Biohybrid Nanoparticle-Based In Situ Monitoring of In Vivo Drug Delivery
Abstract
:1. Introduction
2. Design of Biohybrid Nanoparticles for Drug Delivery
2.1. Nucleic Acid-Based Nanoparticles (NANPs)
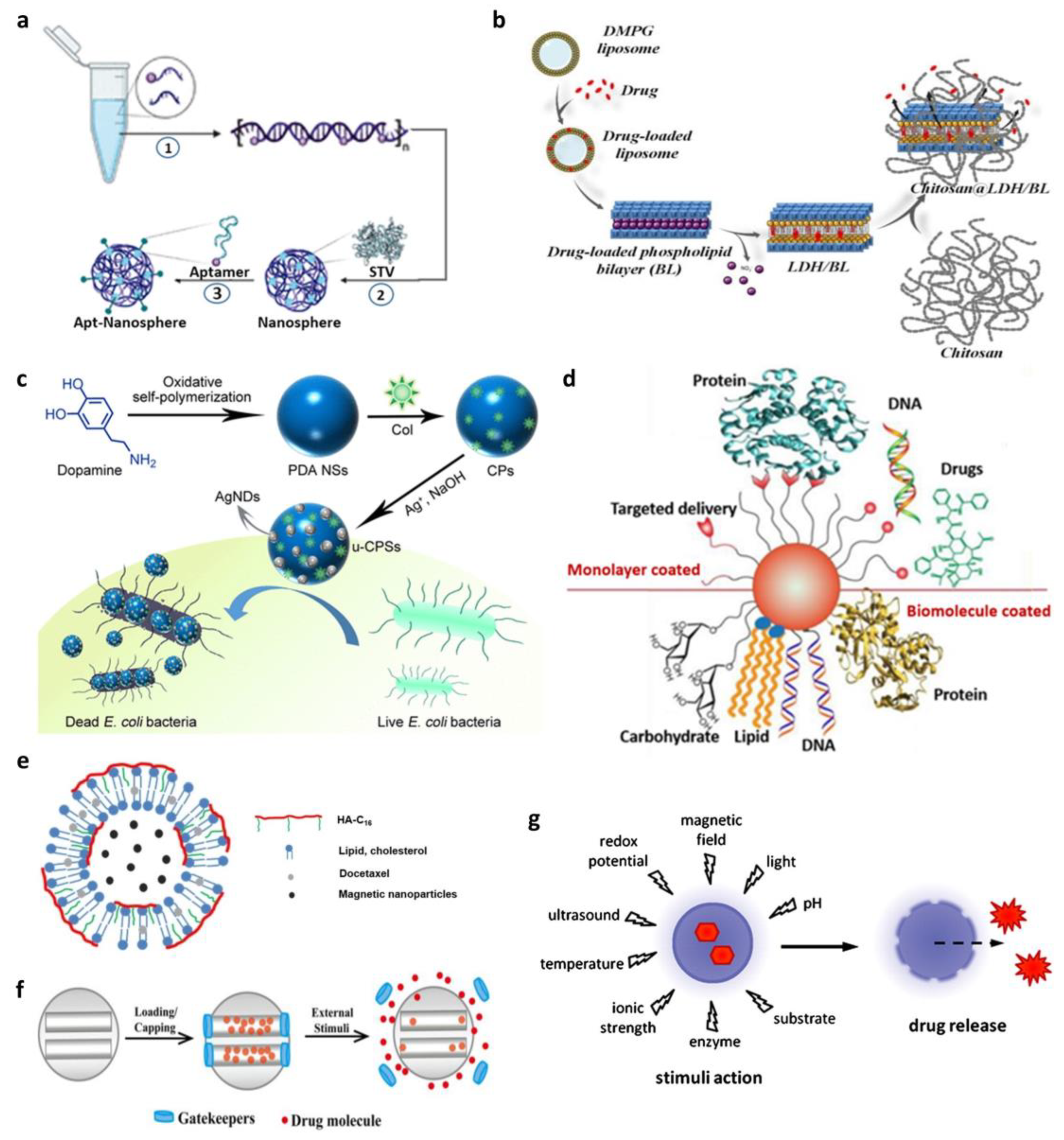
2.2. Liposomes
2.3. Metallic Nanoparticles
2.3.1. Silver Nanoparticles (AgNPs)
2.3.2. Gold Nanoparticles (AuNPs)
2.4. Magnetic Nanoparticles (MNPs)
2.5. Silica Nanoparticles
2.6. Polymeric Nanoparticles
| Nanoparticle | Advantage | Disadvantage | Refs. |
|---|---|---|---|
| Nucleic acid-based nanoparticle | Target specificity Biodegradability and biocompatibility | Potential aggregation with blood cells Adherence to the vessel wall Opsonization with plasma protein | [28,31,82] |
| Liposome | Structural flexibility Ease of conjugation and functionalization with contrast agents and probes Rapid cellular uptake and well-characterized cell internalization mechanism Low immunogenicity Good biocompatibility | High cost Low drug-loading efficiency Limited instability and leakage of loaded materials Rapid clearance | [41,42,83] |
| Silver nanoparticle | Good biocompatibility Direct cancer cell killing capability | Size-dependent cytotoxicity Potential off-target effects with little delivery to the tumor | [49,50,84] |
| Gold nanoparticle | Large surface area Application diversity Suitable for photodynamic therapy Ease of surface modification High stability and biocompatibility | High cost Low biodegradability Potential toxicity depends on their intrinsic characteristics | [59,60,85] |
| Magnetic nanoparticle | Large surface area Small size allows longer circulation and tissue penetration Controlled clustering Application diversity | Lack of colloidal stability Low biocompatibility and biodegradability In vivo toxicity | [69,72,86] |
| Silica nanoparticle (Mesoporous) | Large surface area High stability and biocompatibility Controllable porosity Surface reactivity and ease of functionalization Biodegradability | In vivo toxicity Low drug-loading capacity | [76,77,87] |
| Polymeric nanoparticle | Large drug-loading capacity Stimuli-responsive drug release Precisely controllable size Ease of fusing with other materials | Difficulty of scale-up synthesis Complex synthetic procedure Low biocompatibility | [78,88] |
3. Biohybrid Nanoparticle-Based In Situ Drug Release Monitoring
3.1. Fluorescence-Based In Situ Monitoring of Drug Release
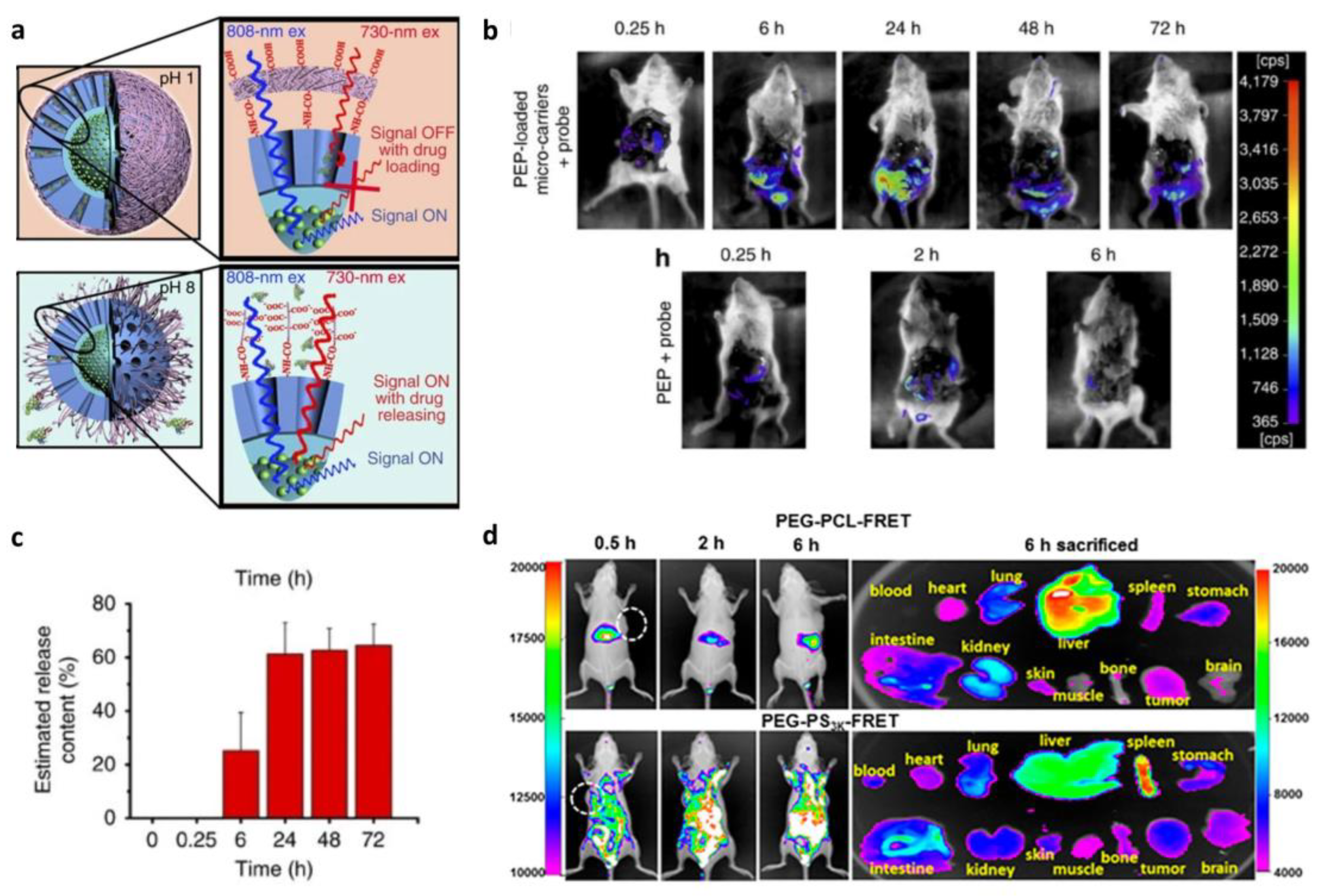
3.1.1. Förster Resonance Energy Transfer (FRET)-Based In Situ Monitoring of Drug Release

3.1.2. Aggregation-Caused Quenching (ACQ)-Based In Situ Monitoring of Drug Release
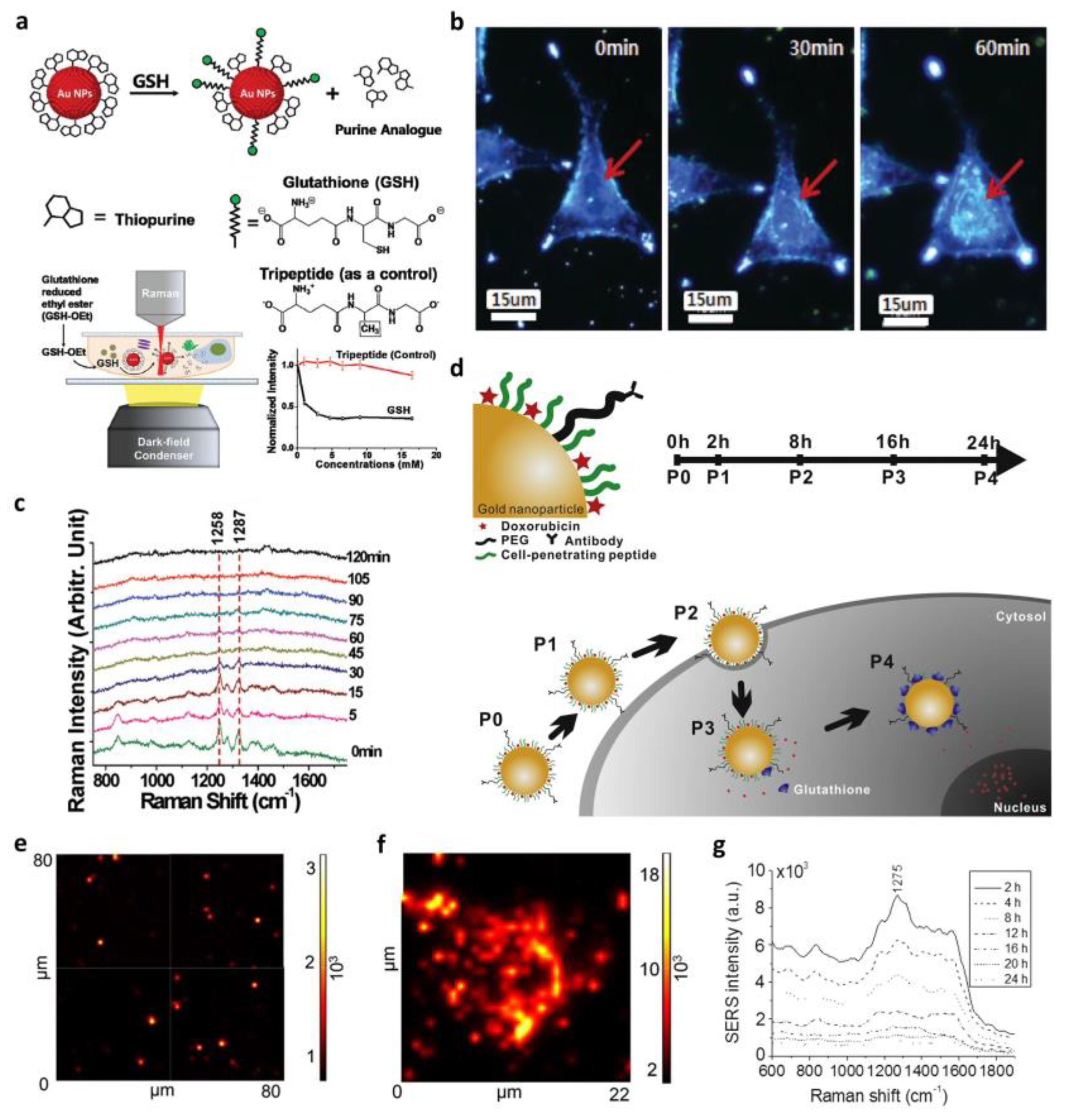
3.2. Surface-Enhanced Raman Spectroscopy (SERS)-Based In Situ Monitoring of Drug Release
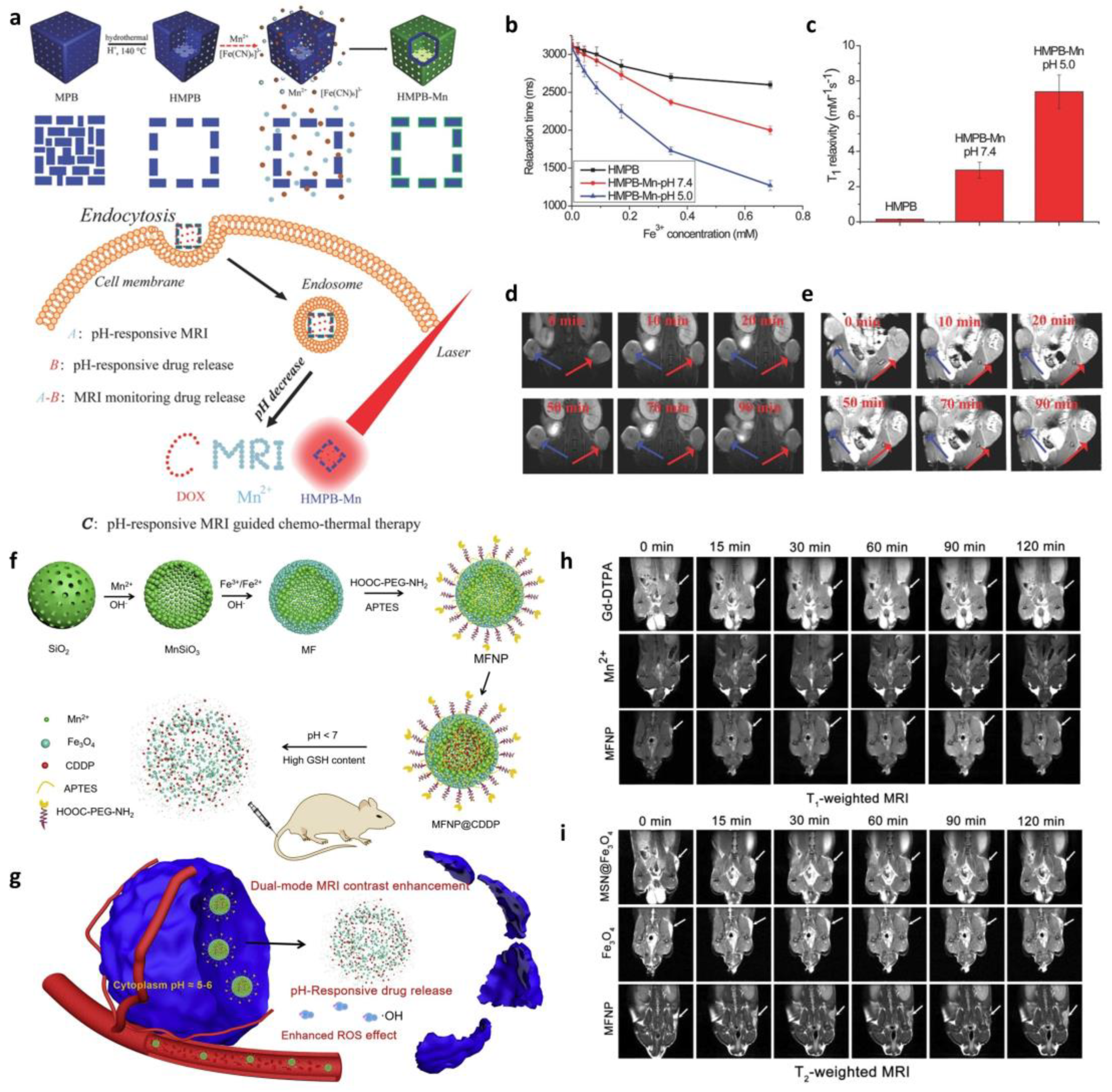
3.3. Magnetic Resonance Imaging (MRI)-Based In Situ Monitoring of Drug Release
| Methods | Contrast Agents | Advantage | Disadvantage | Refs. |
|---|---|---|---|---|
| Fluorescence-based | Various fluorescence-conjugated biomolecules Fluorophore | Non-invasive Radiation-free Available to combine with other imaging methods | Dye cytotoxicity Limited tissue penetration depth | [13,100,105,117] |
| SERS | Raman reporter-conjugated gold nanoparticle | Ease of sample preparation Narrow peak width allowing multitarget detection | Low intensity and poor reproducibility Difficulty of quantitative analysis | [107,111] |
| MRI | Paramagnetic ions (Gadolinium, Manganese, and Iron) | Non-invasive Highly spatial and temporal resolutions Possible to use external magnetic field to manipulate drug carriers and/or cells | Relatively high toxicity of nanoparticles Possibility of signal affected by contrast agents when using superparamagnetic particles Inability to distinguish live cells from dead ones | [112,115] |
