Low-Cost Plant-Based Metal and Metal Oxide Nanoparticle Synthesis and Their Use in Optical and Electrochemical (Bio)Sensors
Abstract
:1. Introduction
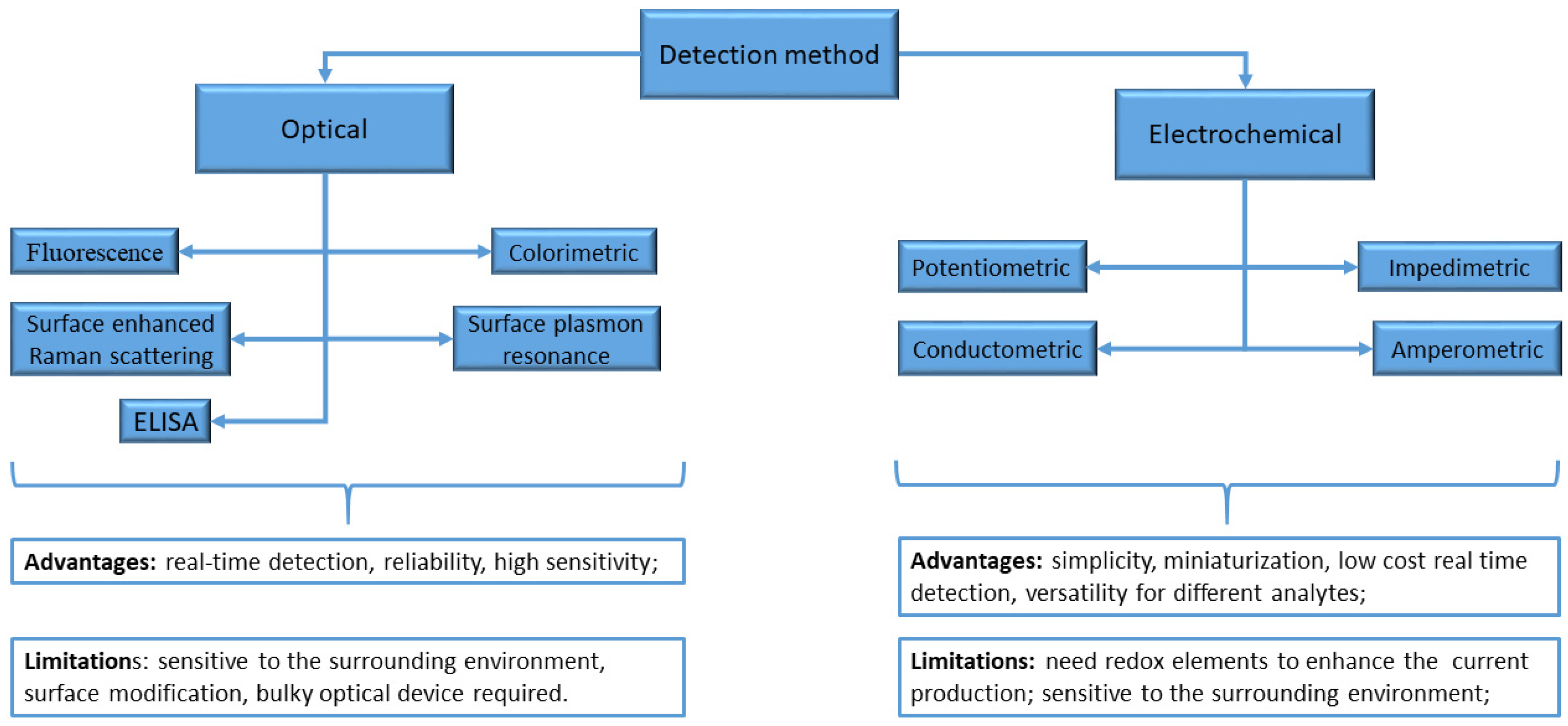
2. Sensors and Biosensors
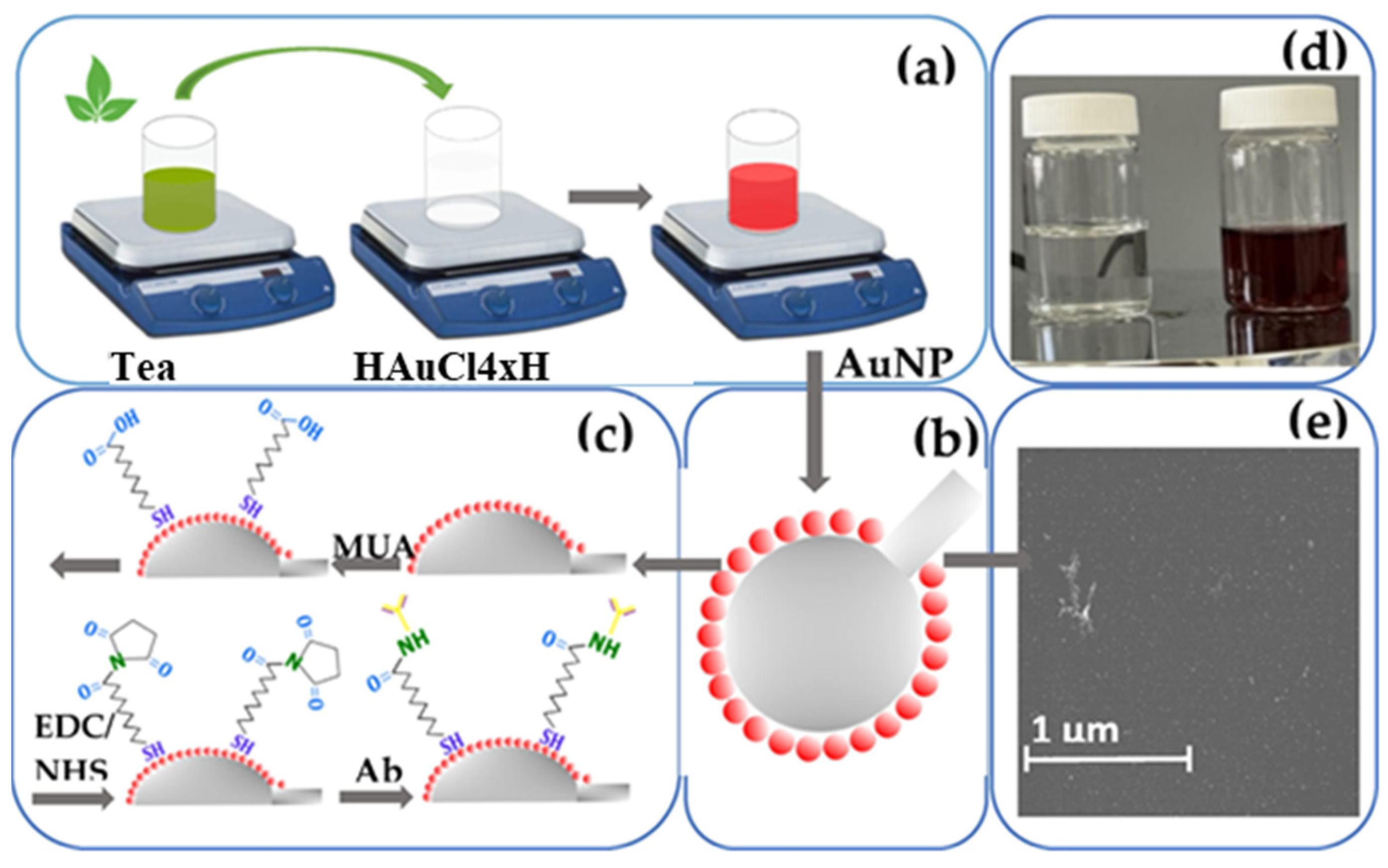
3. Green Synthesis of Metallic Nanoparticles from Plant Extract
