Detection of Dibutyl Phthalate in Surface Water by Fluorescence Polarization Immunoassay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
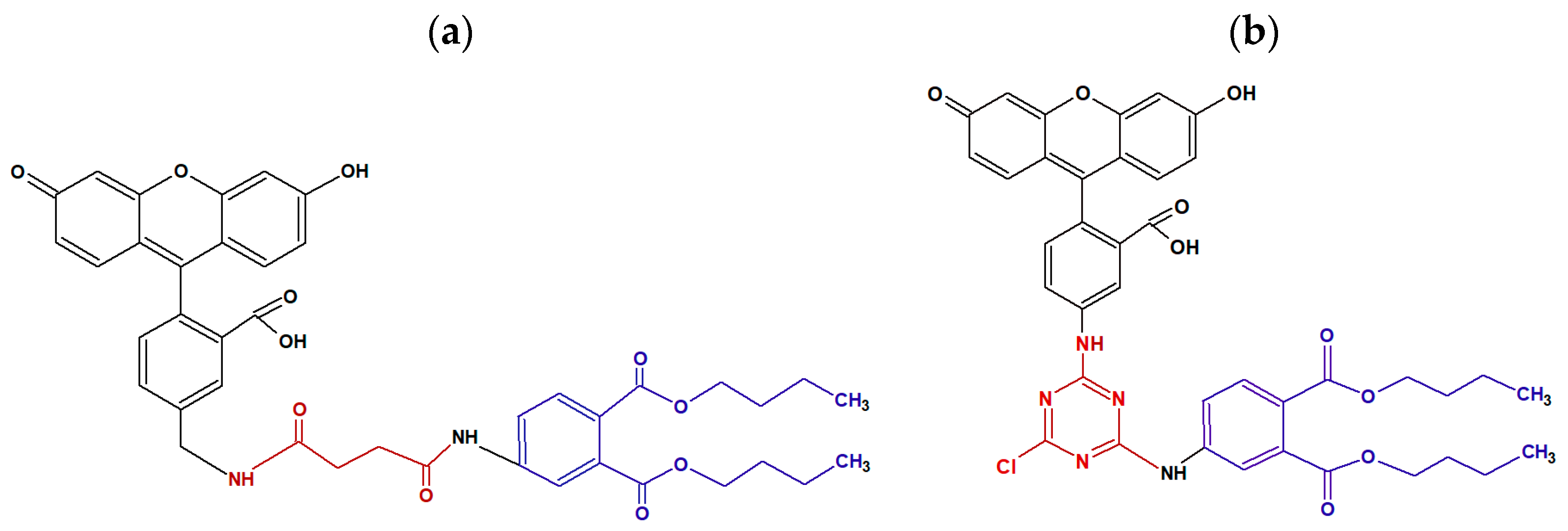
2.2. Synthesis of the Fluorescein Labeled Antigen (Tracer)
Purification of the Tracer
2.3. Producing Monoclonal Antibody
2.4. FPIA for DBP
2.4.1. Kinetics of MAbs—Tracer Interaction
2.4.2. Competitive FPIA
2.5. FPIA Specificity
2.6. Preparation of Water Samples and Recovery Test
2.7. Determination of DBP in Water Samples by GC-MS
3. Results and Discussion
3.1. Obtaining and Characterization of the Specific Reagents
3.2. Studying of Binding Kinetic DBP-AMF and DBP-DTAF with Monoclonal Antibody
3.3. Optimization FPIA for DBP Determination and Studying of Method Crossreactivity
3.4. Determination DBP in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsu, L.H.H.; Aryasomayajula, A.; Selvaganapathy, P.R. A review of sensing systems and their need for environmental water monitoring. Crit. Rev. Biomed. Eng. 2016, 44, 357–382. [Google Scholar] [CrossRef]
- Galindo-Miranda, J.M.; Guízar-González, C.; Becerril-Bravo, E.J.; León-Becerril, E.; Vallejo-Rodríguez, R. Occurrence of emerging contaminants in environmental surface waters and their analytical methodology—A review. Water Sci. Technol. Water Supply 2019, 19, 1871–1884. [Google Scholar] [CrossRef]
- Bonato, M.; Corrà, F.; Bellio, M.; Irato, P.; Santovito, G. Pfas environmental pollution and antioxidant responses: An overview of the impact on human field. Int. J. Environ. Res. Public Health 2020, 17, 8020. [Google Scholar] [CrossRef] [PubMed]
- Savoca, D.; Barreca, S.; Lo Coco, R.; Punginelli, D.; Orecchio, S.; Maccotta, A. Environmental Aspect Concerning Phthalates Contamination: Analytical Approaches and Assessment of Biomonitoring in the Aquatic Environment. Environments 2023, 10, 99. [Google Scholar] [CrossRef]
- Kannan, K.; Stathis, A.; Mazzella, M.J.; Andra, S.S.; Barr, D.B.; Hecht, S.S.; Merrill, L.S.; Galusha, A.L.; Parsons, P.J. Quality assurance and harmonization for targeted biomonitoring measurements of environmental organic chemicals across the Children’s Health Exposure Analysis Resource laboratory network. Int. J. Hyg. Environ. Health 2021, 234, 113741. [Google Scholar] [CrossRef] [PubMed]
- Dueñas-Moreno, J.; Vázquez-Tapia, I.; Mora, A.; Cervantes-Avilés, P.; Mahlknecht, J.; Capparelli, M.V.; Kumar, M.; Wang, C. Occurrence, ecological and health risk assessment of phthalates in a polluted urban river used for agricultural land irrigation in central Mexico. Environ. Res. 2023, 240, 117454. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Zhang, H.; Zhang, C.; Chen, Y.; Li, C.; Hu, Y.; Yu, X.; Zhang, B.; Lin, X. A Nanozymatic-Mediated Smartphone Colorimetric Sensing Platform for the Detection of Dimethyl Phthalate (DMP) and Dibutyl Phthalate (DBP). Biosensors 2023, 13, 919. [Google Scholar] [CrossRef]
