Multi-Functional Nano-Doped Hollow Fiber from Microfluidics for Sensors and Micromotors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
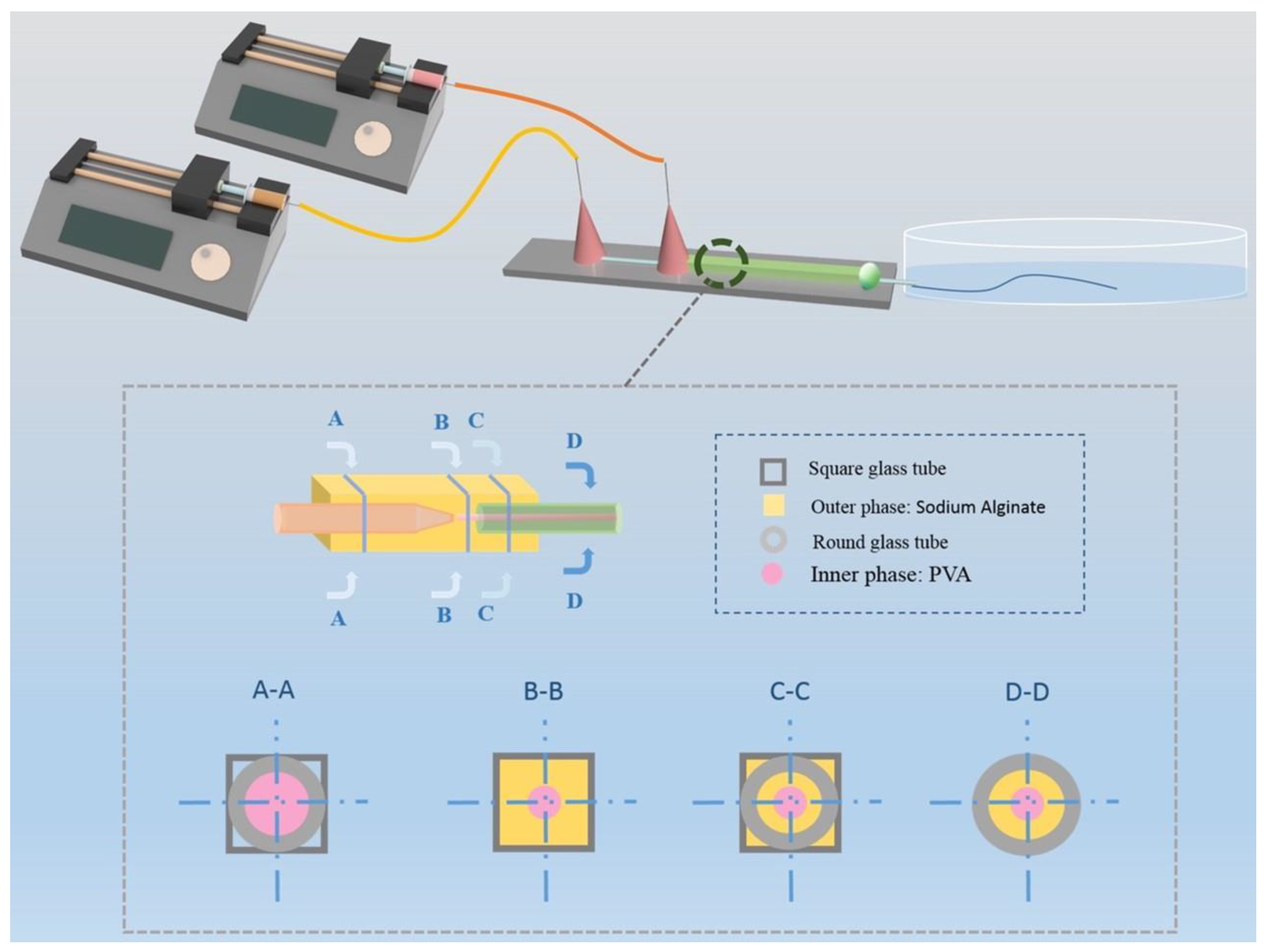
2.2.1. Design of the Microfluidic Chip
2.2.2. Fabrication of Hollow Fiber
2.2.3. Characterization of Hollow Fiber
2.2.4. Mechanical Properties of Hollow Fiber
2.2.5. Measurement of Hollow Fiber Diameter
2.2.6. Performance Testing of Wearable Fiber Sensors
2.2.7. Motion Testing of Micromotors
3. Results and Discussion
3.1. Fabrication and Morphology Characterization of Hollow Fibers
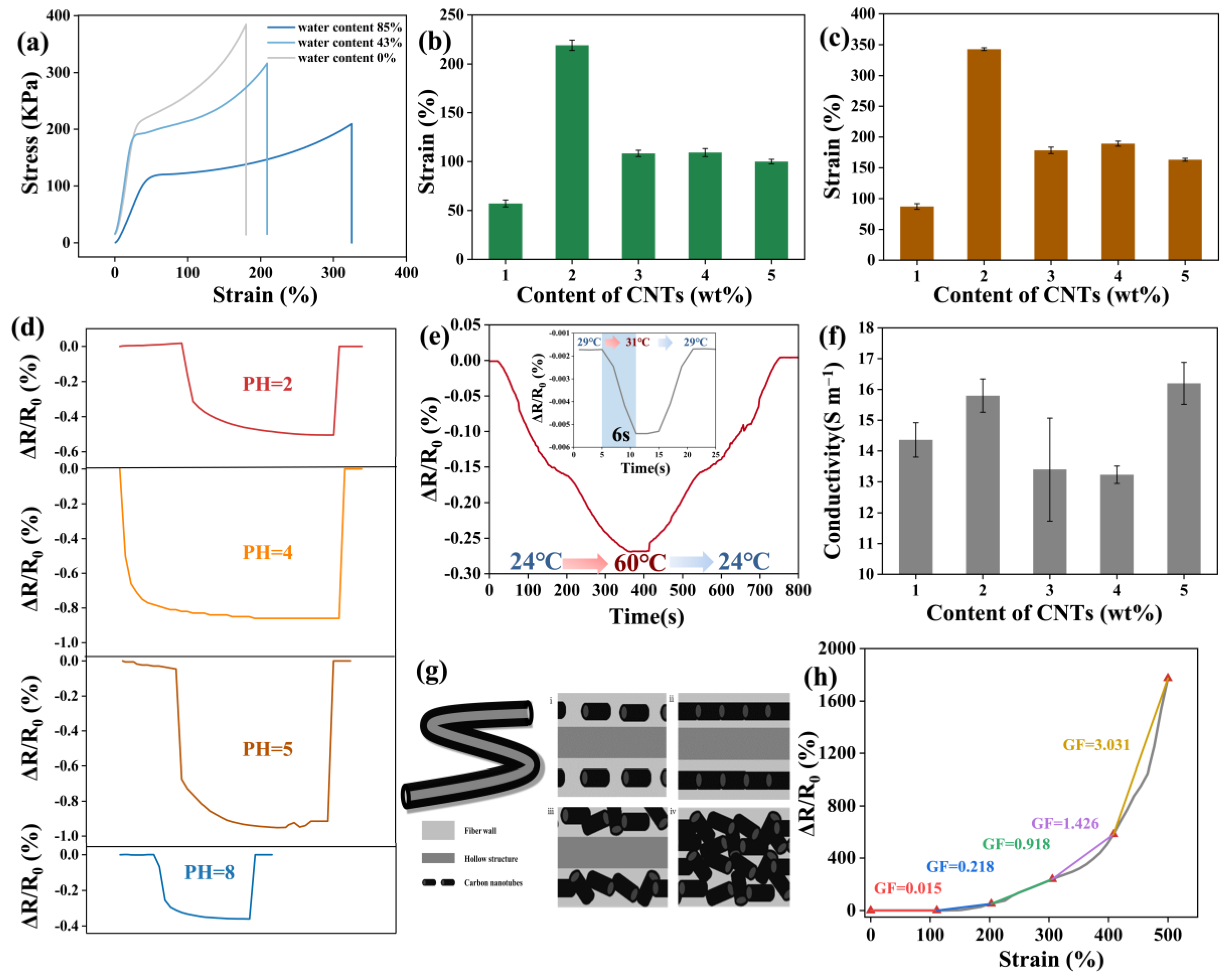
3.2. Mechanical Properties of Hollow Fiber
3.3. Wearable Fiber Sensor
3.3.1. Stability Test of HollowFiber-CNTs
3.3.2. Electrical Conductivity of HollowFiber-CNTs
3.3.3. Wearable Hollow Fiber Sensors for Detecting Human Motion
3.4. Micromotors
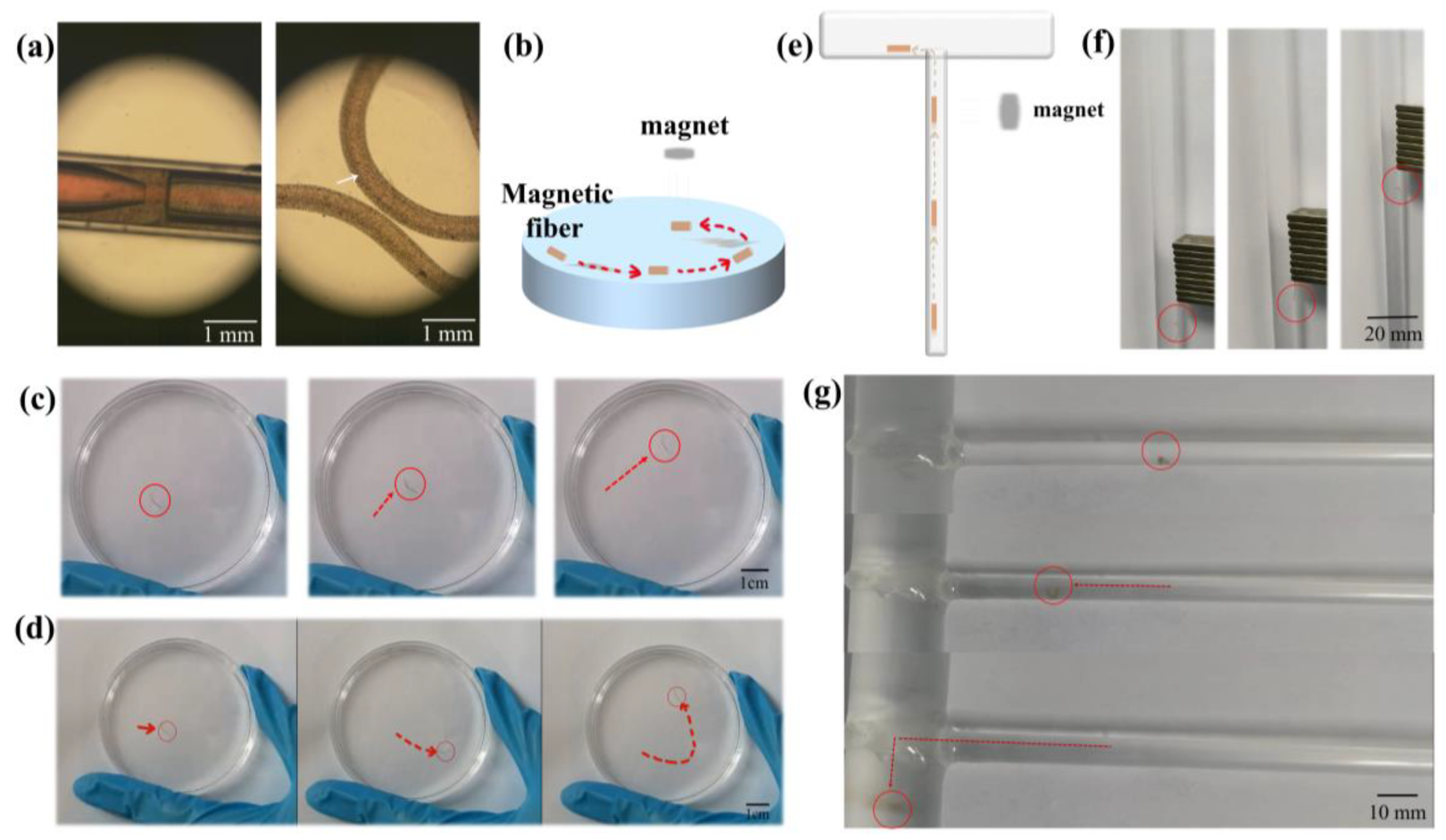
3.4.1. Magnetic-Driven Micromotors
3.4.2. Bubble-Propelled Micromotors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Talebian, S.; Shim, I.K.; Kim, S.C.; Spinks, G.M.; Vine, K.L.; Foroughi, J. Coaxial mussel-inspired biofibers: Making of a robust and efficacious depot for cancer drug delivery. J. Mater. Chem. B 2020, 8, 5064–5079. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.O.; Antunes, J.C.; Felgueiras, H.P. Recent advances in fiber–hydrogel composites for wound healing and drug delivery systems. Antibiotics 2021, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Mirani, B.; Pagan, E.; Shojaei, S.; Dabiri, S.M.H.; Savoji, H.; Mehrali, M.; Sam, M.; Alsaif, J.; Bhiladvala, R.B.; Dolatshahi-Pirouz, A. Facile method for fabrication of meter-long multifunctional hydrogel fibers with controllable biophysical and biochemical features. ACS Appl. Mater. Interfaces 2020, 12, 9080–9089. [Google Scholar] [CrossRef] [PubMed]
- Mazari-Arrighi, E.; Okitsu, T.; Teramae, H.; Aoyagi, H.; Kiyosawa, M.; Yano, M.; Chatelain, F.; Fuchs, A.; Takeuchi, S. In vitro proliferation and long-term preservation of functional primary rat hepatocytes in cell fibers. Sci. Rep. 2022, 12, 8813. [Google Scholar] [CrossRef] [PubMed]