| Ultrasound | Microbubbles | Radiation-free Can detect single cells Relatively inexpensive Allows imaging of soft tissues | Low resolution Restricted to specific parts of body Difficulty of quantification Contrast agent can transfer to non-target cells | [118,119] |
4. Fate of Biohybrid Nanoparticles
4.1. Tracking-Based In Vivo Drug Delivery Monitoring
4.2. Degradation-Based In Vivo Drug Delivery Monitoring
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnology 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.S.; Fenniri, H.; Singh, B. Nanotechnology-based drug delivery systems. J. Occup. Med. Toxicol. 2007, 2, 16. [Google Scholar] [CrossRef]
- Kingsley, J.D.; Dou, H.; Morehead, J.; Rabinow, B.; Gendelman, H.E.; Destache, C.J. Nanotechnology: A Focus on Nanoparticles as a Drug Delivery System. J. Neuroimmune Pharmacol. 2006, 1, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Bellier, N.; Baipaywad, P.; Ryu, N.; Lee, J.Y.; Park, H. Recent biomedical advancements in graphene oxide- and reduced graphene oxide-based nanocomposite nanocarriers. Biomater. Res. 2022, 26, 65. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Tak, E.; Cho, Y.J.; Kim, J.; Lee, J.; Lee, R.; Lee, K.; Kwon, M.; Yoon, Y.-I.; Lee, S.-G.; et al. Nano-biomarker-Based Surface-Enhanced Raman Spectroscopy for Selective Diagnosis of Gallbladder and Liver Injury. BioChip J. 2022, 16, 49–57. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Kim, M.I. Using Nanomaterials in Colorimetric Toxin Detection. BioChip J. 2021, 15, 123–134. [Google Scholar] [CrossRef]
- Singh, R.K.; Knowles, J.C.; Kim, H.-W. Advances in nanoparticle development for improved therapeutics delivery: Nanoscale topographical aspect. J. Tissue Eng. 2019, 10, 2041731419877528. [Google Scholar] [CrossRef]
- Yang, G.; Phua, S.Z.F.; Bindra, A.K.; Zhao, Y. Degradability and Clearance of Inorganic Nanoparticles for Biomedical Applications. Adv. Mater. 2019, 31, 1805730. [Google Scholar] [CrossRef]
- Wei, Y.; Quan, L.; Zhou, C.; Zhan, Q. Factors relating to the biodistribution & clearance of nanoparticles & their effects on in vivo application. Nanomedicine 2018, 13, 1495–1512. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef]
- Hahn, M.A.; Singh, A.K.; Sharma, P.; Brown, S.C.; Moudgil, B.M. Nanoparticles as contrast agents for in-vivo bioimaging: Current status and future perspectives. Anal. Bioanal. Chem. 2011, 399, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, Z.; Xue, K.; Li, J.; Wang, W.; Ma, J.; Ma, C.; Bai, X.; Huang, Y.; Pan, X.; et al. Development of Aggregation-Caused Quenching Probe-Loaded Pressurized Metered-Dose Inhalers with Fluorescence Tracking Potentials. AAPS PharmSciTech 2020, 21, 296. [Google Scholar] [CrossRef]
- Kaeokhamloed, N.; Legeay, S.; Roger, E. FRET as the tool for in vivo nanomedicine tracking. J. Control. Release 2022, 349, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.; Downs, J.; Maples, D.; Ranjan, A. MRI-guided monitoring of thermal dose and targeted drug delivery for cancer therapy. Pharm. Res. 2013, 30, 2709–2717. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tang, Y.; Dai, S.; Kleitz, F.; Qiao, S.Z. Smart surface-enhanced Raman scattering traceable drug delivery systems. Nanoscale 2016, 8, 12803–12811. [Google Scholar] [CrossRef]
- Huang, F.; Watson, E.; Dempsey, C.; Suh, J. Real-Time Particle Tracking for Studying Intracellular Trafficking of Pharmaceutical Nanocarriers. In Cellular and Subcellular Nanotechnology: Methods and Protocols; Weissig, V., Elbayoumi, T., Olsen, M., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 211–223. [Google Scholar]
- Zheng, F.; Xiong, W.; Sun, S.; Zhang, P.; Zhu, J.J. Recent advances in drug release monitoring. Nanophotonics 2019, 8, 391–413. [Google Scholar] [CrossRef]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef]