4. Metallic Nanoparticle-Based Sensors
4.1. Ag Nanoparticles
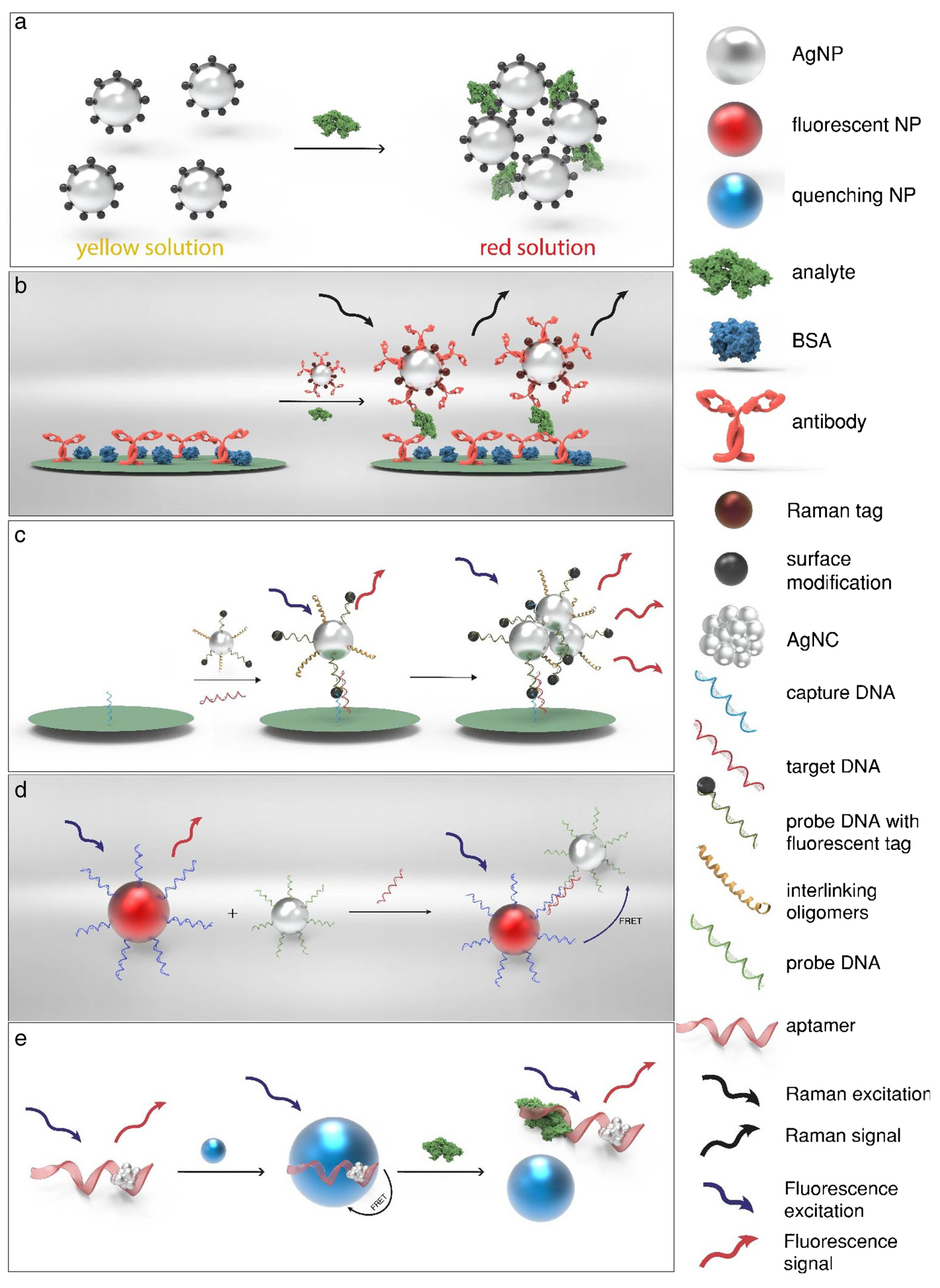
4.1.1. Optical (Bio)Sensors Based on Ag Nanoparticles
- 1.
- Detection of Hg2+ as a water pollutant by AgNPs.
- 2.
- Detection of ammonia by AgNPs
- 3.
- Detection of different heavy metal ions as water pollutants by AgNPs
- 4.
