Needle Tip Tracking through Photoluminescence for Minimally Invasive Surgery
Abstract
:1. Introduction
2. Materials and Methods
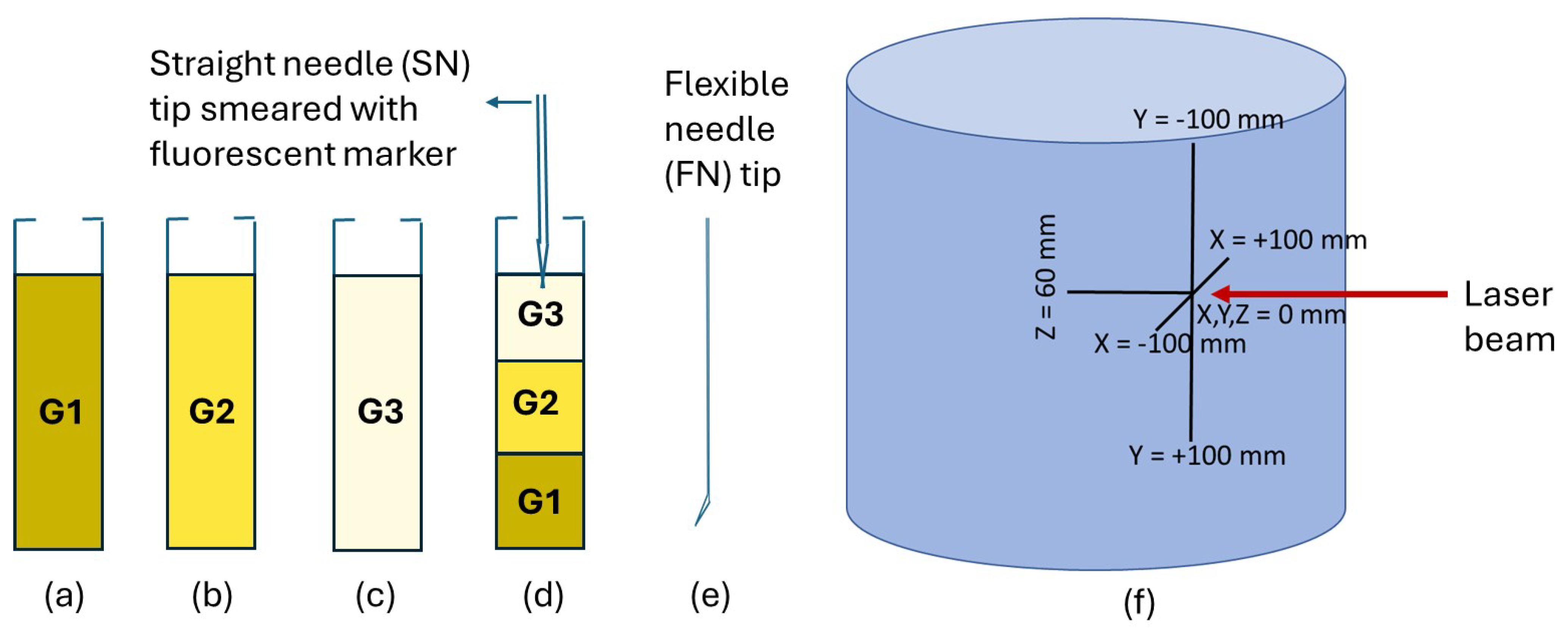
2.1. Preparation of Gelatin Tissues and Needle Setup
2.2. Photoluminescent Markers
2.3. 3D Needle Tip Tracking Using Luminescence
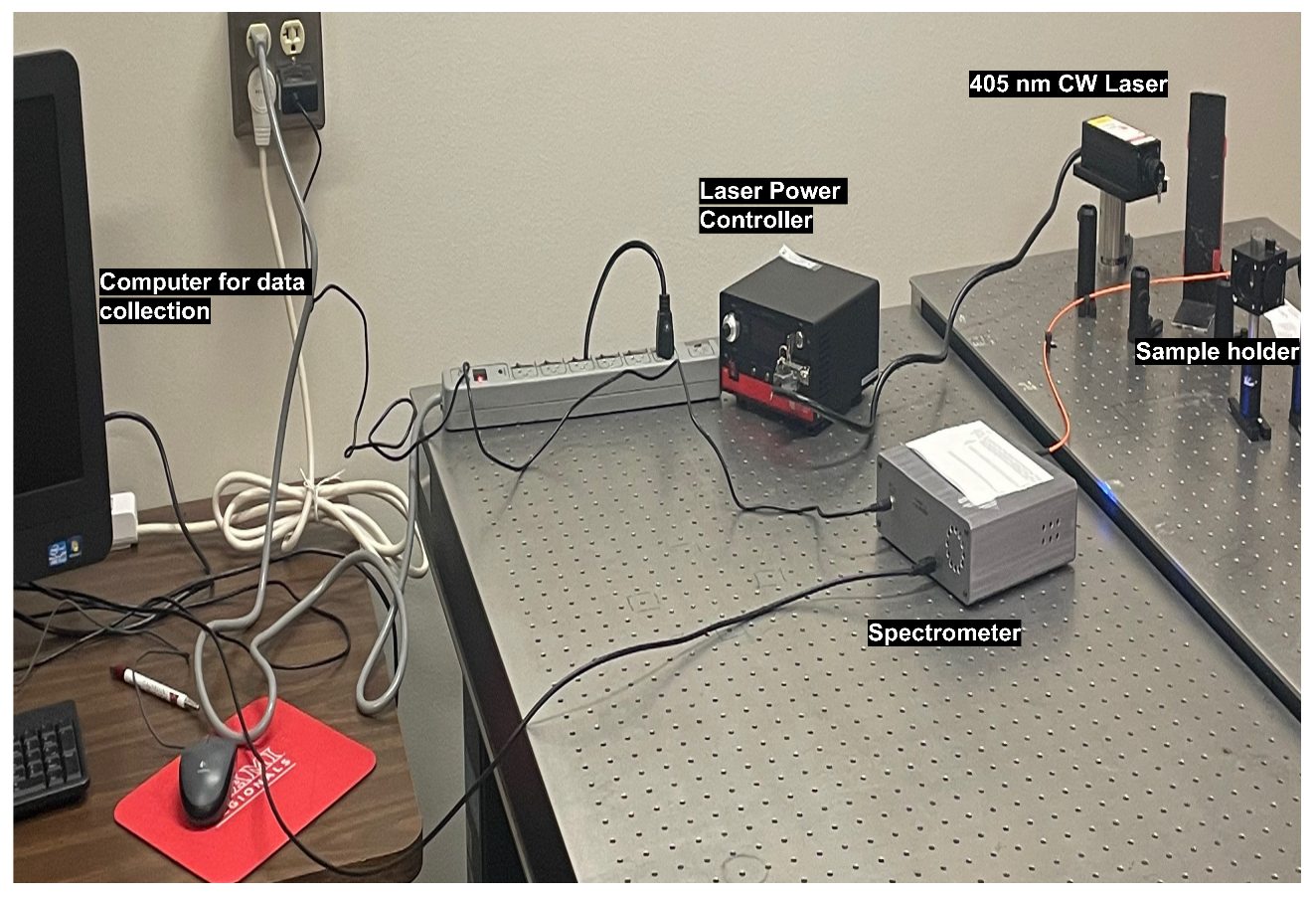
2.4. Experimental Methods for Spectroscopy
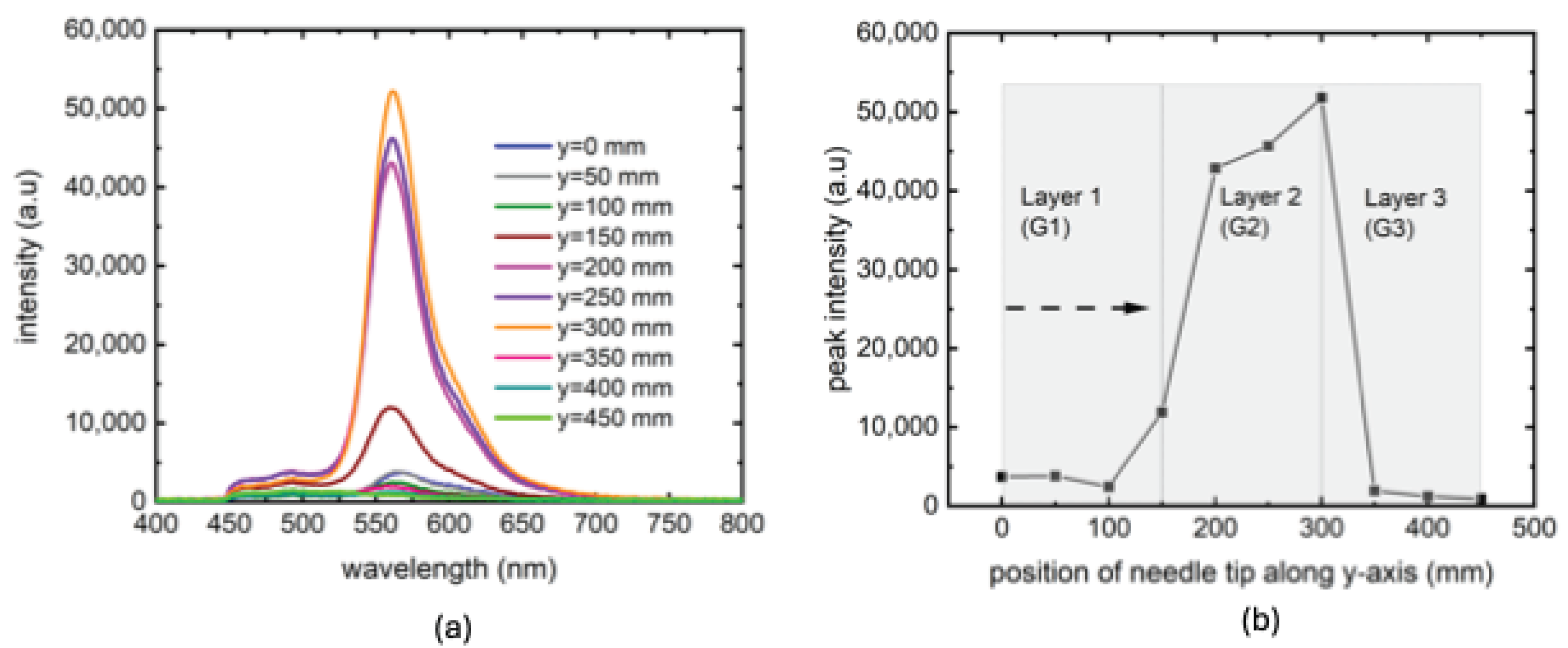
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Baere, T.; Roux, C.; Noel, G.; Delpla, A.; Deschamps, F.; Varin, E.; Tselikas, L. Robotic assistance for percutaneous needle insertion in the kidney: Preclinical proof on a swine animal model. Eur. Radiol. Exp. 2022, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Narayan, M.; Fey, A.M.; Choti, M.A. Data-driven detection of adverse events in robotic needle steering. In Proceedings of the 2018 International Symposium on Medical Robotics (ISMR), Atlanta, GA, USA, 1–3 March 2018; pp. 1–6. [Google Scholar]
- Kuo, C.H.; Dai, J.S. Robotics for minimally invasive surgery: A historical review from the perspective of kinematics. In Proceedings of the International Symposium on History of Machines and Mechanisms, HMM, Tainan, Taiwan, 11–14 November 2008; Springer: Berlin/Heidelberg, Germany, 2009; pp. 337–354. [Google Scholar]
- Issatayeva, A.; Amantayeva, A.; Blanc, W.; Tosi, D.; Molardi, C. Design and analysis of a fiber-optic sensing system for shape reconstruction of a minimally invasive surgical needle. Sci. Rep. 2021, 11, 8609. [Google Scholar] [CrossRef]