- Haque, S.T.; Chowdhury, E.H. Recent Progress in Delivery of Therapeutic and Imaging Agents Utilizing Organic-Inorganic Hybrid Nanoparticles. Curr. Drug Deliv. 2018, 15, 485–496. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Corbo, C.; Molinaro, R.; Lakey, J.R.T. Biohybrid Nanoparticles to Negotiate with Biological Barriers. Small 2019, 15, 1902333. [Google Scholar] [CrossRef]
- Li, M.; Luo, Z.; Zhao, Y. Hybrid Nanoparticles as Drug Carriers for Controlled Chemotherapy of Cancer. Chem. Rec. 2016, 16, 1833–1851. [Google Scholar] [CrossRef] [PubMed]
- Banskota, S.; Yousefpour, P.; Chilkoti, A. Cell-Based Biohybrid Drug Delivery Systems: The Best of the Synthetic and Natural Worlds. Macromol. Biosci. 2017, 17, 1600361. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hableel, G.; Zhao, E.R.; Jokerst, J.V. Multifunctional nanomedicine with silica: Role of silica in nanoparticles for theranostic, imaging, and drug monitoring. J. Colloid Interface Sci. 2018, 521, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Yafout, M.; Ousaid, A.; Khayati, Y.; El Otmani, I.S. Gold nanoparticles as a drug delivery system for standard chemotherapeutics: A new lead for targeted pharmacological cancer treatments. Sci. Afr. 2021, 11, e00685. [Google Scholar] [CrossRef]
- Schröfel, A.; Kratošová, G.; Šafařík, I.; Šafaříková, M.; Raška, I.; Shor, L.M. Applications of biosynthesized metallic nanoparticles—A review. Acta Biomater. 2014, 10, 4023–4042. [Google Scholar] [CrossRef]
- Byun, M.J.; Lim, J.; Kim, S.-N.; Park, D.-H.; Kim, T.-H.; Park, W.; Park, C.G. Advances in Nanoparticles for Effective Delivery of RNA Therapeutics. BioChip J. 2022, 16, 128–145. [Google Scholar] [CrossRef]
- Jaeger, L.; Chworos, A. The architectonics of programmable RNA and DNA nanostructures. Curr. Opin. Struct. Biol. 2006, 16, 531–543. [Google Scholar] [CrossRef]
- Narwade, M.; Shaikh, A.; Gajbhiye, K.R.; Kesharwani, P.; Gajbhiye, V. Advanced cancer targeting using aptamer functionalized nanocarriers for site-specific cargo delivery. Biomater. Res. 2023, 27, 42. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, J.; Noh, S.; Sohn, H.; Lee, T. Recent Development of Aptasensor for Influenza Virus Detection. BioChip J. 2020, 14, 327–339. [Google Scholar] [CrossRef]
- Li, J.; Fan, C.; Pei, H.; Shi, J.; Huang, Q. Smart Drug Delivery Nanocarriers with Self-Assembled DNA Nanostructures. Adv. Mater. 2013, 25, 4386–4396. [Google Scholar] [CrossRef]
- Lee, D.; Baek, S.; Kim, Y.-Y.; Bang, Y.; Jung, H.N.; Im, H.-J.; Song, Y.-K. Self-Assembled DNA–Protein Hybrid Nanospheres: Biocompatible Nano-Drug-Carriers for Targeted Cancer Therapy. ACS Appl. Mater. Interfaces 2022, 14, 37493–37503. [Google Scholar] [CrossRef]
- Nam, H.; Jeon, H.; Kim, H.; Yoon, H.Y.; Kim, S.H.; Lee, J.B. Module-assembly of injectable cellular DNA hydrogel via clickable cells and DNA scaffolds. Chem. Eng. J. 2023, 452, 139492. [Google Scholar] [CrossRef]
- Xue, C.; Zhang, S.; Yu, X.; Hu, S.; Lu, Y.; Wu, Z.S. Periodically Ordered, Nuclease-Resistant DNA Nanowires Decorated with Cell-Specific Aptamers as Selective Theranostic Agents. Angew. Chem. Int. Ed. Engl. 2020, 59, 17540–17547. [Google Scholar] [CrossRef] [PubMed]
- Lerner, D.A.; Bégu, S.; Aubert-Pouëssel, A.; Polexe, R.; Devoisselle, J.M.; Azaïs, T.; Tichit, D. Synthesis and Properties of New Multilayer Chitosan@layered Double Hydroxide/Drug Loaded Phospholipid Bilayer Nanocomposite Bio-Hybrids. Materials 2020, 13, 3565. [Google Scholar] [CrossRef] [PubMed]
