A Point-of-Care Testing Device Utilizing Graphene-Enhanced Fiber Optic SPR Sensor for Real-Time Detection of Infectious Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. SARS-CoV-2 Culture and Inactivation
2.3. Graphene-Based FO-SPR Sensing Probe Fabrication
2.4. SARS-CoV-2 Antibody Bio-Functionalization on the FO-SPR Sensing Probes
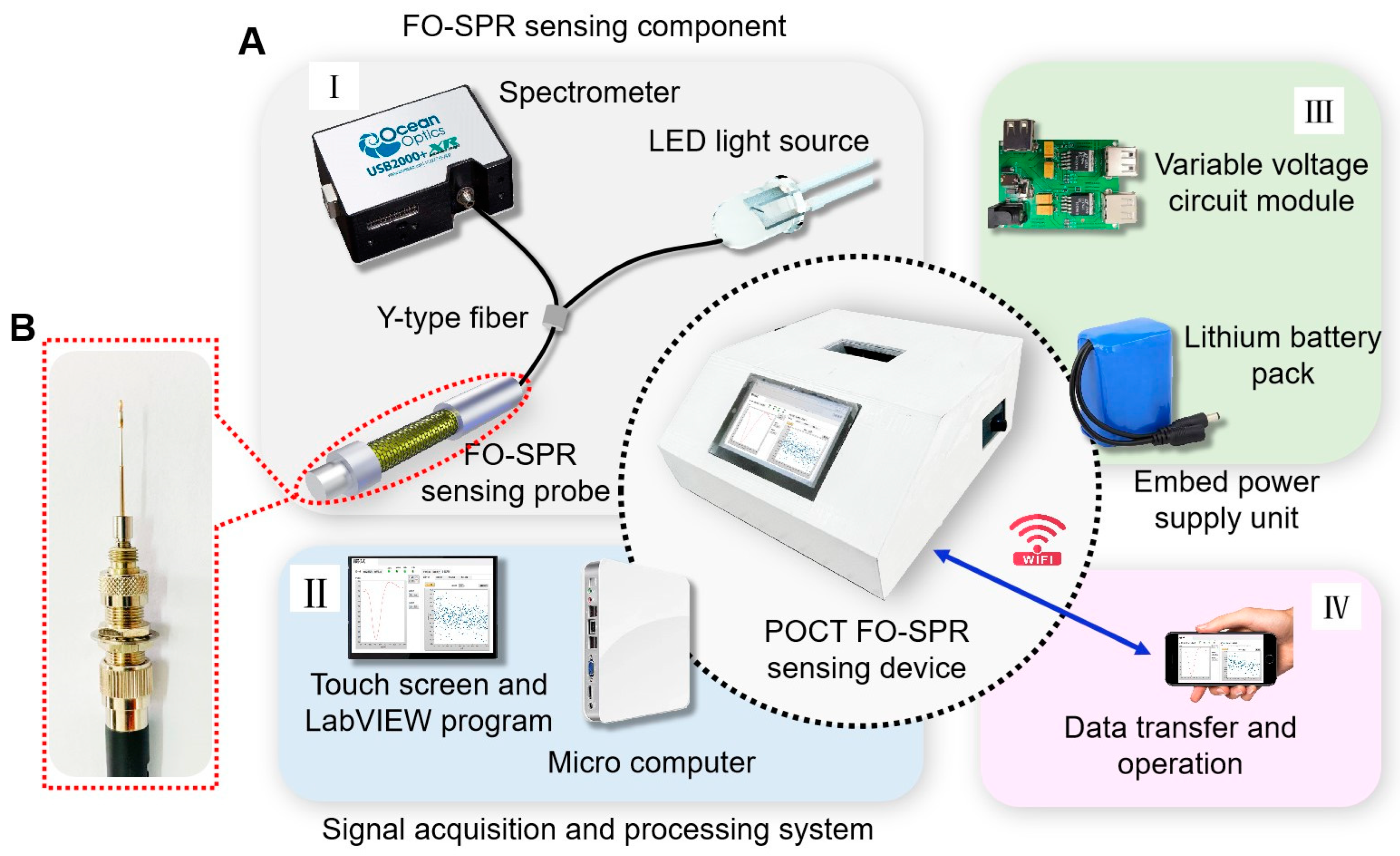
2.5. Portable FO-SPR Device Development
3. Results and Discussion
3.1. Pathogens Detection and Data Analysis
3.2. FO-SPR Sensing Probe Characterization
3.3. Experimental Verification of FO-SPR Sensing Device Performance
3.3.1. Real-Time Detection of SARS-CoV-2 Spike S1 Protein
3.3.2. Real-Time Detection of Inactivated SARS-CoV-2 Virus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Jong, M.D.; Simmons, C.P.; Thanh, T.T.; Hien, V.M.; Smith, G.J.D.; Chau, T.N.B.; Hoang, D.M.; Van Vinh Chau, N.; Khanh, T.H.; Dong, V.C.; et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006, 12, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- De Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.M.; Lai, S.T.; Poon, L.L.M.; Guan, Y.; Yam, L.Y.C.; Lim, W.; Nicholls, J.; Yee, W.K.S.; Yan, W.W.; Cheung, M.T. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 2003, 361, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Kevadiya, B.D.; Machhi, J.; Herskovitz, J.; Oleynikov, M.D.; Blomberg, W.R.; Bajwa, N.; Soni, D.; Das, S.; Hasan, M.; Patel, M.; et al. Diagnostics for SARS-CoV-2 infections. Nat. Mater. 2021, 20, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xia, Y.; Tang, Y.; Zhang, W.L.; Yeh, Y.T.; Lu, H.; Zheng, S.Y. A Nanostructured Microfluidic Immunoassay Platform for Highly Sensitive Infectious Pathogen Detection. Small 2017, 13, 1700425. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-care diagnostics for infectious diseases: From methods to devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef]
- Xiao, M.; Tian, F.; Liu, X.; Zhou, Q.; Pan, J.; Luo, Z.; Yang, M.; Yi, C. Virus Detection: From State of the Art Laboratories to Smartphone-Based Point-of-Care Testing. Adv. Sci. 2022, 9, 2105904. [Google Scholar] [CrossRef]
- Luppa, P.B.; Müller, C.; Schlichtiger, A.; Schlebusch, H. Point-of-care testing (POCT): Current techniques and future perspectives. TrAC Trends Anal. Chem. 2011, 30, 887–898. [Google Scholar] [CrossRef]
- Liu, J.; Geng, Z.; Fan, Z.; Liu, J.; Chen, H. Point-of-care testing based on smartphone: The current state-of-the-art (2017–2018). Biosens. Bioelectron. 2019, 132, 17–37. [Google Scholar] [CrossRef]
