G.M.; Van Der Veen, A.G.; Ploegh, H.L. A microengraving method for rapid selection of single cells producing antigen-specific antibodies. Nat. Biotechnol. 2006, 24, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.; Ozawa, T.; Tajiri, K.; Obata, T.; Kondo, S.; Kinoshita, K.; Kadowaki, S.; Takahashi, K.; Sugiyama, T.; Kishi, H.; et al. A rapid and efficient single-cell manipulation method for screening antigen-specific antibody–secreting cells from human peripheral blood. Nat. Med. 2009, 15, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Ogunniyi, A.O.; Story, C.M.; Papa, E.; Guillen, E.; Love, J.C. Screening individual hybridomas by microengraving to discover monoclonal antibodies. Nat. Protoc. 2009, 4, 767–782. [Google Scholar] [CrossRef] [Green Version]
- Revzin, A.; Sekine, K.; Sin, A.; Tompkins, R.G.; Toner, M. Development of a microfabricated cytometry platform for characterization and sorting of individual leukocytes. Lab Chip 2005, 5, 30–37. [Google Scholar] [CrossRef]
- Zhu, H.; Stybayeva, G.; Silangcruz, J.; Yan, J.; Ramanculov, E.; Dandekar, S.; George, M.D.; Revzin, A. Detecting Cytokine Release from Single T-cells. Anal. Chem. 2009, 81, 8150–8156. [Google Scholar] [CrossRef] [Green Version]
- Yamamura, S.; Kishi, H.; Tokimitsu, Y.; Kondo, S.; Honda, R.; Rao, S.R.; Omori, M.; Tamiya, E.; Muraguchi, A. Single-Cell Microarray for Analyzing Cellular Response. Anal. Chem. 2005, 77, 8050–8056. [Google Scholar] [CrossRef]
- Jin, A.; Ozawa, T.; Tajiri, K.; Obata, T.; Kishi, H.; Muraguchi, A. Rapid isolation of antigen-specific antibody-secreting cells using a chip-based immunospot array. Nat. Protoc. 2011, 6, 668–676. [Google Scholar] [CrossRef]
- Kwon, D.; Kim, J.; Chung, S.; Park, I. A nanowire-integrated microfluidic device for hydrodynamic trapping and anchoring of bacterial cells. In Proceedings of the 2014 IEEE 27th International Conference on Micro ElectroMechanical Systems (MEMS), San Francisco, CA, USA, 26–30 January 2014; pp. 246–249. [Google Scholar] [CrossRef]
- Narayanamurthy, V.; Nagarajan, S.; Khan, A.Y.F.; Samsuri, F.; Sridhar, T.M. Microfluidic hydrodynamic trapping for single cell analysis: Mechanisms, methods and applications. Anal. Methods 2017, 9, 3751–3772. [Google Scholar] [CrossRef]
- Dura, B.; Dougan, S.K.; Barisa, M.; Hoehl, M.M.; Lo, C.T.; Ploegh, H.L.; Voldman, J. Profiling lymphocyte interactions at the single-cell level by microfluidic cell pairing. Nat. Commun. 2015, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Dura, B.; Servo, M.M.; Barry, R.M.; Ploeghf, H.L.; Dougand, S.K.; Voldman, J. Longitudinal multiparameter assay of lymphocyte interactions from onset by microfluidic cell pairing and culture. Proc. Natl. Acad. Sci. USA 2016, 113, E3599–E3608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Liu, X.; Li, P.; Liu, F.; Kojima, M.; Huang, Q.; Arai, T. On-Chip Cell–Cell Interaction Monitoring at Single-Cell Level by Efficient Immobilization of Multiple Cells in Adjustable Quantities. Anal. Chem. 2020, 92, 11607–11616. [Google Scholar] [CrossRef]