- Ran, H.H.; Cheng, X.; Gao, G.; Sun, W.; Jiang, Y.W.; Zhang, X.; Jia, H.R.; Qiao, Y.; Wu, F.G. Colistin-Loaded Polydopamine Nanospheres Uniformly Decorated with Silver Nanodots: A Nanohybrid Platform with Improved Antibacterial and Antibiofilm Performance. ACS Appl. Bio Mater. 2020, 3, 2438–2448. [Google Scholar] [CrossRef]
- Rana, S.; Bajaj, A.; Mout, R.; Rotello, V.M. Monolayer coated gold nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2012, 64, 200–216. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Zheng, S.; Han, J.; Le, V.H.; Park, J.-O.; Park, S. Nanohybrid magnetic liposome functionalized with hyaluronic acid for enhanced cellular uptake and near-infrared-triggered drug release. Colloids Surf. B Biointerfaces 2017, 154, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Milla, P.; Dosio, F.; Cattel, L. PEGylation of Proteins and Liposomes: A Powerful and Flexible Strategy to Improve the Drug Delivery. Curr. Drug Metab. 2012, 13, 105–119. [Google Scholar] [CrossRef]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Pelucelli, A.; Zoroddu, M.A. An updated overview on metal nanoparticles toxicity. Semin. Cancer Biol. 2021, 76, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-F.; Chen, C. Fate and Toxicity of Metallic and Metal-Containing Nanoparticles for Biomedical Applications. Small 2011, 7, 2965–2980. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef] [PubMed]
- Lekha, D.C.; Shanmugam, R.; Madhuri, K.; Dwarampudi, L.P.; Bhaskaran, M.; Kongara, D.; Tesfaye, J.L.; Nagaprasad, N.; Bhargavi, V.L.N.; Krishnaraj, R. Review on Silver Nanoparticle Synthesis Method, Antibacterial Activity, Drug Delivery Vehicles, and Toxicity Pathways: Recent Advances and Future Aspects. J. Nanomater. 2021, 2021, 4401829. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Mudila, H.; Gupta, G.; Sharma, A.K.; Kumar, D.; Bakshi, H.A.; Negi, P.; Kapoor, D.N.; Chellappan, D.K.; et al. Emerging trends in clinical implications of bio-conjugated silver nanoparticles in drug delivery. Colloid Interface Sci. Commun. 2020, 35, 100244. [Google Scholar] [CrossRef]
- Lu, B.; Lu, F.; Zou, Y.; Liu, J.; Rong, B.; Li, Z.; Dai, F.; Wu, D.; Lan, G. In Situ reduction of silver nanoparticles by chitosan-l-glutamic acid/hyaluronic acid: Enhancing antimicrobial and wound-healing activity. Carbohydr. Polym. 2017, 173, 556–565. [Google Scholar] [CrossRef]
- Qiu, L.; Li, J.-W.; Hong, C.-Y.; Pan, C.-Y. Silver Nanoparticles Covered with pH-Sensitive Camptothecin-Loaded Polymer Prodrugs: Switchable Fluorescence “Off” or “On” and Drug Delivery Dynamics in Living Cells. ACS Appl. Mater. Interfaces 2017, 9, 40887–40897. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, S.B.; Adnan, R.; Rameez Khan, R.M.; Rashid, M. Gold, Silver, and Palladium Nanoparticles: A Chemical Tool for Biomedical Applications. Front. Chem. 2020, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Eom, G.; Hwang, A.; Kim, H.; Moon, J.; Kang, H.; Jung, J.; Lim, E.-K.; Jeong, J.; Park, H.G.; Kang, T. Ultrasensitive Detection of Ovarian Cancer Biomarker Using Au Nanoplate SERS Immunoassay. BioChip J. 2021, 15, 348–355. [Google Scholar] [CrossRef]
- Heo, S.E.; Ha, J.W. Single-Particle Study: Refractive Index Sensitivity of Localized Surface Plasmon Resonance Inflection Points in Mesoporous Silica-Coated Gold Nanorods. BioChip J. 2022, 16, 183–190. [Google Scholar] [CrossRef]
- Haute, D.V.; Berlin, J.M. Challenges in realizing selectivity for nanoparticle biodistribution and clearance: Lessons from gold nanoparticles. Ther. Deliv. 2017, 8, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ren, L.; Wang, H. Strategies in the design of gold nanoparticles for intracellular targeting: Opportunities and challenges. Ther. Deliv. 2017, 8, 879–897. [Google Scholar] [CrossRef]
- Nicol, J.R.; Dixon, D.; Coulter, J.A. Gold nanoparticle surface functionalization: A necessary requirement in the development of novel nanotherapeutics. Nanomedicine 2015, 10, 1315–1326. [Google Scholar] [CrossRef]