- Shang, H.; Zhang, X.; Ding, M.; Zhang, A.; Wang, C. Dual-mode paper biosensing platform based on Oxidase-like CoFeMn nanozymes for point-of-care detection of glutathione. ACS Appl. Nano Mater. 2023, 6, 8907–8915. [Google Scholar] [CrossRef]
- Huang, X.Y.; Chen, L.Z.; Zhi, W.X.; Zeng, R.M.; Ji, G.X.; Cai, H.H.; Xu, J.; Wang, J.Y.; Chen, S.Z.; Tang, Y.; et al. Urchin-Shaped Au–Ag@Pt Sensor Integrated Lateral Flow Immunoassay for multimodal detection and specific discrimination of clinical multiple bacterial infections. Anal. Chem. 2023, 95, 13101–13112. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Ganguli, A.; Nguyen, J.; Brisbin, R.; Shanmugam, K.; Hirschberg, D.L.; Wheeler, M.B.; Bashir, R.; Nash, D.M.; Cunningham, B.T. Smartphone-based multiplex 30-min nucleic acid test of live virus from nasal swab extract. Lab Chip 2020, 20, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Gahlaut, S.K.; Pathak, A.; Gupta, B.D.; Singh, J.P. Portable fiber-optic SPR platform for the detection of NS1 antigen for dengue diagnosis. Biosens. Bioelectron. 2022, 196, 113720. [Google Scholar] [CrossRef] [PubMed]
- Fathi, F.; Rashidi, M.R.; Omidi, Y. Ultra-sensitive detection by metal nanoparticles-mediated enhanced SPR biosensors. Talanta 2019, 192, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Dillen, A.; Scarpellini, C.; Daenen, W.; Driesen, S.; Zijlstra, P.; Lammertyn, J. Integrated Signal Amplification on a Fiber Optic SPR Sensor Using Duplexed Aptamers. ACS Sens. 2023, 8, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Mostufa, S.; Akib, T.B.A.; Rana, M.M.; Islam, M.R. Highly Sensitive TiO2/Au/Graphene Layer-Based Surface Plasmon Resonance Biosensor for Cancer Detection. Biosensors 2022, 12, 603. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Shin, J.; Sim, J.; Cho, H.; Hong, S. Reusable surface plasmon resonance biosensor chip for the detection of H1N1 influenza virus. Biosens. Bioelectron. 2020, 168, 112561. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Srivastava, S.K. Silicon Nitride-BP-Based Surface Plasmon Resonance Highly Sensitive Biosensor for Virus SARS-CoV-2 Detection. Plasmonics 2022, 17, 1065–1077. [Google Scholar] [CrossRef]
- Liang, G.; Luo, Z.; Liu, K.; Wang, Y.; Dai, J.; Duan, Y. Fiber Optic Surface Plasmon Resonance–Based Biosensor Technique: Fabrication, Advancement, and Application. Crit. Rev. Anal. Chem. 2015, 46, 213–223. [Google Scholar] [CrossRef]
- Li, X.; Nguyen, L.V.; Hill, K.; Ebendorff-Heidepriem, H.; Schartner, E.P.; Zhao, Y.; Zhou, X.; Zhang, Y.; Warren-Smith, S.C. All-fiber all-optical quantitative polymerase chain reaction (qPCR). Sens. Actuators B Chem. 2020, 323, 128681. [Google Scholar] [CrossRef]