- Rossa, C.; Tavakoli, M. Issues in closed-loop needle steering. Control Eng. Pract. 2017, 62, 55–69. [Google Scholar] [CrossRef]
- De Jong, T.L.; Van De Berg, N.J.; Tas, L.; Moelker, A.; Dankelman, J.; Van Den Dobbelsteen, J.J. Needle placement errors: Do we need steerable needles in interventional radiology? In Medical Devices: Evidence and Research; Taylor and Francis: Abingdon-on-Thames, UK, 2018; pp. 259–265. [Google Scholar]
- Shahriari, N.; Heerink, W.; van Katwijk, T.; Hekman, E.; Oudkerk, M.; Misra, S. Computed tomography (CT)–compatible remote center of Motion Needle Steering Robot: Fusing CT Images and Electromagnetic Sensor Data. Med. Eng. Phys. 2017, 45, 71–77. [Google Scholar]
- Gao, S.; Wang, Y.; Ma, X.; Zhou, H.; Jiang, Y.; Yang, K.; Lu, L.; Wang, S.; Nephew, B.C.; Fichera, L.; et al. Intraoperative laparoscopic photoacoustic image guidance system in the da Vinci surgical system. Biomed. Opt. Express 2023, 14, 4914–4928. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, J.; Xia, J.; Yao, J.; Shi, J.; Li, C. Photoacoustic imaging with limited sampling: A review of machine learning approaches. Biomed. Opt. Express 2023, 14, 1777–1799. [Google Scholar] [CrossRef]
- Abolhassani, N.; Patel, R.; Moallem, M. Control of soft tissue deformation during robotic needle insertion. Minim. Invasive Ther. Allied Technol. 2006, 15, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Jiang, S.; Liang, D.; Yang, Z.; Yu, Y.; Wang, W. Modeling of path planning and needle steering with path tracking in anatomical soft tissues for minimally invasive surgery. Med. Eng. Phys. 2017, 41, 35–45. [Google Scholar] [CrossRef]