- Gole, J.; Gore, A.; Richards, A.; Chiu, Y.-J.; Fung, H.-L.; Bushman, D.; Chiang, H.-I.; Chun, J.; Lo, Y.-H.; Zhang, K. Massively parallel polymerase cloning and genome sequencing of single cells using nanoliter microwells. Nat. Biotechnol. 2013, 31, 1126–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Wang, Z.; Li, Z.; Kim, J.; Deng, Y.; Li, Y.; Heath, J.R.; Wei, W.; Lu, S.; Shi, Q. High-throughput screening of rare metabolically active tumor cells in pleural effusion and peripheral blood of lung cancer patients. Proc. Natl. Acad. Sci. USA 2017, 114, 2544–2549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Dong, X.; Tu, Y.; Miao, G.; Zhang, Z.; Zhang, L.; Wei, Z.; Yu, D.; Qiu, X. Methods and platforms for analysis of nucleic acids from single-cell based on microfluidics. Microfluid. Nanofluidics 2021, 25, 1–19. [Google Scholar] [CrossRef]
- Bose, S.; Wan, Z.; Carr, A.; Rizvi, A.H.; Vieira, G.; Pe’Er, D.; Sims, P.A. Scalable microfluidics for single-cell RNA printing and sequencing. Genome Biol. 2015, 16, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Sims, P.A. An Automated Microwell Platform for Large-Scale Single Cell RNA-Seq. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gierahn, T.M.; Wadsworth, M.H., II; Hughes, T.K.; Bryson, B.D.; Butler, A.; Satija, R.; Fortune, S.; Love, J.C.; Shalek, A.K. Seq-Well: Portable, low-cost RNA sequencing of single cells at high throughput. Nat. Methods 2017, 14, 395–398. [Google Scholar] [CrossRef]
- Pai, J.A.; Satpathy, A.T. High-throughput and single-cell T cell receptor sequencing technologies. Nat. Methods 2021, 18, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.K.; Wadsworth, M.H.; Gierahn, T.M.; Do, T.; Weiss, D.; Andrade, P.R.; Ma, F.; Silva, B.J.D.A.; Shao, S.; Tsoi, L.C.; et al. Second-Strand Synthesis-Based Massively Parallel scRNA-Seq Reveals Cellular States and Molecular Features of Human Inflammatory Skin Pathologies. Immunity 2020, 53, 878–894.e7. [Google Scholar] [CrossRef]
- Tokimitsu, Y.; Kishi, H.; Kondo, S.; Honda, R.; Tajiri, K.; Motoki, K.; Ozawa, T.; Kadowaki, S.; Obata, T.; Fujiki, S.; et al. Single lymphocyte analysis with a microwell array chip. Cytom. Part A 2007, 71, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Kinoshita, K.; Kadowaki, S.; Tajiri, K.; Kondo, S.; Honda, R.; Ikemoto, M.; Piao, L.; Morisato, A.; Fukurotani, K.; et al. MAC-CCD system: A novel lymphocyte microwell-array chip system equipped with CCD scanner to generate human monoclonal antibodies against influenza virus. Lab Chip 2009, 9, 158–163. [Google Scholar] [CrossRef]