- Orecchio, S.; Indelicato, R.; Barreca, S. Determination of Selected Phthalates by Gas Chromatography–Mass Spectrometry in Personal Perfumes. J. Toxicol. Environ. Health Part A 2015, 78, 1008–1018. [Google Scholar] [CrossRef]
- Amorello, D.; Indelicato, R.; Barreca, S.; Orecchio, S.; Orecchio, S. Analytical Method for Quantification of Several Phthalate Acid Esters by Gas Chromatography-Mass Spectrometry in Coffee Brew Samples. ChemistryOpen 2022, 12, e202200082. [Google Scholar] [CrossRef]
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef]
- Naveen, K.V.; Saravanakumar, K.; Zhang, X.; Sathiyaseelan, A.; Wang, M. Impact of environmental phthalate on human health and their bioremediation strategies using fungal cell factory—A review. Environ. Res. Part 1 2022, 214, 113781. [Google Scholar] [CrossRef] [PubMed]
- Baloyi, N.D.; Tekere, M.; Maphangwa, K.W.; Masindi, V. Insights into the Prevalence and Impacts of Phthalate Esters in Aquatic Ecosystems. Front. Environ. Sci. 2021, 9, 684190. [Google Scholar] [CrossRef]
- Berenstein, G.; Hughes, E.A.; Zalts, A.; Basack, S.; Bonesi, S.M.; Montserrat, J.M. Environmental fate of dibutylphthalate in agricultural plastics: Photodegradation, migration and ecotoxicological impact on soil. Chemosphere 2022, 290, 133221. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhu, F.; Zhu, C.; Chen, Z.; Liu, S.; Wang, C.; Gu, C. Dibutyl phthalate release from polyvinyl chloride microplastics: Influence of plastic properties and environmental factors. Water Res. 2021, 204, 117597. [Google Scholar] [CrossRef] [PubMed]
- Li, E.H.; Xu, B.H.; Wei, H.B.; Bai, Y.C.; Zhang, Q.; Yu, W.W.; Xu, Z.H.; Qi, X.L.; Zhang, D.H.; Wang, H. Molecular mechanism of di-n-butyl phthalate promotion of bladder cancer development. Toxicol. In Vitro 2023, 86, 105508. [Google Scholar] [CrossRef]
- Xie, F.; Chen, X.; Weng, S.; Xia, T.; Sun, X.; Luo, T.; Li, P. Effects of two environmental endocrine disruptors di-n-butyl phthalate (DBP) and mono-n-butyl phthalate (MBP) on human sperm functions in vitro. Reprod. Toxicol. 2019, 83, 1–7. [Google Scholar] [CrossRef]
- Benjamin, S.; Masai, E.; Kamimura, N.; Takahashi, K.; Anderson, R.C.; Faisalm, P.A. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mater. 2017, 340, 360–383. [Google Scholar] [CrossRef]
- Mínguez-Alarcón, L.; Burns, J.; Williams, P.L.; Korrick, S.A.; Lee, M.M.; Bather, J.R.; Kovalev, S.V.; Sokolov, S.A.; Lebedev, A.T.; Smigulina, L.; et al. Urinary phthalate metabolite concentrations during four windows spanning puberty (prepuberty through sexual maturity) and association with semen quality among young Russian men. Intern. J. Hyg. Environ. Health 2022, 243, 113977. [Google Scholar] [CrossRef]
- Rastegari, F.; Amin, M.; Ebrahim, K. Risk of Phthalate Exposure among Hospitalized Patient via Intravenous Fluids Receiving. Iran. J. Toxicol. 2017, 11, 33–38. [Google Scholar] [CrossRef]
- Jiang, N.; Song, P.; Li, X.; Zhu, L.; Wang, J.; Yin, X.; Wang, J. Dibutyl phthalate induced oxidative stress and genotoxicity on adult zebrafish (Danio rerio) brain. J. Hazard. Mater. 2022, 424, 12774. [Google Scholar] [CrossRef]