- Amina, S.J.; Guo, B. A Review on the Synthesis and Functionalization of Gold Nanoparticles as a Drug Delivery Vehicle. Int. J. Nanomed. 2020, 15, 9823–9857. [Google Scholar] [CrossRef]
- Li, S.; Bouchy, S.; Penninckx, S.; Marega, R.; Fichera, O.; Gallez, B.; Feron, O.; Martinive, P.; Heuskin, A.-C.; Michiels, C.; et al. Antibody-functionalized gold nanoparticles as tumor-targeting radiosensitizers for proton therapy. Nanomedicine 2019, 14, 317–333. [Google Scholar] [CrossRef]
- Zong, J.; Cobb, S.L.; Cameron, N.R. Peptide-functionalized gold nanoparticles: Versatile biomaterials for diagnostic and therapeutic applications. Biomater. Sci. 2017, 5, 872–886. [Google Scholar] [CrossRef] [PubMed]
- Akbal Vural, O. Evaluation of protein functionalized gold nanoparticles to improve tamoxifen delivery: Synthesis, characterization, and biocompatibility on breast cancer cells. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 1437–1448. [Google Scholar] [CrossRef]
- Taton, T.A. Preparation of Gold Nanoparticle–DNA Conjugates. Curr. Protoc. Nucleic Acid Chem. 2002, 9, 12.2.1–12.2.12. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Mao, X.; Zeng, Q.; Wang, S.; Kawde, A.-N.; Liu, G. Aptamer-Functionalized Gold Nanoparticles as Probes in a Dry-Reagent Strip Biosensor for Protein Analysis. Anal. Chem. 2009, 81, 669–675. [Google Scholar] [CrossRef]
- Lee, W.-J.; Kim, K.-J.; Hossain, M.K.; Cho, H.-Y.; Choi, J.-W. DNA–Gold Nanoparticle Conjugates for Intracellular miRNA Detection Using Surface-Enhanced Raman Spectroscopy. BioChip J. 2022, 16, 33–40. [Google Scholar] [CrossRef]
- Lee, S.H.; Bae, K.H.; Kim, S.H.; Lee, K.R.; Park, T.G. Amine-functionalized gold nanoparticles as non-cytotoxic and efficient intracellular siRNA delivery carriers. Int. J. Pharm. 2008, 364, 94–101. [Google Scholar] [CrossRef]
- Wang, X.; Cai, X.; Hu, J.; Shao, N.; Wang, F.; Zhang, Q.; Xiao, J.; Cheng, Y. Glutathione-Triggered “Off–On” Release of Anticancer Drugs from Dendrimer-Encapsulated Gold Nanoparticles. J. Am. Chem. Soc. 2013, 135, 9805–9810. [Google Scholar] [CrossRef]
- Mornet, S.; Vasseur, S.; Grasset, F.; Veverka, P.; Goglio, G.; Demourgues, A.; Portier, J.; Pollert, E.; Duguet, E. Magnetic nanoparticle design for medical applications. Prog. Solid State Chem. 2006, 34, 237–247. [Google Scholar] [CrossRef]
- Ha, Y.; Kim, I. Recent Developments in Innovative Magnetic Nanoparticles-Based Immunoassays: From Improvement of Conventional Immunoassays to Diagnosis of COVID-19. BioChip J. 2022, 16, 351–365. [Google Scholar] [CrossRef]
- Correa, S.; Puertas, S.; Gutiérrez, L.; Asín, L.; Martínez de la Fuente, J.; Grazú, V.; Betancor, L. Design of stable magnetic hybrid nanoparticles of Si-entrapped HRP. PLoS ONE 2019, 14, e0214004. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Namvar, F.; Nadi, B.; Rahman, M.Z.A.; Amin, J. Synthesis, Surface Modification and Characterisation of Biocompatible Magnetic Iron Oxide Nanoparticles for Biomedical Applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [PubMed]
- Abarca-Cabrera, L.; Fraga-García, P.; Berensmeier, S. Bio-nano interactions: Binding proteins, polysaccharides, lipids and nucleic acids onto magnetic nanoparticles. Biomater. Res. 2021, 25, 12. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.M.; Shi, L.; Quina, F.H.; Rosenzweig, Z. Stöber Synthesis of Monodispersed Luminescent Silica Nanoparticles for Bioanalytical Assays. Langmuir 2005, 21, 4277–4280. [Google Scholar] [CrossRef] [PubMed]