- Detection of H2O2 by AgNPs
4.1.2. Ag-Based Electrochemical (Bio)Sensors for Pharmaceutical and Bioactive Molecules
4.2. Au Nanoparticles
4.2.1. Optical Sensors Based on Au Nanoparticles
4.2.2. Electrochemical Biosensor Based on Au Nanoparticles
4.3. Non-Noble Metals
4.3.1. Ni and NiO Nanoparticles
4.3.2. Zn and ZnO Nanoparticles
| MNP Systems | Type of Extract | Detected Analyte | Linear Range | LOD | Reference |
|---|---|---|---|---|---|
| AgNP–Hedy-arum | Hedysarum aqueous; Soap-root extract | Hg(II) | 10–100 µM | 2.2 μM | [60] |
| AgNP–AB | Agaricus Bispores | Hg(II) | 10–90 µM | 2.1 μM | [61] |
| AgNP–CLW | Cauliflower-Brassica oleracea var. botrytis | Hg(II) | 0.49 μM | [62] | |
| AgNP–Aw | Achillea Wilhelmsii | Hg(II) | 100 nM–100 µM 10–700 µM | 28 nM on solution 0.3 μM on paper | [63] |
| AgNP–AC | Acacia chundra | Hg(II) | 0.28 μM | [64] | |
| AgNP–CJ | Citrus japonica (CJ) | Hg(II) | 0.3–7.3 µM | 0.09 μM | [65] |
| AgNP–MD | Mimosa diplotricha | Hg(II) | 5–45 μM | 1.46 μM | [66] |
| AgNP–M | Molasses | Hg(II) | 0.01–1 μM | 0.02 μM | [67] |
| AgNP–TC | Terminalia chebula | Ammonia | 0–100 ppm | 50 ppm | [68] |
| AgNP–GG | Cyamopsis tetragonaloba | Ammonia | 1–50 ppm | 1 ppm | [69] |
| AgNP–SG | Sugarcane leaves | H2O2 | 0–200 mM | 30 mM | [70] |
| Ammonia | 0–50 ppm | 5 ppm | |||
| AgNP–MOF | Moringa oleifera flower | Cu(IV) | 1–12 mM | 0.249 mM | [71] |
| AgNP–AS | Allium sativum | Cd(II) | 10–90 μM | 0.277 μM | [73] |
| AgNP–LE | Lycopersicon esculentum | Cr(III) | 10–90 μM | 0.804 μM | [74] |
| AgNP–LBG | Ceratonia siliqua | H2O2 | 0.01–1 mM | 0.01 mM | [75] |
| AgNP–Algae | Noctiluca scintillans | H2O2 | 4.70–32 nM | 1.34 nM | [76] |
| AgNP–rGOx | Tea | H2O2 | 0.002–20 mM | 0.73 μM | [80] |
| Ag–GO | Andrographis paniculata | H2O2 | 0–15 μM | 2.65 μM | [81] |
| AgNPs–GCE | C. sempervirens pollen | H2O2 | 5 μM–2.5 mM | 0.23 μM | [82] |
| AgNP–xGnP | Araucaria angustifolia | paracetamol | 4.98 × 10−6–3.38 × 10−5 mol L−1 | 8.50 × 10−8 mol L−1 | [83] |
| AgNps/f-MWCNT | Cinnamomum tamala | BPA | 3.9 fM–102.4 nM | 0.38 nM | [84] |
| AgNps/f-MWCNT | Moringa oleifera extract | BPA | 0.3–8 µM | 0.22 µM | [86] |
| AuNP–GG | Guar Gum (GG) | ammonia | - | 1 ppb | [89] |
| AuNP–CMGK | Gum Karaya | Cu(II) | 10–1000 nM | 10 nM | [90] |
| AuNP–WTB | Willow tree bark | cysteine | 2 × 10−7–20 × 10−7 mol/L | 0.63 × 10−7 mol/L | [91] |
| AuNP–tea | Green tea | CD44 antigen | 42.9 aM–100 nM | 0.111 pM | [92] |
| AuNP–tea | Green tea | Acetamiprid | 3.0 × 10−8–4.0 × 10−6 M | 1.76 × 10−8 M | [93] |
| AuNP–rGO | E. tereticornis | L-tryptophan | 0.5–500 µmol/L | 0.39 µmol/L | [94] |
| AuNP–rGO | Rose | glucose | 1–8 mM | 10 µM | [95] |
| AuNP–GO | Bischofia javanica Blume | chloramphenicol | 1.5–2.95 μM | 0.25 μM | [96] |
| AuNP–CNT–SPE | Sargassum sp. | glucose | 1–7 mM | 50 µM | [97] |
| NiNP–NS | Nigella sativa | glucose | 50–600 µM | 3.2 µM | [98] |
| NiONP–TS | Trigonella subenervis | glucose | 10–200 μM | 3.2 µM | [99] |
| NiNP–PP | Pomelo Peel | glucose | 15.84 μM–6.48 mM | 4.8 µM | [100] |
| NiONP–TE | Tagetes erecta L. | glucose | 0.1–1 mM | <83 µM | [101] |
| GCE/ZnO–NDCS/GOx | peach juice | glucose | 0.2–12 mM | 6.3 µM | [107] |
| GCE/MWCNTs/ZnO NPs | Carica papaya | Silymarin | 0.014–0.152 mg/L | 0.062 mg/L | [108] |
| GPE/ZnO NPs | Citrus sinensis | formaldehyde | 0–100 mM | 18 μM | [109] |
| ZnONPs | Ixora Coccinea | Ethanol | 40–800 ppm | 200 ppm | [111] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milone, A.; Monteduro, A.G.; Rizzato, S.; Leo, A.; Di Natale, C.; Kim, S.S.; Maruccio, G. Advances in Materials and Technologies for Gas Sensing from Environmental and Food Monitoring to Breath Analysis. Adv. Sustain. Syst. 2023, 7, 2200083. [Google Scholar] [CrossRef]
- Wasilewski, T.; Neubauer, D.; Kamysz, W.; Gębicki, J. Recent progress in the development of peptide-based gas biosensors for environmental monitoring. Case Stud. Chem. Environ. Eng. 2022, 5, 100197. [Google Scholar] [CrossRef]
- Palumbo, M.; Attolico, G.; Capozzi, V.; Cozzolino, R.; Corvino, A.; de Chiara, M.L.V.; Pace, B.; Pelosi, S.; Ricci, I.; Romaniello, R.; et al. Emerging postharvest technologies to enhance the shelf-life of fruit and vegetables: An overview. Foods 2022, 11, 3925. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.iso.org/home.html (accessed on 16 October 2023).
- Available online: https://www.astm.org/ (accessed on 16 October 2023).