- Kortenkamp, A.; Koch, H.M. Refined reference doses and new procedures for phthalate mixture risk assessment focused on male developmental toxicity. Int. J. Hyg. Environ. Health 2020, 224, 113428. [Google Scholar] [CrossRef]
- Bodar, C.W.M. Environmental Risk Limits for Dibutylphthalate (DBP). RIVM Letter Report 601782009/2008. 2008. Available online: https://www.rivm.nl/bibliotheek/rapporten/601782009.pdf (accessed on 18 October 2023).
- National Research Council. Phthalates and Cumulative Risk Assessment: The Tasks Ahead; The National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- SANPIN 1.2.3685-21; Hygienic Standards and Requirements for Ensuring the Safety and (or) Harmlessness of Environmental Factors for Humans. Federal Service for Supervision of Consumer Rights Protection and Human Well-Being: Moscow, Russia, 2021.
- US EPA. United States Environmental Protection Agency (US). National primary drinking water regulations. Final Rule. Fed. Regist. 1991, 56, 30266–30281. [Google Scholar]
- Liu, L.; Tibbs, J.; Li, N.; Bacon, A.; Shepherd, S.; Lee, H.; Chauhan, N.; Demirci, U.; Wang, X.; Cunningham, B.T. A photonic resonator interferometric scattering microscope for label-free detection of nanometer-scale objects with digital precision in point-of-use environments. Biosens. Bioelectron. 2023, 228, 115197. [Google Scholar] [CrossRef] [PubMed]
- Prakashan, D.; PR, R.; Gandhi, S. A Systematic Review on the Advanced Techniques of Wearable Point-of-Care Devices and Their Futuristic Applications. Diagnostics 2023, 13, 916. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, K.; Kanungo, L. Chemiluminescence and Fluorescence Biosensors for food application: A Review. Sens. Actuators Rep. 2023, 5, 100137. [Google Scholar] [CrossRef]
- Soheilifar, S.; Rajabi-Moghaddam, M.-J.; Karimi, G.; Mohammadinejad, A.; Motamedshariaty, V.S.; Mohajeri, S.A. Application of molecularly imprinted polymer in solid-phase microextraction coupled with HPLC-UV for analysis of dibutyl phthalate in bottled water and soft drink samples. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 552–560. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhang, W.; Shen, X.F.; Qiao, J.Y.; Pang, Y.H. Magnetic covalent organic frameworks for rapid solid-phase extraction of phthalate esters and bisphenol A in beverage samples. Anal. Methods 2023, 15, 1135–1144. [Google Scholar] [CrossRef]
- Cao, X.-L. Determination of phthalates and adipate in bottled water by headspace solid-phase microextraction and gas chromatography/mass spectrometry. J. Chromatogr. A 2008, 1178, 231–238. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, Y.; Li, F.; Jia, M.; Shi, G. A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor. Open Chem. 2019, 17, 392–400. [Google Scholar] [CrossRef]
- Conde-Díaz, A.; Santana-Mayor, Á.; Herrera-Herrera, A.V.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.Á. Assessment of endocrine disruptor pollutants and their metabolites in environmental water samples using a sustainable natural deep eutectic solvent-based analytical methodology. Chemosphere 2023, 338, 139480. [Google Scholar] [CrossRef]