- Janik, M.; Hamidi, S.V.; Koba, M.; Perreault, J.; Walsh, R.; Bock, W.J.; Śmietana, M. Real-time isothermal DNA amplification monitoring in picoliter volumes using an optical fiber sensor. Lab Chip 2021, 21, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gong, P.; Zhao, Q.; Zhou, X.; Zhang, Y.; Zhao, Y. Plug-in optical fiber SPR biosensor for lung cancer gene detection with temperature and pH compensation. Sens. Actuators B Chem. 2022, 359, 131596. [Google Scholar] [CrossRef]
- Peeters, B.; Safdar, S.; Daems, D.; Goos, P.; Spasic, D.; Lammertyn, J. Solid-Phase PCR-Amplified DNAzyme Activity for Real-Time FO-SPR Detection of the MCR-2 Gene. Anal. Chem. 2020, 92, 10783–10791. [Google Scholar] [CrossRef] [PubMed]
- Pollet, J.; Delport, F.; Janssen, K.P.F.; Jans, K.; Maes, G.; Pfeiffer, H.; Wevers, M.; Lammertyn, J. Fiber optic SPR biosensing of DNA hybridization and DNA–protein interactions. Biosens. Bioelectron. 2009, 25, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.O.; Pandey, G.R.; Patil, A.G.; Borse, V.B.; Deshmukh, P.K.; Patil, D.R.; Tade, R.S.; Nangare, S.N.; Khan, Z.G.; Patil, A.M.; et al. Graphene-based nanocomposites for sensitivity enhancement of surface plasmon resonance sensor for biological and chemical sensing: A review. Biosens. Bioelectron. 2019, 139, 111324. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.Q.; Yi, Z.; Zhang, J.G.; Xu, X.B.; Tang, B.; Li, G.; Li, G.F.; Zeng, L.C.; Chen, J.; Sun, T.Y. A tunable broadband absorber in the terahertz band based on the proportional structure of a single layer of graphene. Diam. Relat. Mater. 2023, 140, 110481. [Google Scholar] [CrossRef]
- Morales-Narváez, E.; Baptista-Pires, L.; Zamora-Gálvez, A.; Merkoçi, A. Graphene-based biosensors: Going simple. Adv. Mater. 2017, 29, 1604905. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, K.; Zhang, C.; Lin, J.; Sun, S.; Li, Q.; Du, L.; Zhang, C.; Liu, J.; Gao, F.; et al. Parapoxvirus-based therapy eliminates SARS-CoV-2 loaded fine aerosol and blocks viral transmission in hamster models. Front. Microbiol. 2022, 13, 1086627. [Google Scholar] [CrossRef]
- Qian, S.; Chen, X.; Jiang, S.; Pan, Q.; Gao, Y.; Wang, L.; Peng, W.; Liang, S.; Zhu, J.; Liu, S. Direct detection of charge and discharge process in supercapacitor by fiber-optic LSPR sensors. Nanophotonics 2020, 9, 1071–1079. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Cheng, J.Y.; Yi, Z.; Tang, B.; Chen, J.; Zhang, J.G.; Xu, X.B.; Tang, C.J.; Sun, T.Y. Spectrally selective solar absorber and thermal infrared suppression based on hollow cylindrical microstructures. Opt. Commun. 2023, 549, 129910. [Google Scholar] [CrossRef]