- Dupont, C.D.; Scully, I.L.; Zimnisky, R.M.; Monian, B.; Rossitto, C.P.; O’Connell, E.B.; Jansen, K.U.; Anderson, A.S.; Love, J.C. Two Vaccines for Staphylococcus aureus Induce a B-Cell-Mediated Immune Response. mSphere 2018, 3, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Xue, Q.; Eisele, M.R.; Sulistijo, E.S.; Brower, K.; Han, L.; Amir, E.-A.D.; Pe’Er, D.; Miller-Jensen, K.; Fan, R. Highly multiplexed profiling of single-cell effector functions reveals deep functional heterogeneity in response to pathogenic ligands. Proc. Natl. Acad. Sci. USA 2015, 112, E607–E615. [Google Scholar] [CrossRef] [Green Version]
- McWhorter, F.Y.; Smith, T.D.; Luu, T.U.; Rahim, M.K.; Haun, J.B.; Liu, W.F. Macrophage secretion heterogeneity in engineered microenvironments revealed using a microwell platform. Integr. Biol. 2016, 8, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Bettini, E.; Paczkowski, P.; Qiong, X.; Kaiser, A.; McConnell, T.; Kodrasi, O.; Quigley, M.F.; Heath, J.; Fan, R.; et al. Single-cell multiplexed cytokine profiling of CD19 CAR-T cells reveals a diverse landscape of polyfunctional antigen-specific response. J. Immunother. Cancer 2017, 5, 1–16. [Google Scholar] [CrossRef]
- Torres, A.J.; Hill, A.S.; Love, J.C. Nanowell-Based Immunoassays for Measuring Single-Cell Secretion: Characterization of Transport and Surface Binding. Anal. Chem. 2014, 86, 11562–11569. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Kim, H.; Doh, J. Multifunctional Microwell Arrays for Single Cell Level Functional Analysis of Lymphocytes. Bioconjugate Chem. 2018, 29, 672–679. [Google Scholar] [CrossRef]
- Fitzgerald, V.; Manning, B.; O’Donnell, B.; O’Reilly, B.; O’Sullivan, D.; O’Kennedy, R.; Leonard, P. Exploiting Highly Ordered Subnanoliter Volume Microcapillaries as Microtools for the Analysis of Antibody Producing Cells. Anal. Chem. 2015, 87, 997–1003. [Google Scholar] [CrossRef]
- Stevens, M.; Oomens, L.; Broekmaat, J.; Weersink, J.; Abali, F.; Swennenhuis, J.; Tibbe, A. VyCAP’s puncher technology for single cell identification, isolation, and analysis. Cytom. Part A 2018, 93, 1255–1259. [Google Scholar] [CrossRef] [Green Version]
- Abali, F.; Broekmaat, J.; Tibbe, A.; Schasfoort, R.B.M.; Zeune, L.; Terstappen, L.W. A microwell array platform to print and measure biomolecules produced by single cells. Lab Chip 2019, 19, 1850–1859. [Google Scholar] [CrossRef]
- Abali, F.; Stevens, M.; Tibbe, A.; Terstappen, L.; van der Velde, P.; Schasfoort, R. Isolation of single cells for protein therapeutics using microwell selection and Surface Plasmon Resonance imaging. Anal. Biochem. 2017, 531, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Naguro, I.; Herr, A.E. In Situ Single-Cell Western Blot on Adherent Cell Culture. Angew. Chem. Int. Ed. 2019, 58, 13929–13934. [Google Scholar] [CrossRef]
- Gomez Martinez, A.E.; Herr, A.E. Programmed Cell-Death Mechanism Analysis Using Same-Cell, Multimode DNA and Proteoform Electrophoresis. ACS Meas. Sci. Au 2021, 1, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.M.; Liyu, A.V.; Tsai, C.-F.; Moore, R.J.; Orton, D.J.; Chrisler, W.B.; Gaffrey, M.J.; Liu, T.; Smith, R.D.; Kelly, R.T.; et al. Automated Coupling of Nanodroplet Sample Preparation with Liquid Chromatography–Mass Spectrometry for High-Throughput Single-Cell Proteomics. Anal. Chem. 2020, 92, 10588–10596. [Google Scholar] [CrossRef]