- Ealias, A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar]
- Pal, G.; Rai, P.; Pandey, A. Green synthesis of nanoparticles: A greener approach for a cleaner future. In Green synthesis, characterization and applications of nanoparticles. In Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–26. [Google Scholar]
- Dutta, D.; Das, B.M. Scope of green nanotechnology towards amalgamation of green chemistry for cleaner environment: A review on synthesis and applications of green nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100418. [Google Scholar] [CrossRef]
- Patil, S.; Chandrasekaran, R. Biogenic nanoparticles: A comprehensive perspective in synthesis, characterization, application and its challenges. J. Genet. Eng. Biotechnol. 2020, 18, 67. [Google Scholar] [CrossRef]
- Das, R.K.; Pachapur, V.L.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Cledon, M.; Dalila, L.M.A.; Sarma, S.J.; Brar, S.K. Biological synthesis of metallic nanoparticles: Plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 18. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green synthesis of metallic nanoparticles: Applications and limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Al-Tamimi, S.A. Biogenic green synthesis of metal oxide nanoparticles using oat biomass for ultrasensitive modified polymeric sensors. Green Chem. Lett. Rev. 2021, 14, 166–179. [Google Scholar] [CrossRef]
- Mandal, D.; Mishra, S.; Singh, R.K. Green synthesized nanoparticles as potential nanosensors. In Environmental, Chemical and Medical Sensors; Springer: Singapore, 2018; pp. 137–164. [Google Scholar]
- Hacke, A.C.M.; Lima, D.; Kuss, S. Green synthesis of electroactive nanomaterials by using plant-derived natural products. J. Electroanal. Chem. 2022, 922, 116786. [Google Scholar] [CrossRef]
- Jianrong, C.; Yuqing, M.; Nongyue, H.; Xiaohua, W.; Sijiao, L. Nanotechnology and biosensors. Biotechnol. Adv. 2004, 22, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Oprea, D.; Sanz, C.G.; Barsan, M.M.; Enache, T.A. PC-12 Cell Line as a Neuronal Cell Model for Biosensing Applications. Biosensors 2022, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- El-Aassar, M.R.; Ibrahim, O.M.; Fouda, M.M.G.; El-Beheri, N.G.; Agwa, M.M. Wound healing of nanofiber comprising Polygalacturonic/Hyaluronic acid embedded silver nanoparticles: In-vitro and in-vivo studies. Carbohydr. Polym. 2020, 238, 116175. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Al-Kadmy, I.M.S.; Khazaal, S.S.; Abbas, S.; Suhail, A.; El-Mokhtar, M.A.; Abd Ellah, N.H.; Ahmed, E.A.; Abd-ellatief, R.B.; El-Masry, E.A.; et al. Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2021, 11, 10751. [Google Scholar] [CrossRef] [PubMed]
- Sreelekha, E.; Bini, G.; Aswathi, S.; Sajina, N.; Beena, M. A comparative study on the synthesis, characterization, and antioxidant activity of green and chemically synthesized silver nanoparticles. BioNanoScience 2021, 11, 489–496. [Google Scholar] [CrossRef]
- Scandurra, A.; Censabella, M.; Gulino, A.; Grimaldi, M.G.; Ruffino, F. Gold nanoelectrode arrays dewetted onto graphene paper for selective and direct electrochemical determination of glyphosate in drinking water. Sens. Bio-Sens. Res. 2022, 36, 100496. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Maruccio, G.; Narang, J. Electrochemical Sensors: From Working Electrodes to Functionalization and Miniaturized Devices; Woodhead Publishing: Cambridge, UK, 2022. [Google Scholar]
- Herrera-Domínguez, M.; Morales-Luna, G.; Mahlknecht, J.; Cheng, Q.; Aguilar-Hernández, I.; Ornelas-Soto, N. Optical Biosensors and Their Applications for the Detection of Water Pollutants. Biosensors 2023, 13, 370. [Google Scholar] [CrossRef]
- Emami, T.; Madani, R.; Golchinfar, F.; Shoushtary, A.; Amini, S.M. Comparison of Gold Nanoparticle Conjugated Secondary Antibody with Non-Gold Secondary Antibody in an ELISA Kit Model. Monoclon. Antibodies Immunodiagn. Immunother. 2015, 34, 366–370. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Jasim, N.O.; Hasan, T.K.; Flieh, H. Characterization of silver nano particles synthesized by leaves green tea extract. J. Glob. Pharma Technol. 2018, 10, 423–427. [Google Scholar]
- Malik, S.; Niazi, M.; Khan, M.; Rauff, B.; Anwar, S.; Amin, F.; Hanif, R. Cytotoxicity study of gold nanoparticle synthesis using Aloe vera, honey, and Gymnema sylvestre leaf extract. ACS Omega 2023, 8, 6325–6336. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, E.Y.; Lee, J. Non-toxic nanoparticles from phytochemicals: Preparation and biomedical application. Bioprocess Biosyst. Eng. 2014, 37, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Hong, Y.N.; Weyers, A.; Kim, Y.S.; Linhardt, R.J. Polysaccharides and phytochemicals: A natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol. 2011, 5, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, T.; Nadhman, A. Applications of plant terpenoids in the synthesis of colloidal silver nanoparticles. Adv. Colloid Interface Sci. 2016, 234, 132–141. [Google Scholar]
- Venkata, A.L.K.; Sivaram, S.; Sajeet, M.; Sanjay, P.M.; Srilakshman, G.; Muthuraman, M.S. Review on terpenoid mediated nanoparticles: Significance, mechanism, and biomedical applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2022, 13, 033003. [Google Scholar] [CrossRef]