- Nguyen, N.H.L.; Kim, S.; Lindemann, G.; Berry, V. COVID-19 Spike Protein Induced Phononic Modification in Antibody Coupled Graphene for Viral Detection Application. ACS Nano 2021, 15, 11743–11752. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yi, Y.T.; Zhang, J.G.; Xu, X.B.; Tang, B.; Li, G.F.; Zeng, L.C.; Chen, J.; Sun, T.Y.; Yi, Z. A four-narrowband terahertz tunable absorber with perfect absorption and high sensitivity. Mater. Res. Bull. 2024, 170, 112572. [Google Scholar] [CrossRef]
- Qiu, S.; Leng, Y.K.; Yuan, J.H.; Zhang, Z.C.; Zhou, X.; Liu, B.; Mei, C.; Ya, B.B.; Wang, K.R.; Sang, X.Z.; et al. Ultrahigh-sensitivity label-free single mode-tapered multimode-single mode fiber U-shaped biosensor for Staphylococcus aureus detection. Sens. Actuators B Chem. 2023, 375, 132927. [Google Scholar] [CrossRef]
- Karpińska, K.S.; Kudła, P.; Orzeł, U.; Narajczyk, M.; Niedziółka, M.J.; Pałys, B.; Filipek, S.; Ebner, A.; Jönsson, J.N. Investigation of Peptides for Molecular Recognition of C-Reactive Protein-Theoretical and Experimental Studies. Anal. Chem. 2023, 95, 14475–14483. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.R.A.; Hjort, R.G.; Pola, C.C.; Parate, K.; Reis, E.L.; Soares, N.F.F.; McLamore, E.S.; Claussen, J.C.; Gomes, C.L. Laser-Induced Graphene Electrochemical Immunosensors for Rapid and Label-Free Monitoring of Salmonella enterica in Chicken Broth. ACS Sens. 2020, 5, 1900–1911. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]
- Xu, S.; Zhan, J.; Man, B.; Jiang, S.; Yue, W.; Gao, S.; Guo, C.; Liu, H.; Li, Z.; Wang, J.; et al. Real-time reliable determination of binding kinetics of DNA hybridization using a multi-channel graphene biosensor. Nat. Commun. 2017, 8, 14902. [Google Scholar] [CrossRef]
- Wang, S.; Hossain, M.Z.; Shinozuka, K.; Shimizu, N.; Kitada, S.; Suzuki, T.; Ichige, R.; Kuwana, A.; Kobayashi, H. Graphene field-effect transistor biosensor for detection of biotin with ultrahigh sensitivity and specificity. Biosens. Bioelectron. 2020, 165, 112363. [Google Scholar] [CrossRef]
- Kwong Hong Tsang, D.; Lieberthal, T.J.; Watts, C.; Dunlop, I.E.; Ramadan, S.; del Rio Hernandez, A.E.; Klein, N. Chemically Functionalised Graphene FET Biosensor for the Label-free Sensing of Exosomes. Sci. Rep. 2019, 9, 112363. [Google Scholar] [CrossRef]
- Ni, Z.; Wang, H.; Kasim, J.; Fan, H.; Yu, T.; Wu, Y.H.; Feng, Y.; Shen, Z. Graphene thickness determination using reflection and contrast spectroscopy. Nano Lett. 2007, 7, 2758–2763. [Google Scholar] [CrossRef]