- Janjua, T.I.; Cao, Y.; Yu, C.; Popat, A. Clinical translation of silica nanoparticles. Nat. Rev. Mater. 2021, 6, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Feng, N. Mesoporous silica nanoparticles: Synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert Opin. Drug Deliv. 2019, 16, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Bugno, J.; Hsu, H.-j.; Hong, S. Recent advances in targeted drug delivery approaches using dendritic polymers. Biomater. Sci. 2015, 3, 1025–1034. [Google Scholar] [CrossRef]
- Ray, P.; Ferraro, M.; Haag, R.; Quadir, M. Dendritic Polyglycerol-Derived Nano-Architectures as Delivery Platforms of Gemcitabine for Pancreatic Cancer. Macromol. Biosci. 2019, 19, 1900073. [Google Scholar] [CrossRef]
- Keller, A.; Linko, V. Challenges and Perspectives of DNA Nanostructures in Biomedicine. Angew. Chem. Int. Ed. 2020, 59, 15818–15833. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Tan, P.; Li, H.; Wang, J.; Gopinath, S.C.B. Silver nanoparticle in biosensor and bioimaging: Clinical perspectives. Biotechnol. Appl. Biochem. 2021, 68, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Expert Opin. Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Stueber, D.D.; Villanova, J.; Aponte, I.; Xiao, Z.; Colvin, V.L. Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends. Pharmaceutics 2021, 13, 943. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liao, Z.; Li, M.; Zhang, H.; Li, T.; Qin, X.; Li, S.; Wu, C.; You, F.; Liao, X.; et al. Mesoporous Silica Nanoparticles-Based Nanoplatforms: Basic Construction, Current State, and Emerging Applications in Anticancer Therapeutics. Adv. Healthc. Mater. 2023, 12, 2201884. [Google Scholar] [CrossRef]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef]
- Niu, G.; Chen, X. The Role of Molecular Imaging in Drug Delivery. Drug Deliv. 2009, 3, 109–113. [Google Scholar]
- Jokerst, J.V.; Gambhir, S.S. Molecular Imaging with Theranostic Nanoparticles. Acc. Chem. Res. 2011, 44, 1050–1060. [Google Scholar] [CrossRef]
- He, H.; Zhang, X.; Du, L.; Ye, M.; Lu, Y.; Xue, J.; Wu, J.; Shuai, X. Molecular imaging nanoprobes for theranostic applications. Adv. Drug Deliv. Rev. 2022, 186, 114320. [Google Scholar] [CrossRef]
- Yoon, S.; Cheon, S.Y.; Park, S.; Lee, D.; Lee, Y.; Han, S.; Kim, M.; Koo, H. Recent advances in optical imaging through deep tissue: Imaging probes and techniques. Biomater. Res. 2022, 26, 57. [Google Scholar] [CrossRef] [PubMed]
- Haizan, I.; Park, D.H.; Choi, M.Y.; Lee, H.; Choi, J.-H. Nanomaterials-Based Exosomes for the Diagnostics and Drug Deliveries of Central Nervous System Diseases. BioChip J. 2023, 17, 293–307. [Google Scholar] [CrossRef]
- Watson, P.; Jones, A.T.; Stephens, D.J. Intracellular trafficking pathways and drug delivery: Fluorescence imaging of living and fixed cells. Adv. Drug Deliv. Rev. 2005, 57, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Deng, H.; Huang, P.; Sun, P.; Huang, X.; Su, Y.; Zhu, X.; Shen, J.; Yan, D. Real-time self-tracking of an anticancer small molecule nanodrug based on colorful fluorescence variations. RSC Adv. 2016, 6, 12472–12478. [Google Scholar] [CrossRef]
- White, N.S.; Errington, R.J. Fluorescence techniques for drug delivery research: Theory and practice. Adv. Drug Deliv. Rev. 2005, 57, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; He, B.; Tao, J.; He, Y.; Deng, H.; Wang, X.; Zheng, Y. Application of Förster Resonance Energy Transfer (FRET) technique to elucidate intracellular and In Vivo biofate of nanomedicines. Adv. Drug Deliv. Rev. 2019, 143, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, D.; Sun, D.; Gu, J. Current status of in vivo bioanalysis of nano drug delivery systems. J. Pharm. Anal. 2020, 10, 221–232. [Google Scholar] [CrossRef]
- Morton, S.W.; Zhao, X.; Quadir, M.A.; Hammond, P.T. FRET-enabled biological characterization of polymeric micelles. Biomaterials 2014, 35, 3489–3496. [Google Scholar] [CrossRef]