- Keijok, W.J.; Pereira, R.H.A.; Alvarez, L.A.C.; Prado, A.R.; da Silva, A.R.; Ribeiro, J.; de Oliveira, J.P.; Guimarães, M.C.C. Controlled biosynthesis of gold nanoparticles with Coffea arabica using factorial design. Sci. Rep. 2019, 9, 16019. [Google Scholar] [CrossRef]
- Baghaienezhad, M.; Boroghani, M.; Anabestani, R. Silver nanoparticles synthesis by coffee residues extract and their antibacterial activity. Nanomed. Res. J. 2020, 5, 29–34. [Google Scholar]
- Zameer, S.; Yamamoto, Y. Carbohydrate-directed synthesis of silver and gold nanoparticles: Effect of the structure of carbohydrates and reducing agents on the size and morphology of the composites. Carbohydr. Res. 2011, 346, 651–658. [Google Scholar]
- Maruyama, T.; Fujimoto, Y.; Maekawa, T. Synthesis of gold nanoparticles using various amino acids. J. Colloid Interface Sci. 2015, 447, 254–257. [Google Scholar] [CrossRef]
- Rashid, M.U.; Khairul, H.B.; Quayum, M.E. Synthesis of silver nano particles (Ag-NPs) and their uses for quantitative analysis of vitamin C tablets. Dhaka Univ. J. Pharm. Sci. 2013, 12, 29–33. [Google Scholar] [CrossRef]
- Daizy, P. Honey mediated green synthesis of silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 1078–1081. [Google Scholar]
- Willner, I.; Baron, R.; Willner, B. Growing metal nanoparticles by enzymes. Adv. Mater. 2006, 18, 1109–1120. [Google Scholar] [CrossRef]
- Khan, A.U.; Wei, Y.; Ahmad, A.; Khan, Z.U.H.; Tahir, K.; Khan, S.U.; Muhammad, N.; Khan, F.U.; Yuan, Q. Enzymatic browning reduction in white cabbage, potent antibacterial and antioxidant activities of biogenic silver nanoparticles. J. Mol. Liq. 2016, 215, 39–46. [Google Scholar] [CrossRef]
- Tarannum, N.; Divya and Gautam, Y.K. Facile green synthesis and applications of silver nanoparticles: A state-of-the-art review. RSC Adv. 2019, 9, 34926. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jun, B.-H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape-and size-dependent refractive index sensitivity of gold nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef]
- Yang, Z.-X.; Zhong, W.; Au, C.; Wanga, J.-Y.; Du, Y.-W. An environment-benign solvothermal method for the synthesis of flower-like hierarchical nickel and zinc compounds and their transformation to nanoporous NiO and ZnO. CrystEngComm 2011, 13, 1831–1837. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Behbudi, G.; Gholami, A.; Hashemi, S.A.; Nejad, Z.M.; Bahrani, S.; Chiang, W.-H.; Wei, L.C.; Omidifar, N. Shape-controlled synthesis of zinc nanostructures mediating macromolecules for biomedical applications. Biomater. Res. 2022, 26, 4. [Google Scholar] [CrossRef]
- Anu, R.; Yadav, K.; Jagadevan, S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar]
- Samson, R.; Navale, G.R.; Dharne, M.S. Biosensors: Frontiers in rapid detection of COVID-19. 3 Biotech 2020, 10, 385. [Google Scholar] [CrossRef]
- Wang, S.; Payne, G.F.; Bentley, W.E. Quorum sensing communication: Molecularly connecting cells, their neighbors, and even devices. Annu. Rev. Chem. Biomol. Eng. 2020, 11, 447–468. [Google Scholar] [CrossRef]
- Rohiwal, S.S.; Dvorakova, N.; Klima, J.; Vaskovicova, M.; Senigl, F.; Slouf, M.; Pavlova, E.; Stepanek, P.; Babuka, D.; Benes, H.; et al. Polyethylenimine based magnetic nanoparticles mediated non-viral CRISPR/Cas9 system for genome editing. Sci. Rep. 2020, 10, 4619. [Google Scholar] [CrossRef]
- Manhas, J.; Edelstein, H.I.; Leonard, J.N.; Morsut, L. The evolution of synthetic receptor systems. Nat. Chem. Biol. 2022, 18, 244–255. [Google Scholar] [CrossRef]
- McCarty, N.S.; Graham, A.E.; Studená, L.; Ledesma-Amaro, R. Multiplexed CRISPR technologies for gene editing and transcriptional regulation. Nat. Commun. 2020, 11, 1281. [Google Scholar] [CrossRef]
- Mousavi, P.S.; Smith, S.J.; Chen, J.B.; Karlikow, M.; Tinafar, A.; Robinson, C.; Liu, W.; Ma, D.; Green, A.A.; Kelley, S.O.; et al. A multiplexed, electrochemical interface for gene-circuit-based sensors. Nat. Chem. 2020, 12, 48–55. [Google Scholar] [CrossRef]
- Ahmar, S.; Mahmood, T.; Fiaz, S.; Mora-Poblete, F.; Shafique, M.S.; Chattha, M.S.; Jung, K.-H. Advantage of nanotechnology-based genome editing system and its application in crop improvement. Front. Plant Sci. 2021, 12, 663849. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lu, Y.; He, L.; Pang, J.; Yang, F.; Liu, Y. Colorimetric sensor array based on gold nanoparticles: Design principles and recent advances. Trends Anal. Chem. 2019, 122, 115754. [Google Scholar] [CrossRef]
- Montes-Garcia, V.; Squillaci, M.A.; Diez-Castellnou, M.; Ong, Q.K.; Stellacci, F.; Samori, P. Chemical sensing with Au and Ag nanoparticles. Chem. Soc. Rev. 2021, 50, 1269–1304. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, S.; Astudillo-Calderón, S.; Pintos, B.; Pérez-Urria, E.; Manzanera, J.A.; Martín, L.; Gomez-Garay, A. Role of Synthetic Plant Extracts on the Production of Silver-Derived Nanoparticles. Plants 2021, 10, 1671. [Google Scholar] [CrossRef]
- Beck, F.; Loessl, M.; Baeumner, A.J. Signaling strategies of silver nanoparticles in optical and electrochemical biosensors: Considering their potential for the point-of-care. Microchim. Acta 2023, 190, 91. [Google Scholar] [CrossRef]