- Wu, G.; Tang, X.; Meyyappan, M.; Lai, K.W.C. Doping effects of surface functionalization on graphene with aromatic molecule and organic solvents. Appl. Surf. Sci. 2017, 425, 713–721. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, L.; Yang, M.; Zheng, Y.; Li, L.; Gao, L.; Nerngchamnong, N.; Nai, C.T.; Sangeeth, C.S.S.; Feng, Y.P.; et al. Giant enhancement in vertical conductivity of stacked CVD graphene sheets by self-assembled molecular layers. Nat. Commun. 2014, 5, 5461. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, C.; Jiang, X.; Zhang, P.; Chen, S.; Su, F.; Wang, H.; Liu, W.; He, X.; Chen, L.; et al. High-intensity vector signals for detecting SARS-CoV-2 RNA using CRISPR/Cas13a couple with stabilized graphene field-effect transistor. Biosens. Bioelectron. 2023, 222, 114979. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Jiang, M.; Jiang, S.; Du, L.; Cheng, Y.; Li, P.; Wang, C. Design and fabrication of Gr/Ag-coated tilted grating sensor for ultra-sensitive detection of DNA hybridization. Sens. Actuators B Chem. 2022, 359, 131587. [Google Scholar] [CrossRef]
- Ang, W.L.; Lim, R.R.X.; Ambrosi, A.; Bonanni, A. Rapid electrochemical detection of COVID-19 genomic sequence with dual-function graphene nanocolloids based biosensor. FlatChem 2022, 32, 100336. [Google Scholar] [CrossRef]
- Romagnoli, A.; D’Agostino, M.; Pavoni, E.; Ardiccioni, C.; Motta, S.; Crippa, P.; Biagetti, G.; Notarstefano, V.; Rexha, J.; Perta, N.; et al. SARS-CoV-2 multi-variant rapid detector based on graphene transistor functionalized with an engineered dimeric ACE2 receptor. Nano Today 2023, 48, 101729. [Google Scholar] [CrossRef]
- Xiao, C.Q.; Jiang, F.L.; Zhou, B.; Li, R.; Liu, Y. Immobilization of Escherichia coli for detection of phage T4 using surface plasmon resonance. Sci. China Chem. 2012, 55, 1931–1939. [Google Scholar] [CrossRef]
- Makhneva, E.; Farka, Z.; Skládal, P.; Zajíčková, L. Cyclopropylamine plasma polymer surfaces for label-free SPR and QCM immunosensing of Salmonella. Sens. Actuators B Chem. 2018, 276, 447–455. [Google Scholar] [CrossRef]
- Svärd, A.; Neilands, J.; Palm, E.; Svensäter, G.; Bengtsson, T.; Aili, D. Protein-Functionalized Gold Nanoparticles as Refractometric Nanoplasmonic Sensors for the Detection of Proteolytic Activity of Porphyromonas gingivalis. ACS Appl. Nano Mater. 2020, 3, 9822–9830. [Google Scholar] [CrossRef]