- Utagawa, Y.; Ino, K.; Kumagai, T.; Hiramoto, K.; Takinoue, M.; Nashimoto, Y.; Shiku, H. Electrochemical glue for binding chitosan–alginate hydrogel fibers for cell culture. Micromachines 2022, 13, 420. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, Y.; Zhou, A.; Zhang, Y.; Xu, Y.; Ning, X. Alginate functionalized biomimetic 3D scaffold improves cell culture and cryopreservation for cellular therapy. Int. J. Biol. Macromol. 2022, 211, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Fatahian, S.A.; Motavalizadehkakhky, A.; Hosseiny, M.; Nouri, S.M.M.; Zhiani, R.; Sohrabpour, M.; Khanmohammadi, M. Upregulation of biochemical and biophysical properties of cell-laden microfiber, silk-hyaluronic acid composite. Int. J. Biol. Macromol. 2022, 211, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Merenda, A.; Riyadh, A.-A.; Dumée, L.F.; Zhang, X.; Thang, S.H.; Pham, H.; Kong, L. Towards next generation high throughput ion exchange membranes for downstream bioprocessing: A review. J. Membr. Sci. 2022, 647, 120325. [Google Scholar] [CrossRef]
- Li, H.-N.; Yang, J.; Xu, Z.-K. Hollow fiber membranes with Janus surfaces for continuous deemulsification and separation of oil-in-water emulsions. J. Membr. Sci. 2020, 602, 117964. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.-X.; Wang, M.; Xu, Z.-L.; Wei, Y.-M.; Shen, B.-J.; Zhu, K.-K. High-flux NaA zeolite pervaporation membranes dynamically synthesized on the alumina hollow fiber inner-surface in a continuous flow system. J. Membr. Sci. 2019, 570, 445–454. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.; Li, X.; Dong, J.; Zhao, X.; Zhang, Q. Wearable and robust polyimide hydrogel fiber textiles for strain sensors. ACS Appl. Mater. Interfaces 2021, 13, 43323–43332. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zou, Y.; Ji, Y.; Zhong, K.; Du, X.; Du, Z.; Cheng, X.; Wang, S. Tough and extremely temperature-tolerance nanocomposite organohydrogels as ultrasensitive wearable sensors for wireless human motion monitoring. Compos. Part A Appl. Sci. Manuf. 