- Carro, N.; Fernández, R.; Sóñora, S.; Cobas, J.; García, I.; Ignacio, M.; Mouteira, A. Optimization of micro-QuEChERS extraction coupled with gas chromatography-mass spectrometry for the fast determination of phthalic acid esters in mussel samples. Anal. Methods 2023, 15, 1836–1845. [Google Scholar] [CrossRef]
- Bazarsadueva, S.V.; Taraskin, V.V.; Budaeva, O.D.; Nikitina, E.P.; Zhigzhitzhapova, S.V.; Shiretorova, V.G.; Bazarzhapov TZh Radnaeva, L.D. First Data on PAE Levels in Surface Water in Lakes of the Eastern Coast of Baikal. Int. J. Environ. Res. Public Health 2023, 20, 1173. [Google Scholar] [CrossRef]
- Ai, S.; Gao, X.; Wang, X.; Li, J.; Fan, B.; Zhao, S.; Liu, Z. Exposure and tiered ecological risk assessment of phthalate esters in the surface water of Poyang Lake, China. Chemosphere 2021, 262, 127864. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, C.; Chen, Y.; Kong, F.; Xu, Z. Detection of dibutyl phthalate in food samples by fluorescence ratio immunosensor based on dual-emission carbon quantum dot labelled aptamers. Food Agric. Immunol. 2020, 31, 813–826. [Google Scholar] [CrossRef]
- Chen, B.; Li, L.; Yang, Q.; Zhang, M. Self-corrected dual-optical immunosensors using carbon dots@SiO2@MnO2 improving diethyl phthalate detection accuracy. Talanta 2023, 261, 124652. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, W.; Jiang, H.; Wang, Z.; Wang, Z.; Guo, P.; Sun, S.; Ding, S. Indirect Competitive Enzyme-Linked Immunosorbent Assay for the Detection of Dibutyl Phthalate in White Wine, Compared With GC-MS. Food Anal. Methods 2014, 7, 1619–1626. [Google Scholar] [CrossRef]
- Sun, R.; Zhuang, H. Biotin-Streptavidin Enzyme-linked Immunosorbent Assay for the Determination of Dibutyl Phthalate in Beverages and Drinking Water Using a Specific Polyclonal Antibody. Food Anal. Methods 2015, 8, 1990–1999. [Google Scholar] [CrossRef]
- Kuang, H.; Liu, L.; Xu, L.; Ma, W.; Guo, L.; Wang, L.; Xu, C. Development of an Enzyme-Linked Immunosorbent Assay for Dibutyl Phthalate in Liquor. Sensors 2013, 13, 8331–8339. [Google Scholar] [CrossRef]
- Berlina, A.N.; Ragozina, M.Y.; Komova, N.S.; Serebrennikova, K.V.; Zherdev, A.V.; Dzantiev, B.B. Development of Lateral Flow Test-System for the Immunoassay of Dibutyl Phthalate in Natural Waters. Biosensors 2022, 12, 1002. [Google Scholar] [CrossRef]
- Baranovskaya, V.S.; Berlina, A.N.; Eremin, S.A. A Fluorescence Polarization Immunoassay Procedure for Determining Dibutyl Phthalate in Water. J. Anal. Chem. 2022, 77, 466–472. [Google Scholar] [CrossRef]
- Zhang, J.; Li, T.; Zhang, T.; Xue, P.; Guan, T.; Yuan, Y.; Yu, H. Receptor-Based Fluorescence Polarization Assay to Detect Phthalate Esters in Chinese Spirits. Food Anal. Methods 2017, 10, 1293–1300. [Google Scholar] [CrossRef]