- Leavesley, S.J.; Rich, T.C. Overcoming limitations of FRET measurements. Cytom. Part A 2016, 89, 325–327. [Google Scholar] [CrossRef]
- Periasamy, A.; Mazumder, N.; Sun, Y.; Christopher, K.G.; Day, R.N. FRET Microscopy: Basics, Issues and Advantages of FLIM-FRET Imaging. In Advanced Time-Correlated Single Photon Counting Applications; Becker, W., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 249–276. [Google Scholar]
- Suryaprakash, S.; Lao, Y.H.; Cho, H.Y.; Li, M.; Ji, H.Y.; Shao, D.; Hu, H.; Quek, C.H.; Huang, D.; Mintz, R.L.; et al. Engineered Mesenchymal Stem Cell/Nanomedicine Spheroid as an Active Drug Delivery Platform for Combinational Glioblastoma Therapy. Nano Lett. 2019, 19, 1701–1705. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, L.; Yang, J.; Bo, R.; Du, H.; Lin, K.; Zhang, D.; Ramachandran, M.; Shen, Y.; Xu, Y.; et al. Rotatable Aggregation-Induced-Emission/Aggregation-Caused-Quenching Ratio Strategy for Real-Time Tracking Nanoparticle Dynamics. Adv. Funct. Mater. 2020, 30, 1910348. [Google Scholar] [CrossRef]
- He, H.; Wang, L.; Ma, Y.; Yang, Y.; Lv, Y.; Zhang, Z.; Qi, J.; Dong, X.; Zhao, W.; Lu, Y.; et al. The biological fate of orally administered mPEG-PDLLA polymeric micelles. J. Control. Release 2020, 327, 725–736. [Google Scholar] [CrossRef]
- Yuan, H.; Zhao, W.; Wu, W. How can aggregation-caused quenching based bioimaging of drug nanocarriers be improved? Ther. Deliv. 2020, 11, 809–812. [Google Scholar] [CrossRef]
- Qi, J.; Hu, X.; Dong, X.; Lu, Y.; Lu, H.; Zhao, W.; Wu, W. Towards more accurate bioimaging of drug nanocarriers: Turning aggregation-caused quenching into a useful tool. Adv. Drug Deliv. Rev. 2019, 143, 206–225. [Google Scholar] [CrossRef]
- Yuen, C.; Zheng, W.; Huang, Z. Surface-Enhanced Raman Scattering: Principles, Nanostructures, Fabrications, and Biomedical Applications. J. Innov. Opt. Health Sci. 2008, 01, 267–284. [Google Scholar] [CrossRef]
- Jaworska, A.; Fornasaro, S.; Sergo, V.; Bonifacio, A. Potential of Surface Enhanced Raman Spectroscopy (SERS) in Therapeutic Drug Monitoring (TDM). A Critical Review. Biosensors 2016, 6, 47. [Google Scholar] [CrossRef]
- Ock, K.; Jeon, W.I.; Ganbold, E.O.; Kim, M.; Park, J.; Seo, J.H.; Cho, K.; Joo, S.-W.; Lee, S.Y. Real-Time Monitoring of Glutathione-Triggered Thiopurine Anticancer Drug Release in Live Cells Investigated by Surface-Enhanced Raman Scattering. Anal. Chem. 2012, 84, 2172–2178. [Google Scholar] [CrossRef]
- Hossain, M.K.; Cho, H.Y.; Kim, K.J.; Choi, J.W. In situ monitoring of doxorubicin release from biohybrid nanoparticles modified with antibody and cell-penetrating peptides in breast cancer cells using surface-enhanced Raman spectroscopy. Biosens. Bioelectron. 2015, 71, 300–305. [Google Scholar] [CrossRef]
- Zheng, X.-S.; Jahn, I.J.; Weber, K.; Cialla-May, D.; Popp, J. Label-free SERS in biological and biomedical applications: Recent progress, current challenges and opportunities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 197, 56–77. [Google Scholar] [CrossRef]
- Grover, V.P.B.; Tognarelli, J.M.; Crossey, M.M.E.; Cox, I.J.; Taylor-Robinson, S.D.; McPhail, M.J.W. Magnetic Resonance Imaging: Principles and Techniques: Lessons for Clinicians. J. Clin. Exp. Hepatol. 2015, 5, 246–255. [Google Scholar] [CrossRef]
- Long, C.M.; Bulte, J.W.M. In vivo tracking of cellular therapeutics using magnetic resonance imaging. Expert Opin. Biol. Ther. 2009, 9, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Gao, W.; Ma, M.; Wu, M.; Zhang, L.; Zheng, Y.; Chen, H.; Shi, J. A Prussian Blue-Based Core–Shell Hollow-Structured Mesoporous Nanoparticle as a Smart Theranostic Agent with Ultrahigh pH-Responsive Longitudinal Relaxivity. Adv. Mater. 2015, 27, 6382–6389. [Google Scholar] [CrossRef]