2022, 157, 106905. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, Y.; Zhu, J.; Le, K.; Servati, P.; Jiang, F. Tough and Ultrastretchable Liquid-Free Ion Conductor Strengthened by Deep Eutectic Solvent Hydrolyzed Cellulose Microfibers. Adv. Funct. Mater. 2022, 32, 2202533. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, W.; Zhao, Q.; Zhang, L. Macromolecular Phase-Transition-Driven Motility of Hollow Hydrogel Tubes. ACS Appl. Polym. Mater. 2020, 2, 3054–3059. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.; Chen, B.; Luo, Y. Magnetically-driven drug and cell on demand release system using 3D printed alginate based hollow fiber scaffolds. Int. J. Biol. Macromol. 2021, 168, 38–45. [Google Scholar] [CrossRef]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-based nanomaterials: Multifunctional materials for biomedical engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite hydrogels: 3D polymer–nanoparticle synergies for on-demand drug delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef]
- Yi, F.-L.; Meng, F.-C.; Li, Y.-Q.; Huang, P.; Hu, N.; Liao, K.; Fu, S.-Y. Highly stretchable CNT Fiber/PAAm hydrogel composite simultaneously serving as strain sensor and supercapacitor. Compos. Part B Eng. 2020, 198, 108246. [Google Scholar] [CrossRef]
- Roy, S.; David-Pur, M.; Hanein, Y. Carbon nanotube-based ion selective sensors for wearable applications. ACS Appl. Mater. Interfaces 2017, 9, 35169–35177. [Google Scholar] [CrossRef]
- Lu, Y.; Yue, Y.; Ding, Q.; Mei, C.; Xu, X.; Wu, Q.; Xiao, H.; Han, J. Self-recovery, fatigue-resistant, and multifunctional sensor assembled by a nanocellulose/carbon nanotube nanocomplex-mediated hydrogel. ACS Appl. Mater. Interfaces 2021, 13, 50281–50297. [Google Scholar] [CrossRef]