- Iha, K.; Inada, M.; Kawada, N.; Nakaishi, K.; Watabe, S.; Tan, Y.H.; Shen, C.; Ke, L.Y.; Yoshimura, T.; Ito, E. Ultrasensitive ELISA Developed for Diagnosis. Diagnostics 2019, 9, 78. [Google Scholar] [CrossRef]
- Xiong, Y.; Leng, Y.; Li, X.; Huang, X.; Xiong, Y. Emerging strategies to enhance the sensitivity of competitive ELISA for detection of chemical contaminants in food samples. TrAC Trends Anal. Chem. 2020, 126, 115861. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, B.; Sun, J.; Yi, Y.; Li, M.; Du, D.; Zhu, F.; Luan, J. Highly efficient detection of salbutamol in environmental water samples by an enzyme immunoassay. Sci. Total Environ. 2018, 613, 861–865. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, M.; Wang, W.; Jiang, Q.; Wang, F.; Pang, D.-W.; Liu, X. Plasmonic and photothermal immunoassay via Enzyme-Triggered Crystal Growth on gold nanostars. Anal. Chem. 2019, 91, 2086–2092. [Google Scholar] [CrossRef]
- Smith, D.S.; Eremin, S.A. Fluorescence polarization immunoassays and related methods for simple, high-throughput screening of small molecules. Anal. Bioanal. Chem. 2008, 394, 1499–1507. [Google Scholar] [CrossRef]
- Pourfarzaneh, M.; White, G.; Landon, J.; Smith, D. Cortisol directly determined in serum by fluoroimmunoassay with magnetizable solid phase. Clin. Chem. 1980, 26, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Tolmacheva, N.G.; Pirogov, A.V.; Shpigun, O.A. The use of microemulsions for the extraction of phtalic acid esters from soils with subsequent decomposition of microemulsions, simultaneous pre-concentration of dialkhylphthalates in organic phase and following GC-MS determination of target compounds. Mosc. Univ. Chem. Bull. 2017, 58, 83–88. [Google Scholar]
- Pirogov, A.V.; Tolmacheva, N.G.; Shpigun, O.A. Extraction and Subsequent Determination of Dialkyl Phthalates in the Soil Using Gas Chromatography Combined with Tandem Mass Spectrometry. Mosc. Univ. Chem. Bull. 2014, 69, 158–162. [Google Scholar] [CrossRef]
- Lacko, M.; Michalczuk, B.; Matejc, S.; Spanel, P. Ion chemistry of phthalates in selected ion flow tube mass spectrometry: Isomeric effects and secondary reactions with water vapour. Phys. Chem. Chem. Phys. 2020, 22, 16345. [Google Scholar] [CrossRef]
- Duan, C.; Zhang, H.; Zhang, Y.; Li, Q.; Li, P.; Mari, G.M.; Eremin, S.A.; Shen, J.; Wang, Z. A Robust Homogeneous Fluorescence Polarization Immunoassay for Rapid Determination of Erythromycin in Milk. Foods 2023, 12, 1581. [Google Scholar] [CrossRef]
- Raysyan, A.; Zwigart, S.D.; Eremin, S.A.; Schneider, R.J. BPA Endocrine Disruptor Detection at the Cutting Edge: FPIA and ELISA Immunoassays. Biosensors 2023, 13, 664. [Google Scholar] [CrossRef]
- Hua, L.; Wang, D.; Wang, K.; Wang, Y.; Gu, J.; Zhang, Q.; You, Q.; Wang, L. Design of Tracers in Fluorescence Polarization Assay for Extensive Application in Small Molecule Drug Discovery. J. Med. Chem. 2023, 66, 10934–10958. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, S.; De Ruyck, K.; Beloglazova, N.; Eremin, S.A.; De Saeger, S.; Zhang, S.; Shen, J.; Wang, Z. Fluorescence polarization assays for chemical contaminants in food and environmental analyses. Trends Anal. Chem. 2019, 114, 293–313. [Google Scholar] [CrossRef]