- Woo, J.; Williams, S.M.; Markillie, L.M.; Feng, S.; Tsai, C.-F.; Aguilera-Vazquez, V.; Sontag, R.L.; Moore, R.J.; Hu, D.; Mehta, H.S.; et al. High-throughput and high-efficiency sample preparation for single-cell proteomics using a nested nanowell chip. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Teh, S.Y.; Lin, R.; Hung, L.H.; Lee, A.P. Droplet Microfluidics. Lab Chip 2008, 8, 198–220. [Google Scholar] [CrossRef] [PubMed]
- Matuła, K.; Rivello, F.; Huck, W.T.S. Single-Cell Analysis Using Droplet Microfluidics. Adv. Biosyst. 2019, 4, e1900188. [Google Scholar] [CrossRef] [Green Version]
- Tang, F.; Barbacioru, C.; Wang, Y.; Nordman, E.; Lee, C.; Xu, N.; Wang, X.; Bodeau, J.; Tuch, B.B.; Siddiqui, A.; et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 2009, 6, 377–382. [Google Scholar] [CrossRef]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015, 161, 1202–1214. [Google Scholar] [CrossRef] [Green Version]
- Zilionis, R.; Nainys, J.; Veres, A.; Savova, V.; Zemmour, D.; Klein, R.Z.A.V.V.S.A.M.; Mazutis, R.Z.J.N.L. Single-cell barcoding and sequencing using droplet microfluidics. Nat. Protoc. 2017, 12, 44–73. [Google Scholar] [CrossRef]
- Gawad, C.; Koh, W.; Quake, S.R. Single-cell genome sequencing: Current state of the science. Nat. Rev. Genet. 2016, 17, 175–188. [Google Scholar] [CrossRef]
- Lan, F.; Demaree, B.; Ahmed, N.; Abate, A.R. Single-cell genome sequencing at ultra-high-throughput with microfluidic droplet barcoding. Nat. Biotechnol. 2017, 35, 640–646. [Google Scholar] [CrossRef] [Green Version]
- Zheng, G.X.Y.; Terry, J.M.; Belgrader, P.; Ryvkin, P.; Bent, Z.W.; Wilson, R.; Ziraldo, S.B.; Wheeler, T.D.; McDermott, G.P.; Zhu, J.; et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ruff, D.W.; Dhingra, D.M.; Thompson, K.; Marin, J.A.; Ooi, A.T. High-throughput multimodal single-cell targeted DNA and Surface Protein Analysis Using the Mission Bio Tapestri Platform. Methods Mol. Biol. 2022, 2386, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Minussi, D.C.; Nicholson, M.D.; Ye, H.; Davis, A.; Wang, K.; Baker, T.; Tarabichi, M.; Sei, E.; Du, H.; Rabbani, M.; et al. Breast tumours maintain a reservoir of subclonal diversity during expansion. Nature 2021, 592, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Liu, Y.; Zhang, M.; Xu, X.; Chen, Y.; Zhang, H.; Yang, C.J. Microfluidic single-cell transcriptomics: Moving towards multimodal and spatiotemporal omics. Lab Chip 2021, 21, 3829–3849. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, J.; Wu, L.; Guo, J.; Song, Y.; Tian, T.; Wang, W.; Zhu, Z.; Yang, C. Microfluidic Single-Cell Omics Analysis. Small 2020, 16, e1903905. [Google Scholar] [CrossRef] [PubMed]