- Hadjerci, O.; Hafiane, A.; Morette, N.; Novales, C.; Vieyres, P.; Delbos, A. Assistive system based on nerve detection and needle navigation in ultrasound images for regional anesthesia. Expert Syst. Appl. 2016, 61, 64–77. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Suzuki, M.; Kato, A.; Hatano, M.; Konishi, K.; Hashizume, M.; Fujie, M.G. Enhanced targeting in breast tissue using a robotic tissue preloading-based needle insertion system. IEEE Trans. Robot. 2012, 28, 710–722. [Google Scholar] [CrossRef]
- Brown, P.J.; Tan, H.; Stitzel, J.D. Displacement control device for dynamic tissue deformation in MRI-biomed 2010. Biomed. Sci. Instrum. 2010, 46, 99–104. [Google Scholar]
- Vrooijink, G.; Abayazid, M. Needle path planning and steering in a three-dimensional non-static environment using two-dimensional ultrasound images. Int. J. Robot. Res. 2014, 33, 1361–1374. [Google Scholar] [CrossRef]
- Barnoy, Y.; Erin, O.; Raval, S.; Pryor, W.; Mair, L.O.; Weinberg, I.N.; Diaz-Mercado, Y.; Krieger, A.; Hager, G.D. Control of Magnetic Surgical Robots With Model-Based Simulators and Reinforcement Learning. IEEE Trans. Med. Robot. Bionics 2022, 4, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Scali, M.; Pusch, T.; Breedveld, P.; Dodou, D. Ovipositor-inspired steerable needle: Design and preliminary experimental evaluation. Bioinspir. Biomimetics 2017, 13, 016006. [Google Scholar] [CrossRef] [PubMed]