- Prosposito, P.; Burratti, L.; Venditti, I. Silver Nanoparticles as Colorimetric Sensors for Water Pollutants. Chemosensors 2020, 8, 26. [Google Scholar] [CrossRef]
- Forough, M.; Farhadi, K. Biological and green synthesis of silver nanoparticles. Turk. J. Eng. Environ. Sci. 2010, 34, 281–287. [Google Scholar]
- Farhadi, K.; Forough, M.; Molaei, R.; Hajizadeh, S.; Rafipour, A. Highly selective Hg2+ colorimetric sensor using green synthesized and unmodified silver nanoparticles. Sens. Actuators B Chem. 2012, 161, 880–885. [Google Scholar] [CrossRef]
- Sebastian, M.; Aravind, A.; Mathew, B. Green Silver Nanoparticles Based Dual Sensor for Toxic Hg(II) Ions. Nanotechnology 2018, 29, 355502. [Google Scholar] [CrossRef]
- Kadam, J.; Dhawal, P.; Barve, S.; Kakodkar, S. Green synthesis of silver nanoparticles using cauliflower waste and their multifaceted applications in photocatalytic degradation of methylene blue dye and Hg2+ biosensing. SN Appl. Sci. 2020, 2, 738. [Google Scholar] [CrossRef]
- Mavaei, M.; Chahardoli, A.; Fattahi, A.; Khoshroo, A. A Simple Method for Developing a Hand-Drawn Paper-Based Sensor for Mercury; Using Green Synthesized Silver Nanoparticles and Smartphone as a Hand-Held-Device for Colorimetric Assay. Glob. Chall. 2021, 5, 2000099. [Google Scholar] [CrossRef] [PubMed]
- Ramachandiran, D.; Rajesh, K. Selective colorimetric and fluorimertic sensor of Hg(II) ion from silver nanoparticles using Acacia chundra leaves extract. Mater. Chem. Phys. 2022, 287, 126284. [Google Scholar] [CrossRef]
- Bhagat, S.; Shaikh, H.; Nafady, A.; Sirajuddin; Sherazi, S.T.H.; Bhanger, M.I.; Shah, M.R.; Abro, M.I.; Memon, R.; Bhagat, R. Trace Level Colorimetric Hg2+ Sensor Driven by Citrus japonica Leaf Extract Derived Silver Nanoparticles: Green Synthesis and Application. J. Clust. Sci. 2022, 33, 1865–1875. [Google Scholar] [CrossRef]
- Punnoose, M.S.; Bijimol, D.; Abraham, T.; Plathanam, N.J.; Mathew, B. Green Synthesized Unmodified Silver Nanoparticles as Reproducible Dual Sensor for Mercuric Ions and Catalyst to Abate Environmental Pollutants. BioNanoScience 2021, 11, 739–754. [Google Scholar] [CrossRef]
- Gangarapu, M.; Anaikutti, P.; Sarangapany, S. Sustainable Utilization of Molasses Towards Green Synthesis of Silver Nanoparticles for Colorimetric Heavy Metal Sensing and Catalytic Applications. J. Clust. Sci. 2020, 31, 1137–1145. [Google Scholar]
- Edison, T.N.J.I.; Atchudan, R.; Lee, Y.R. Optical Sensor for Dissolved Ammonia Through the Green Synthesis of Silver Nanoparticles by Fruit Extract of Terminalia chebula. J. Clust. Sci. 2016, 27, 683–690. [Google Scholar] [CrossRef]
- Pandey, S.; Goswami, G.K.; Nanda, K.K. Green synthesis of biopolymer–silver nanoparticle nanocomposite: An optical sensor for ammonia detection. Int. J. Biol. Macromol. 2012, 51, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Srikhao, N.; Kasemsiri, P.; Lorwanishpaisarn, N.; Okhawilai, M. Green synthesis of silver nanoparticles using sugarcane leaves extract for colorimetric detection of ammonia and hydrogen peroxide. Res. Chem. Intermed. 2021, 47, 1269–1283. [Google Scholar] [CrossRef]
- Aadila, K.R.; Pandey, N.; Mussatto, S.I.; Jha, H. Green synthesis of silver nanoparticles using acacia lignin, their cytotoxicity, catalytic, metal ion sensing capability and antibacterial activity. J. Environ. Chem. Eng. 2019, 7, 103296. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M.; Esmail, G.A.; Al-Dhabi, N.A.; Arasu, M.V. Green synthesis and characterization of silver nanoparticles from Moringa oleifera flower and assessment of antimicrobial and sensing properties. J. Photochem. Photobiol. B Biol. 2020, 205, 111836. [Google Scholar] [CrossRef]
- Aravind, A.; Sebastian, M.; Mathew, B. Green silver nanoparticles as a multifunctional sensor for toxic Cd(II) ions. New J. Chem. 2018, 42, 15022–15031. [Google Scholar] [CrossRef]
- Aravind, A.; Sebastian, M.; Mathew, B. Green synthesized unmodified silver nanoparticles as a multi-sensor for Cr(III) ions. Environ. Sci. Water Res. Technol. 2018, 4, 1531–1542. [Google Scholar] [CrossRef]
- Tagad, C.K.; Dugasani, S.R.; Aiyer, R.; Park, S.; Kulkarni, A.; Sabharwal, S. Green synthesis of silver nanoparticles and their application for the development of optical fiber based hydrogen peroxide sensor. Sens. Actuators B 2013, 183, 144–149. [Google Scholar] [CrossRef]
- Elgamouz, A.; Idriss, H.; Nassab, C.; Bihi, A.; Bajou, K.; Hasan, K.; Haija, M.A.; Patole, S.P. Green Synthesis, Characterization, Antimicrobial, Anti-Cancer, and Optimization of Colorimetric Sensing of Hydrogen Peroxide of Algae Extract Capped Silver Nanoparticles. Nanomaterials 2020, 10, 1861. [Google Scholar] [CrossRef]
- Chandraker, S.K.; Lal, M.; Kumar, A.; Shukla, R. Justicia adhatoda L. mediated green synthesis of silver nanoparticles and assessment of their antioxidant, hydrogen peroxide sensing and optical properties. Mater. Technol. 2022, 37, 1355–1365. [Google Scholar] [CrossRef]
- Mahadevan, S.; Vijayakumar, S.; Arulmozhi, P. Green synthesis of silver nano particles from Atalantia monophylla (L.) Correa leaf extract, their antimicrobial activity and sensing capability of H2O2. Microb. Pathog. 2017, 113, 445–450. [Google Scholar] [CrossRef]
- Alipilakkotte, S.; Sreejith, L. Green synthesized PLA/silver nanoparticle probe for sensing of hydrogen peroxide in biological samples. Mater. Lett. 2018, 217, 33–38. [Google Scholar] [CrossRef]