- Estelrich, J.; Sánchez-Martín, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, G.; Du, R.; Xu, R.; Zhu, D.; Qian, J.; Bai, G.; Yang, C.; Zhang, Z.; Zhang, X.; et al. A biodegradable MnSiO3@Fe3O4 nanoplatform for dual-mode magnetic resonance imaging guided combinatorial cancer therapy. Biomaterials 2019, 194, 151–160. [Google Scholar] [CrossRef]
- Dahal, D.; Ray, P.; Pan, D. Unlocking the power of optical imaging in the second biological window: Structuring near-infrared II materials from organic molecules to nanoparticles. WIREs Nanomed. Nanobiotechnology 2021, 13, e1734. [Google Scholar] [CrossRef]
- Paefgen, V.; Doleschel, D.; Kiessling, F. Evolution of contrast agents for ultrasound imaging and ultrasound-mediated drug delivery. Front. Pharmacol. 2015, 6, 197. [Google Scholar] [CrossRef]
- Sennoga, C.A.; Kanbar, E.; Auboire, L.; Dujardin, P.-A.; Fouan, D.; Escoffre, J.-M.; Bouakaz, A. Microbubble-mediated ultrasound drug-delivery and therapeutic monitoring. Expert Opin. Drug Deliv. 2017, 14, 1031–1043. [Google Scholar] [CrossRef]
- Weinstain, R.; Segal, E.; Satchi-Fainaro, R.; Shabat, D. Real-time monitoring of drug release. Chem. Commun. 2010, 46, 553–555. [Google Scholar] [CrossRef]
- Perrigue, P.M.; Murray, R.A.; Mielcarek, A.; Henschke, A.; Moya, S.E. Degradation of Drug Delivery Nanocarriers and Payload Release: A Review of Physical Methods for Tracing Nanocarrier Biological Fate. Pharmaceutics 2021, 13, 770. [Google Scholar] [CrossRef]
- Hofmann-Amtenbrink, M.; Grainger, D.W.; Hofmann, H. Nanoparticles in medicine: Current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1689–1694. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, L.; Wang, W.; Li, X.; Zhang, F. In vivo gastrointestinal drug-release monitoring through second near-infrared window fluorescent bioimaging with orally delivered microcarriers. Nat. Commun. 2017, 8, 14702. [Google Scholar] [CrossRef]
- Kim, S.; Shi, Y.; Kim, J.Y.; Park, K.; Cheng, J.-X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle–cell interaction. Expert Opin. Drug Deliv. 2010, 7, 49–62. [Google Scholar] [CrossRef]
- Sun, X.; Wang, G.; Zhang, H.; Hu, S.; Liu, X.; Tang, J.; Shen, Y. The Blood Clearance Kinetics and Pathway of Polymeric Micelles in Cancer Drug Delivery. ACS Nano 2018, 12, 6179–6192. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, X.; Liu, Z.; Cheng, L. Recent progress of chemodynamic therapy-induced combination cancer therapy. Nano Today 2020, 35, 100946. [Google Scholar] [CrossRef]
- Wang, S.; Yang, L.; Cho, H.-Y.; Dean Chueng, S.-T.; Zhang, H.; Zhang, Q.; Lee, K.-B. Programmed degradation of a hierarchical nanoparticle with redox and light responsivity for self-activated photo-chemical enhanced chemodynamic therapy. Biomaterials 2019, 224, 119498. [Google Scholar] [CrossRef]
- Delaney, L.J.; Eisenbrey, J.R.; Brown, D.; Brody, J.R.; Jimbo, M.; Oeffinger, B.E.; Stanczak, M.; Forsberg, F.; Liu, J.-B.; Wheatley, M.A. Gemcitabine-loaded microbubble system for ultrasound imaging and therapy. Acta Biomater. 2021, 130, 385–394. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, S.; Cho, H.-Y. Biohybrid Nanoparticle-Based In Situ Monitoring of In Vivo Drug Delivery. Biosensors 2023, 13, 1017. https://doi.org/10.3390/bios13121017
Ju S, Cho H-Y. Biohybrid Nanoparticle-Based In Situ Monitoring of In Vivo Drug Delivery. Biosensors. 2023; 13(12):1017. https://doi.org/10.3390/bios13121017
Chicago/Turabian StyleJu, Sohee, and Hyeon-Yeol Cho. 2023. "Biohybrid Nanoparticle-Based In Situ Monitoring of In Vivo Drug Delivery" Biosensors 13, no. 12: 1017. https://doi.org/10.3390/bios13121017
APA StyleJu, S., & Cho, H.-Y. (2023). Biohybrid Nanoparticle-Based In Situ Monitoring of In Vivo Drug Delivery. Biosensors, 13(12), 1017. https://doi.org/10.3390/bios13121017