- Li, A.D.; Putra, K.B.; Chen, L.; Montgomery, J.S.; Shih, A. Mosquito proboscis-inspired needle insertion to reduce tissue deformation and organ displacement. Sci. Rep. 2020, 10, 12248. [Google Scholar] [CrossRef]
- Matheson, E.; Rodriguez y Baena, F. Biologically Inspired Surgical Needle Steering: Technology and Application of the Programmable Bevel-Tip Needle. Biomimetics 2020, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Sugiarto, I.; Putri, K. Analysis of dual peak emission from Rhodamine 6G organic dyes using photoluminescence. Proc. J. Phys. Conf. Ser. 2017, 817, 012047. [Google Scholar] [CrossRef]
- Madsen, J.; Warren, N.J.; Armes, S.P.; Lewis, A.L. Synthesis of rhodamine 6G-based compounds for the ATRP synthesis of fluorescently labeled biocompatible polymers. Biomacromolecules 2011, 12, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.G.; Barth, C.W.; Kitts, C.H.; Mebrat, M.D.; Montaño, A.R.; House, B.J.; McCoy, M.E.; Antaris, A.L.; Galvis, S.; McDowall, I.; et al. Near-infrared nerve-binding fluorophores for buried nerve tissue imaging. Sci. Transl. Med. 2020, 12, eaay0712. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, M.; Ullrich, B.; Murchland, M.; Zhou, X.; Ramkumar, C. Substrate and Excitation Intensity Dependence of Saturable Absorption in Perovskite Quantum Dot Films. Nanomaterials 2023, 13, 871. [Google Scholar] [CrossRef]