- Salazar, P.; Fernández, I.; Rodríguez, M.C.; Creus, A.H.; González-Mora, J.L. One-step green synthesis of silver nanoparticle-modified reduced graphene oxide nanocomposite for H2O2 sensing applications. J. Electroanal. Chem. 2019, 855, 113638. [Google Scholar] [CrossRef]
- Nam, N.T.H.; Dat, N.M.; Hai, N.D.; Huong, L.M.; Tai, L.T.; Dat, N.T.; An, H.; Hung, P.N.P.; Truong, N.T.; Son, N.T.; et al. Green synthesis of silver@graphene oxide nanocomposite for antibacterial, cytotoxicity assessment, and hydrogen peroxide electro-sensing. New J. Chem. 2023, 47, 8090. [Google Scholar] [CrossRef]
- Turunc, E.; Kahraman, O.; Binzet, R. Green synthesis of silver nanoparticles using pollen extract: Characterization, assessment of their electrochemical and antioxidant activities. Anal. Biochem. 2021, 621, 114123. [Google Scholar] [CrossRef]
- Zamarchi, F.; Vieira, I.C. Determination of paracetamol using a sensor based on green synthesis of silver nanoparticles in plant extract. J. Pharm. Biomed. Anal. 2021, 196, 113912. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Chauhan, D.; Mukherjee, M.D.; Ranjan, K.R.; Yadav, A.K.; Solanki, P.R. Development of MWCNT decorated with green synthesized AgNps-based electrochemical sensor for highly sensitive detection of BPA. J. Appl. Electrochem. 2021, 51, 447–462. [Google Scholar] [CrossRef]
- Verma, D.; Dhiman, T.K.; Mukherjee, M.D.; Solanki, P.R. Electrophoretically Deposited Green Synthesized Silver Nanoparticles Anchored in Reduced Graphene Oxide Composite Based Electrochemical Sensor for Detection of Bisphenol A. J. Electrochem. Soc. 2021, 168, 097504. [Google Scholar] [CrossRef]
- Jaballah, M.B.; Messaoud, N.B.; Dridi, C. Development of cost-effective and sustainable sensing nanoplatform based on green AgNPs for the determination of BPA in water. J. Mater. Sci. Mater. Electron. 2022, 33, 6981–6998. [Google Scholar] [CrossRef]
- Teimuri-Mofrad, R.; Hadi, R.; Tahmasebi, B.; Farhoudian, S.; Mehravar, M.; Nasiri, R. Green synthesis of gold nanoparticles using plant extract: Mini-Review. Nanochem. Res. 2017, 2, 8–19. [Google Scholar]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Recent Developments in the Facile Bio-Synthesis of Gold Nanoparticles (AuNPs) and Their Biomedical Applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Goswami, G.K.; Nanda, K.K. Green synthesis of polysaccharide/gold nanoparticle nanocomposite: An efficient ammonia sensor. Carbohydr. Polym. 2013, 94, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Gangapuram, B.R.; Bandi, R.; Dadigala, R.; Kotu, G.M.; Guttena, V. Facile Green Synthesis of Gold Nanoparticles with Carboxymethyl Gum Karaya, Selective and Sensitive Colorimetric Detection of Copper(II) Ions. J. Clust. Sci. 2017, 28, 2873–2890. [Google Scholar] [CrossRef]
- Bahram, M.; Mohammadzadeh, E. Green synthesis of gold nanoparticles with willow tree bark extract: A sensitive colourimetric sensor for cysteine detection. Anal. Methods 2014, 6, 6916. [Google Scholar] [CrossRef]
- Ashikbayeva, Z.; Bekmurzayeva, A.; Myrkhiyeva, Z.; Assylbekova, N.; Atabaev, T.S.; Tosi, D. Green-synthesized gold nanoparticle-based optical fiber ball resonator biosensor for cancer biomarker detection. Opt. Laser Technol. 2023, 161, 109136. [Google Scholar] [CrossRef]
- Li, H.; Hu, W.; Hassan, M.M.; Zhang, Z.; Chen, Q. A facile and sensitive SERS-based biosensor for colormetric detection of acetamiprid in green tea based on unmodified gold nanoparticles. J. Food Meas. Charact. 2019, 13, 259–268. [Google Scholar] [CrossRef]
- Nazarpour, S.; Hajian, R.; Sabzvaria, M.H. A novel nanocomposite electrochemical sensor based on green synthesis of reduced graphene oxide/gold nanoparticles modified screen printed electrode for determination of tryptophan using response surface methodology approach. Microchem. J. 2020, 154, 104634. [Google Scholar] [CrossRef]
- Tabrizia, M.A.; Varkanib, J.N. Green synthesis of reduced graphene oxide decorated with gold nanoparticles and its glucose sensing application. Sens. Actuators B Chem. 2014, 202, 475–482. [Google Scholar] [CrossRef]
- Karthik, R.; Govindasamy, M.; Chen, S.-M.; Mani, V.; Lou, B.-S.; Devasenathipathy, R.; Hou, Y.-S.; Elangovan, A. Green synthesized gold nanoparticles decorated graphene oxide for sensitive determination of chloramphenicol in milk, powdered milk, honey and eye drops. J. Colloid Interface Sci. 2016, 475, 46–56. [Google Scholar] [CrossRef] [PubMed]
- González-Fuentes, F.J.; Molina, G.A.; Silva, R.; López-Miranda, J.L.; Esparza, R.; Hernandez-Martinez, A.R.; Estevez, M. Developing a CNT-SPE Sensing Platform Based on Green Synthesized AuNPs, Using Sargassum sp. Sensors 2020, 20, 6108. [Google Scholar] [CrossRef] [PubMed]
- Messai, Y.; Bezzi, H.; Hellal, N.; Belbacha, W.; Messali, S.; Belghidoum, A.; Foudia, M.; Schmutz, M.; Blanck, C.; Derafa, W.; et al. A novel green synthesized NiO nanoparticles modified glassy carbon electrode for non-enzymatic glucose sensing. Microchem. J. 2022, 178, 107332. [Google Scholar]