- Inci, F.; Saylan, Y.; Kojouri, A.M.; Ogut, M.G.; Denizli, A.; Demirci, U. A disposable microfluidic-integrated hand-held plasmonic platform for protein detection. Appl. Mater. Today 2020, 18, 100478. [Google Scholar] [CrossRef]
- Sarcina, L.; Mangiatordi, G.F.; Torricelli, F.; Bollella, P.; Gounani, Z.; Österbacka, R.; Macchia, E.; Torsi, L. Surface Plasmon Resonance Assay for Label-Free and Selective Detection of HIV-1 p24 Protein. Biosensors 2021, 11, 180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jing, J.Y.; Wang, B.T. Highly Sensitive SPR Biosensor Based on Graphene Oxide and Staphylococcal Protein A Co-Modified TFBG for Human IgG Detection. IEEE Trans. Instrum. Meas. 2019, 68, 3350–3357. [Google Scholar] [CrossRef]
- Cennamo, N.; Pasquardini, L.; Arcadio, F.; Lunelli, L.; Vanzetti, L.; Carafa, V.; Altucci, L.; Zeni, L. SARS-CoV-2 spike protein detection through a plasmonic D-shaped plastic optical fiber aptasensor. Talanta 2021, 233, 122532. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.H.; Ordutowski, H.; Tricht, C.V.; Verbruggen, R.; Gallardo, A.B.; Bulcaen, M.; Ciwinska, M.; Cisneros, C.C.; Devriese, C.; Guluzade, S.; et al. Point-of-care therapeutic drug monitoring of adalimumab by integrating a FO-SPR biosensor in a self-powered microfluidic cartridge. Biosens. Bioelectron. 2022, 206, 114125. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Qian, S.; Zhu, S.; Lu, J.; Hu, Y.; Zhang, C.; Geng, Y.; Chen, X.; Guo, Y.; Chen, Z.; et al. A Point-of-Care Testing Device Utilizing Graphene-Enhanced Fiber Optic SPR Sensor for Real-Time Detection of Infectious Pathogens. Biosensors 2023, 13, 1029. https://doi.org/10.3390/bios13121029
Jiang S, Qian S, Zhu S, Lu J, Hu Y, Zhang C, Geng Y, Chen X, Guo Y, Chen Z, et al. A Point-of-Care Testing Device Utilizing Graphene-Enhanced Fiber Optic SPR Sensor for Real-Time Detection of Infectious Pathogens. Biosensors. 2023; 13(12):1029. https://doi.org/10.3390/bios13121029
Chicago/Turabian StyleJiang, Shiyu, Siyu Qian, Shunning Zhu, Jinxin Lu, Yunxin Hu, Cheng Zhang, Yikai Geng, Xuefeng Chen, Ying Guo, Zhaoliang Chen, and et al. 2023. "A Point-of-Care Testing Device Utilizing Graphene-Enhanced Fiber Optic SPR Sensor for Real-Time Detection of Infectious Pathogens" Biosensors 13, no. 12: 1029. https://doi.org/10.3390/bios13121029
APA StyleJiang, S., Qian, S., Zhu, S., Lu, J., Hu, Y., Zhang, C., Geng, Y., Chen, X., Guo, Y., Chen, Z., Pu, J., Guo, Z., & Liu, S. (2023). A Point-of-Care Testing Device Utilizing Graphene-Enhanced Fiber Optic SPR Sensor for Real-Time Detection of Infectious Pathogens. Biosensors, 13(12), 1029. https://doi.org/10.3390/bios13121029