- Shembekar, N.; Chaipan, C.; Utharala, R.; Merten, C.A. Droplet-based microfluidics in drug discovery, transcriptomics and high-throughput molecular genetics. Lab Chip 2016, 16, 1314–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konry, T.; Dominguez-Villar, M.; Baecher-Allan, C.; Hafler, D.A.; Yarmush, M. Droplet-based microfluidic platforms for single T cell secretion analysis of IL-10 cytokine. Biosens. Bioelectron. 2011, 26, 2707–2710. [Google Scholar] [CrossRef] [Green Version]
- El Debs, B.E.; Utharala, R.; Balyasnikova, I.V.; Griffiths, A.D.; Merten, C.A. Functional single-cell hybridoma screening using droplet-based microfluidics. Proc. Natl. Acad. Sci. USA 2012, 109, 11570–11575. [Google Scholar] [CrossRef] [Green Version]
- Mazutis, L.; Gilbert, J.; Ung, W.; A Weitz, D.; Griffiths, A.D.; A Heyman, J. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 2013, 8, 870–891. [Google Scholar] [CrossRef]
- Shembekar, N.; Hu, H.; Eustace, D.; Merten, C.A. Single-Cell Droplet Microfluidic Screening for Antibodies Specifically Binding to Target Cells. Cell Rep. 2018, 22, 2206–2215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeKosky, B.J.; Ippolito, G.C.; Deschner, R.P.; Lavinder, J.J.; Wine, Y.; Rawlings, B.M.; Varadarajan, N.; Giesecke, C.; Dörner, T.; Andrews, S.F.; et al. High-throughput sequencing of the paired human immunoglobulin heavy and light chain repertoire. Nat. Biotechnol. 2013, 31, 166–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeKosky, B.J.; Kojima, T.; Rodin, A.; Charab, W.; Ippolito, G.C.; Ellington, A.D.; Georgiou, G. In-depth determination and analysis of the human paired heavy- and light-chain antibody repertoire. Nat. Med. 2015, 21, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Gérard, A.; Woolfe, A.; Mottet, G.; Reichen, M.; Castrillon, C.; Menrath, V.; Ellouze, S.; Poitou, A.; Doineau, R.; Briseno-Roa, L.; et al. High-throughput single-cell activity-based screening and sequencing of antibodies using droplet microfluidics. Nat. Biotechnol. 2020, 38, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Kemna, E.W.M.; Schoeman, R.M.; Wolbers, F.; Vermes, I.; Weitz, D.A.; Van Den Berg, A. High-yield cell ordering and deterministic cell-in-droplet encapsulation using Dean flow in a curved microchannel. Lab Chip 2012, 12, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Lagus, T.P.; Edd, J.F. High-throughput co-encapsulation of self-ordered cell trains: Cell pair interactions in microdroplets. RSC Adv. 2013, 3, 20512–20522. [Google Scholar] [CrossRef] [Green Version]
- Ding, R.; Hung, K.-C.; Mitra, A.; Ung, L.W.; Lightwood, D.; Tu, R.; Starkie, D.; Cai, L.; Mazutis, L.; Chong, S.; et al. Rapid isolation of antigen-specific B-cells using droplet microfluidics. RSC Adv. 2020, 10, 27006–27013. [Google Scholar] [CrossRef]