- Huang, C.Y.; Li, H.; Wu, Y.; Lin, C.H.; Guan, X.; Hu, L.; Kim, J.; Zhu, X.; Zeng, H.; Wu, T. Inorganic halide perovskite quantum dots: A versatile nanomaterial platform for electronic applications. Nano Micro Lett. 2023, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Niu, H.; Li, R.; Yang, Z.; Wang, J.; Li, X.; Pan, P.; Liu, J.; Zhou, B. One-pot hydrothermal synthesis of fluorescent carbon quantum dots with tunable emission color for application in electroluminescence detection of dopamine. Biosens. Bioelectron. 2022, 10, 100141. [Google Scholar] [CrossRef]
- Cao, M.; Zhao, X.; Gong, X. Ionic liquid-assisted fast synthesis of carbon dots with strong fluorescence and their tunable multicolor emission. Small 2022, 18, 2106683. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lu, S. The light of carbon dots: From mechanism to applications. Matter 2022, 5, 110–149. [Google Scholar] [CrossRef]
- Hui, X.; Rajendran, P.; Ling, T.; Dai, X.; Xing, L.; Pramanik, M. Ultrasound-guided needle tracking with deep learning: A novel approach with photoacoustic ground truth. Photoacoustics 2023, 34, 100575. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayan, M.; Bhowmick, M. Needle Tip Tracking through Photoluminescence for Minimally Invasive Surgery. Biosensors 2024, 14, 470. https://doi.org/10.3390/bios14100470
Narayan M, Bhowmick M. Needle Tip Tracking through Photoluminescence for Minimally Invasive Surgery. Biosensors. 2024; 14(10):470. https://doi.org/10.3390/bios14100470
Chicago/Turabian StyleNarayan, Meenakshi, and Mithun Bhowmick. 2024. "Needle Tip Tracking through Photoluminescence for Minimally Invasive Surgery" Biosensors 14, no. 10: 470. https://doi.org/10.3390/bios14100470