- Lv, J.; Kong, C.; Yang, C.; Yin, L.; Jeerapan, I.; Pu, F.; Zhang, X.; Yang, S.; Yang, Z. Wearable, stable, highly sensitive hydrogel–graphene strain sensors. Beilstein J. Nanotechnol. 2019, 10, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, Q.; Huang, Y.; Wang, D.; Li, L.; Liu, Z. In situ laser synthesis of Pt nanoparticles embedded in graphene films for wearable strain sensors with ultra-high sensitivity and stability. Carbon 2022, 190, 245–254. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Cao, T.; Wang, T.; Sun, L.; Wang, K.; Fan, X. Antiliquid-interfering, antibacteria, and adhesive wearable strain sensor based on superhydrophobic and conductive composite hydrogel. ACS Appl. Mater. Interfaces 2021, 13, 46022–46032. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Q.; Chen, F.; Zhang, Q.; Wang, K.; Fu, Q. Realizing the full nanofiller enhancement in melt-spun fibers of poly (vinylidene fluoride)/carbon nanotube composites. Nanotechnology 2011, 22, 355707. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Pei, Y.; Xie, M.; Jin, Z.-H.; Xiao, Y.-S.; Wang, Y.; Zhang, L.-N.; Li, Y.; Huang, W.-H. An artificial blood vessel implanted three-dimensional microsystem for modeling transvascular migration of tumor cells. Lab Chip 2015, 15, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.S.; Bakht, S.M.; Aleman, J.; Shin, S.R.; Yue, K.; Sica, M.; Ribas, J.; Duchamp, M.; Ju, J. Elastomeric free-form blood vessels for interconnecting organs on chip systems. Lab Chip 2016, 16, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Wang, P.; Su, Z.; Ma, G.; Zhang, S. Enabling multi-enzyme biocatalysis using coaxial-electrospun hollow nanofibers: Redesign of artificial cells. J. Mater. Chem. B 2014, 2, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Song, W.-L.; Fan, L.-Z. Hollow core–shell SnO2/C fibers as highly stable anodes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 21472–21478. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, A.; Rahimi, K.; Saghandali, F.; Scott, J.; Lovell, E. Nanofluid preparation, stability and performance for CO2 absorption and desorption enhancement: A review. J. Environ. Manag. 2022, 313, 114955. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, J.; Zhang, H.; Wang, X.; Yang, C.; Zhao, Y. Shear-flow-induced graphene coating microfibers from microfluidic spinning. Innovation 2022, 3, 100209. [Google Scholar] [CrossRef]