- Akbari, S.; Pirbodaghi, T. A droplet-based heterogeneous immunoassay for screening single cells secreting antigen-specific antibodies. Lab Chip 2014, 14, 3275–3280. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, C.J. Hydrogel Droplet Microfluidics for High-Throughput Single Molecule/Cell Analysis. Accounts Chem. Res. 2017, 50, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Kim, J.-H.; Zhang, D.; Lee, K.-H.; Cangelosi, G.A.; Soelberg, S.D.; Furlong, C.E.; Chung, J.-H.; Shen, A.Q. Microfluidic one-step synthesis of alginate microspheres immobilized with antibodies. J. R. Soc. Interface 2013, 10, 20130566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kartalov, E.P.; Zhong, J.F.; Scherer, A.; Quake, S.R.; Taylor, C.R.; Anderson, W.F. High-throughput multi-antigen microfluidic fluorescence immunoassays. BioTechniques 2006, 40, 85–90. [Google Scholar] [CrossRef] [Green Version]
- White, A.K.; VanInsberghe, M.; Petriv, I.; Hamidi, M.; Sikorski, D.; Marra, M.A.; Piret, J.; Aparicio, S.; Hansen, C.L. High-throughput microfluidic single-cell RT-qPCR. Proc. Natl. Acad. Sci. USA 2011, 108, 13999–14004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Yang, M.; Deng, Y.; Su, G.; Enninful, A.; Guo, C.C.; Tebaldi, T.; Zhang, D.; Kim, D.; Bai, Z.; et al. High-Spatial-Resolution Multi-Omics Sequencing via Deterministic Barcoding in Tissue. Cell 2020, 183, 1665–1681.e18. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.; Ramalingam, N. Integrated Fluidic Circuits for Single-Cell Omics and Multi-omics Applications. Adv. Exp. Med. Biol. 2019, 1129, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Leonavicius, K.; Nainys, J.; Kuciauskas, D.; Mazutis, L. Multi-omics at single-cell resolution: Comparison of experimental and data fusion approaches. Curr. Opin. Biotechnol. 2019, 55, 159–166. [Google Scholar] [CrossRef]




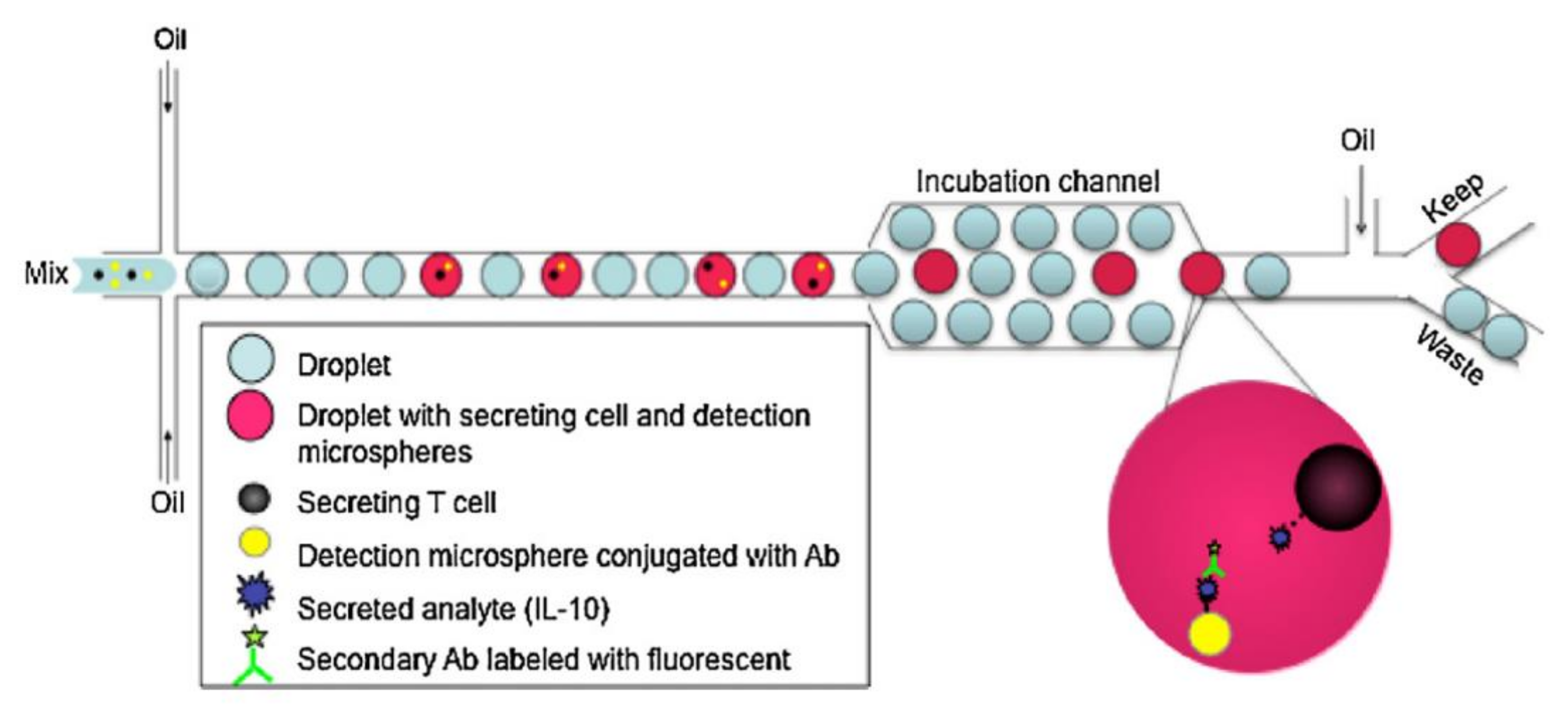
| Microvalves | Microwells | Droplets | |
|---|---|---|---|
| Sample Volume | 2–700 nL/chamber [27,44] | 100 pL–1.5 nL/well [68,82] | 1 fL–10 nL/droplet [19,89] |
| Multiplexing Capability | High | Low | Medium |
| Selective Cell Retrieval | Easy | Medium | Difficult |
| Throughput | 10–300 cells/run [24,115,116] | 100–105 cells/run [66,88] | >10,000 cells/run [92] |
| Scale-up Capacity | Low | Medium | High |
| Ease-of-Operation | Difficult | Easy | Medium |
| Integration Potential | High | Low | Medium |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Chen, J.-Y.; Ataei, M.; Lee, A. Microfluidic Compartmentalization Platforms for Single Cell Analysis. Biosensors 2022, 12, 58. https://doi.org/10.3390/bios12020058
Luo X, Chen J-Y, Ataei M, Lee A. Microfluidic Compartmentalization Platforms for Single Cell Analysis. Biosensors. 2022; 12(2):58. https://doi.org/10.3390/bios12020058
Chicago/Turabian StyleLuo, Xuhao, Jui-Yi Chen, Marzieh Ataei, and Abraham Lee. 2022. "Microfluidic Compartmentalization Platforms for Single Cell Analysis" Biosensors 12, no. 2: 58. https://doi.org/10.3390/bios12020058
APA StyleLuo, X., Chen, J.-Y., Ataei, M., & Lee, A. (2022). Microfluidic Compartmentalization Platforms for Single Cell Analysis. Biosensors, 12(2), 58. https://doi.org/10.3390/bios12020058