- Mahdavi, B.; Paydarfard, S.; Rezaei-Seresht, E.; Baghayeri, M.; Nodehi, M. Green synthesis of NiONPs using Trigonella subenervis extract and its applications as a highly efficient electrochemical sensor, catalyst, and antibacterial agent. Appl. Organomet. Chem. 2021, 35, 6264. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Yu, J.; He, J.; Yang, H.; Ye, Y.; Song, Y. A green and simple strategy to prepare graphene foam-like three-dimensional porous carbon/Ni nanoparticles for glucose sensing. Sens. Actuators B Chem. 2017, 239, 172–179. [Google Scholar] [CrossRef]
- Likasari, I.D.; Astuti, R.W.; Yahya, A.; Isnaini, N.; Purwiandono, G.; Hidayat, H.; Wicaksono, W.P.; Fatimah, I. NiO nanoparticles synthesized by using Tagetes erecta L leaf extract and their activities for photocatalysis, electrochemical sensing, and antibacterial features. Chem. Phys. Lett. 2021, 780, 138914. [Google Scholar] [CrossRef]
- Rajith Kumar, C.R.; Betageri, V.S.; Nagaraju, G.; Pujar, G.H.; Suma, B.P.; Latha, M.S. Photocatalytic, nitrite sensing and antibacterial studies of facile bio-synthesized nickel oxide nanoparticles. J. Sci. Adv. Mater. Devices 2020, 5, 48–55. [Google Scholar] [CrossRef]
- Das, T.R.; Sharma, P.K. Hydrothermal-assisted green synthesis of Ni/Ag@rGO nanocomposite using Punica granatum juice and electrochemical detection of ascorbic acid. Microchem. J. 2020, 156, 104850. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Shirsat, M.D. Optical and structural properties of zinc oxide nanoparticles. Int. J. Adv. Res. Phys. Sci. 2015, 2, 14–18. [Google Scholar]
- Morozov, I.G.; Belousova, O.V.; Ortega, D.; Mafina, M.K.; Kuznetcov, M.V. Structural, optical, XPS and magnetic properties of Zn particles capped by ZnO nanoparticles. J. Alloys Compd. 2015, 633, 237–245. [Google Scholar] [CrossRef]
- Darvishi, E.; Kahrizi, D.; Arkan, E. Comparison of different properties of zinc oxide nanoparticles synthesized by the green (using Juglans regia L. leaf extract) and chemical methods. J. Mol. Liq. 2019, 286, 110831. [Google Scholar] [CrossRef]
- Muthuchamy, N.; Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Lee, Y.R. High-performance glucose biosensor based on green synthesized zinc oxide nanoparticle embedded nitrogen-doped carbon sheet. J. Electroanal. Chem. 2018, 816, 195–204. [Google Scholar] [CrossRef]
- Sharma, D.; Sabela, M.I.; Kanchi, S.; Bisetty, K.; Skelton, A.A.; Honarparvar, B. Green synthesis, characterization and electrochemical sensing of silymarin by ZnO nanoparticles: Experimental and DFT studies. J. Electroanal. Chem. 2018, 808, 160–172. [Google Scholar] [CrossRef]
- Wicaksono, W.P.; Fadilla, N.I.; Zamar, A.A.; Fadillah, G.; Anugrahwati, M.; Anas, A.K.; Kadja, G.T.M. Formaldehyde electrochemical sensor using graphite paste-modified green synthesized zinc oxide nanoparticles. Inorg. Chem. Commun. 2022, 143, 109729. [Google Scholar] [CrossRef]
- Joshi, L.P.; Khatri, B.V.; Gyawali, S.; Gajurel, S.; Chaudhary, D.K. Green Synthesis of Zinc Oxide Nanoparticles Using Ixora Coccinea Leaf Extract for Ethanol Vapour Sensing. J. Phys. Sci. 2021, 32, 15–26. [Google Scholar] [CrossRef]
- Narayana, A.; Bhat, S.A.; Fathima, A.; Lokesh, S.V.; Suryad, S.G.; Yelamaggad, C.V. Green and low-cost synthesis of zinc oxide nanoparticles and their application in transistor-based carbon monoxide sensing. RSC Adv. 2020, 10, 13532–13542. [Google Scholar] [CrossRef]
- Saleh, T.A.; Fadillah, G. Green synthesis protocols, toxicity, and recent progress in nanomaterial-based for environmental chemical sensors applications. Trends Environ. Anal. Chem. 2023, 39, e00204. [Google Scholar] [CrossRef]
- Zagal-Padilla, C.K.; Diaz-Gómez, C.; Gamboa, S.A. Electrochemical characterization of a plasmonic effect ethanol sensor based on two-dimensional ZnO synthesized by green chemistry. Mater. Sci. Semicond. Process. 2022, 137, 106240. [Google Scholar] [CrossRef]
- Rohatgi, V.; Challagulla, N.V.; Pudake, R.N. Volatile Organic Compounds (VOCs) Sensors for Stress Management in Crops. In Biosensors in Agriculture: Recent Trends and Future Perspectives; Springer: Cham, Switzerland, 2021; pp. 81–95. [Google Scholar]
- Gharari, Z.; Hanachi, P.; Walker, T.R. Green synthesized Ag-nanoparticles using Scutellaria multicaulis stem extract and their selective cytotoxicity against breast cancer. Anal. Biochem. 2022, 653, 114786. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciobotaru, I.C.; Oprea, D.; Ciobotaru, C.C.; Enache, T.A. Low-Cost Plant-Based Metal and Metal Oxide Nanoparticle Synthesis and Their Use in Optical and Electrochemical (Bio)Sensors. Biosensors 2023, 13, 1031. https://doi.org/10.3390/bios13121031
Ciobotaru IC, Oprea D, Ciobotaru CC, Enache TA. Low-Cost Plant-Based Metal and Metal Oxide Nanoparticle Synthesis and Their Use in Optical and Electrochemical (Bio)Sensors. Biosensors. 2023; 13(12):1031. https://doi.org/10.3390/bios13121031
Chicago/Turabian StyleCiobotaru, Iulia Corina, Daniela Oprea, Constantin Claudiu Ciobotaru, and Teodor Adrian Enache. 2023. "Low-Cost Plant-Based Metal and Metal Oxide Nanoparticle Synthesis and Their Use in Optical and Electrochemical (Bio)Sensors" Biosensors 13, no. 12: 1031. https://doi.org/10.3390/bios13121031
APA StyleCiobotaru, I. C., Oprea, D., Ciobotaru, C. C., & Enache, T. A. (2023). Low-Cost Plant-Based Metal and Metal Oxide Nanoparticle Synthesis and Their Use in Optical and Electrochemical (Bio)Sensors. Biosensors, 13(12), 1031. https://doi.org/10.3390/bios13121031