- Dong, B.; Zhao, S.; Li, H.; Wen, K.; Ke, Y.; Shen, J.; Zhang, S.; Shi, W.; Wang, Z. Design, synthesis and characterization of tracers and development of a fluorescence polarization immunoassay for the rapid detection of ractopamine in pork. Food Chem. 2019, 271, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Pope, A.J.; Haupts, U.M.; Moore, K.J. Homogeneous fluorescence readouts for miniaturized high-throughput screening: Theory and practice. Drug Discov. Today 1999, 4, 350–362. [Google Scholar] [CrossRef]
- Fluorescence Polarization. Technical Resource Guide, 4th ed.; Invitrogen Corporation: Carlsbad, CA, USA, 2006. [Google Scholar]
- International Union of Pure and Applied Chemistry (IUPAC). Compendium of Analytical Nomenclature (Orange Book); Blackwell Science: Oxford, UK, 1998; p. 223. [Google Scholar]




| Water Sample | Added DBP Concentration, ng/mL | Detected DBP Concentration, ng/mL | Recovery, % |
|---|---|---|---|
| 1 | 30 | 26 ± 1 | 86 ± 4 |
| 90 | 85 ± 3 | 94 ± 4 | |
| 2 | 30 | 34 ± 2 | 113 ± 6 |
| 90 | 98 ± 2 | 109 ± 2 |
| Water Sample | GC-MS, ng/mL | FPIA, ng/mL |
|---|---|---|
| 3 | 10 | ND |
| 4 | 10.6 | 10.5 |
| 5 | 10.6 | 10.0 |
| 6 | 6.0 | ND |
| 7 | 7.5 | ND |
| 8 | 7.3 | ND |
| 9 | 10.5 | 10.0 |
| 10 | 11.2 | 10.0 |
| 11 | 7.1 | ND |
| 12 | 5.3 | ND |
| 13 | 16.5 | 12.5 |
| 14 | 18.1 | 14.2 |
| 15 | 14.0 | 11.0 |
| 16 | 8.0 | ND |
| Antibody | Assay Format | Sample | LOD ng/mL | Range ng/mL | Reference |
|---|---|---|---|---|---|
| Polyclonal | icELISA | White wine | 64.5 | 64.5–1606.2 | [38] |
| Polyclonal | BA-ELISA | Drinking water | 5 | 0.02–8.97 | [39] |
| Monoclonal | ELISA | Baverange | 3.6 | [40] | |
| Monoclonal | ICA | Water | 33.4 | 42.4–1500 | [42] |
| Polyclonal | FPIA | Water | 350 | 500–7500 | [43] |
| Receptor | FPIA | Spirite | 170 | 170–8700 | [44] |
| Monoclonal | FPIA | Water | 7.5 | 12–300 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhametova, L.I.; Karimova, M.R.; Zharikova, O.G.; Pirogov, A.V.; Levkina, V.V.; Chichkanova, E.S.; Liu, L.; Xu, C.; Eremin, S.A. Detection of Dibutyl Phthalate in Surface Water by Fluorescence Polarization Immunoassay. Biosensors 2023, 13, 1005. https://doi.org/10.3390/bios13121005
Mukhametova LI, Karimova MR, Zharikova OG, Pirogov AV, Levkina VV, Chichkanova ES, Liu L, Xu C, Eremin SA. Detection of Dibutyl Phthalate in Surface Water by Fluorescence Polarization Immunoassay. Biosensors. 2023; 13(12):1005. https://doi.org/10.3390/bios13121005
Chicago/Turabian StyleMukhametova, Liliya I., Madina R. Karimova, Olga G. Zharikova, Andrey V. Pirogov, Valentina V. Levkina, Ekaterina S. Chichkanova, Liqiang Liu, Chuanlai Xu, and Sergei A. Eremin. 2023. "Detection of Dibutyl Phthalate in Surface Water by Fluorescence Polarization Immunoassay" Biosensors 13, no. 12: 1005. https://doi.org/10.3390/bios13121005