- Hu, S.; Han, J.; Shi, Z.; Chen, K.; Xu, N.; Wang, Y.; Zheng, R.; Tao, Y.; Sun, Q.; Wang, Z.L. Biodegradable, super-strong, and conductive cellulose macrofibers for fabric-based triboelectric nanogenerator. Nano-Micro Lett. 2022, 14, 115. [Google Scholar] [CrossRef]
- Jiang, M.-Y.; Ju, X.-J.; Deng, K.; Fan, X.-X.; He, X.-H.; Wu, F.; He, F.; Liu, Z.; Wang, W.; Xie, R. The microfluidic synthesis of composite hollow microfibers for k+-responsive controlled release based on a host–guest system. J. Mater. Chem. B 2016, 4, 3925–3935. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jo, A.; Imani, K.B.C.; Kim, D.; Chung, J.-W.; Yoon, J. Microfluidic fabrication of multistimuli-responsive tubular hydrogels for cellular scaffolds. Langmuir 2018, 34, 4351–4359. [Google Scholar] [CrossRef] [PubMed]
- Dabaghi, M.; Saraei, N.; Fusch, G.; Rochow, N.; Brash, J.L.; Fusch, C.; Selvaganapathy, P.R. Microfluidic blood oxygenators with integrated hollow chambers for enhanced air exchange from all four sides. J. Membr. Sci. 2020, 596, 117741. [Google Scholar] [CrossRef]
- Wei, L.; Deng, N.; Wang, X.; Zhao, H.; Yan, J.; Yang, Q.; Kang, W.; Cheng, B. Flexible ordered MnS@ CNC/carbon nanofibers membrane based on microfluidic spinning technique as interlayer for stable lithium-metal battery. J. Membr. Sci. 2021, 637, 119615. [Google Scholar] [CrossRef]
- Bacchin, P.; Snisarenko, D.; Stamatialis, D.; Aimar, P.; Causserand, C. Combining fluorescence and permeability measurements in a membrane microfluidic device to study protein sorption mechanisms. J. Membr. Sci. 2020, 614, 118485. [Google Scholar] [CrossRef]
- Vagner, S.; Patlazhan, S.; Serra, C.; Funfschilling, D.; Kulichikhin, V. Dripping and jetting of semi-dilute polymer solutions co-flowing in co-axial capillaries. Phys. Fluids 2021, 33, 062002. [Google Scholar] [CrossRef]
- Junzong, Z.; Haiying, Q.; Jinsheng, W. Nanoparticle dispersion and coagulation in a turbulent round jet. Int. J. Multiph. Flow 2013, 54, 22–30. [Google Scholar] [CrossRef]
- Meng, Z.-J.; Wang, W.; Xie, R.; Ju, X.-J.; Liu, Z.; Chu, L.-Y. Microfluidic generation of hollow Ca-alginate microfibers. Lab Chip 2016, 16, 2673–2681. [Google Scholar] [CrossRef]
- Daya, R.; Xu, C.; Nguyen, N.-Y.T.; Liu, H.H. Angiogenic hyaluronic acid hydrogels with curcumin-coated magnetic nanoparticles for tissue repair. ACS Appl. Mater. Interfaces 2022, 14, 11051–11067. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Namvar, F.; Nadi, B.; Rahman, M.Z.A.; Amin, J. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Sauret, A.; Cheung Shum, H. All-aqueous multiphase microfluidics. Biomicrofluidics 2013, 7, 061301. [Google Scholar] [CrossRef]
- Yi, Y.; Chiao, M.; Mahmoud, K.A.; Wu, L.; Wang, B. Preparation and characterization of PVA/PVP conductive hydrogels formed by freeze–thaw processes as a promising material for sensor applications. J. Mater. Sci. 2022, 57, 8029–8038. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Guo, J.; Liu, L.; Fu, H.; Yao, J.; Krucińska, I.; Draczynski, Z. Highly strong, tough, and stretchable conductive hydrogels based on silk sericin-mediated multiple physical interactions for flexible sensors. ACS Appl. Polym. Mater. 2021, 4, 618–626. [Google Scholar] [CrossRef]
- Shuai, L.; Guo, Z.H.; Zhang, P.; Wan, J.; Pu, X.; Wang, Z.L. Stretchable, self-healing, conductive hydrogel fibers for strain sensing and triboelectric energy-harvesting smart textiles. Nano Energy 2020, 78, 105389. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.; Lou, H.; Wan, J.; Pu, X. One-step coaxial spinning of core-sheath hydrogel fibers for stretchable ionic strain sensors. Chem. Eng. J. 2023, 458, 141393. [Google Scholar] [CrossRef]
- Fernández-Medina, M.; Ramos-Docampo, M.A.; Hovorka, O.; Salgueiriño, V.; Städler, B. Recent advances in nano-and micromotors. Adv. Funct. Mater. 2020, 30, 1908283. [Google Scholar] [CrossRef]
- Magdanz, V.; Guix, M.; Schmidt, O.G. Tubular micromotors: From microjets to spermbots. Robot. Biomim. 2014, 1, 11. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, J.; Zou, M.; Cai, L.; Zhao, Y. Micromotors from microfluidics. Chem.—Asian J. 2019, 14, 2417–2430. [Google Scholar] [CrossRef]
- Yuan, M.; Gong, M.; Huang, H.; Zhao, Y.; Ying, Y.; Wang, S. Bubble-propelled plasmon-reinforced Pt-ZnIn 2 S 4 micromotors for stirring-free photocatalytic water purification. Inorg. Chem. Front. 2022, 9, 5725–5734. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, G.; Pumera, M. Beyond platinum: Bubble-propelled micromotors based on Ag and MnO2 catalysts. J. Am. Chem. Soc. 2014, 136, 2719–2722. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, J.; Yuan, M.; Yang, H.; Zhao, Y.; Ying, Y.; Wang, S. Large-Scale Self-Assembly of MOFs Colloidosomes for Bubble-Propelled Micromotors and Stirring-Free Environmental Remediation. Angew. Chem. Int. Ed. 2022, 61, e202211163. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Z.; Sun, H.; Lyu, T.; Ma, X.; Guo, J.; Tian, Y. Multi-Functional Nano-Doped Hollow Fiber from Microfluidics for Sensors and Micromotors. Biosensors 2024, 14, 186. https://doi.org/10.3390/bios14040186
Wang Y, Wang Z, Sun H, Lyu T, Ma X, Guo J, Tian Y. Multi-Functional Nano-Doped Hollow Fiber from Microfluidics for Sensors and Micromotors. Biosensors. 2024; 14(4):186. https://doi.org/10.3390/bios14040186
Chicago/Turabian StyleWang, Yanpeng, Zhaoyang Wang, Haotian Sun, Tong Lyu, Xing Ma, Jinhong Guo, and Ye Tian. 2024. "Multi-Functional Nano-Doped Hollow Fiber from Microfluidics for Sensors and Micromotors" Biosensors 14, no. 4: 186. https://doi.org/10.3390/bios14040186
APA StyleWang, Y., Wang, Z., Sun, H., Lyu, T., Ma, X., Guo, J., & Tian, Y. (2024). Multi-Functional Nano-Doped Hollow Fiber from Microfluidics for Sensors and Micromotors. Biosensors, 14(4), 186. https://doi.org/10.3390/bios14040186





