Beta-Barrel Nanopores as Diagnostic Sensors: An Engineering Perspective
Abstract
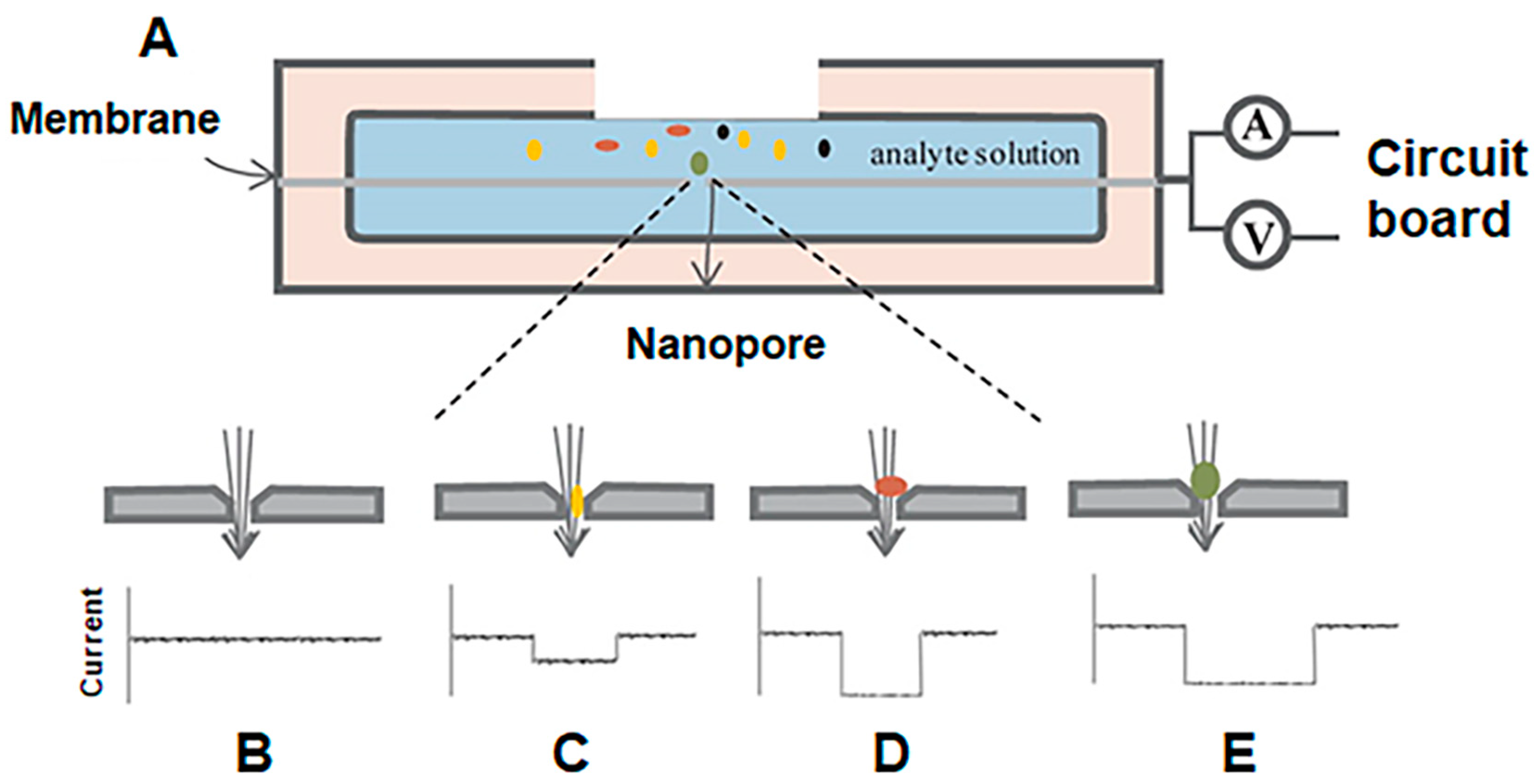
:1. Introduction
2. Modifying the Structure and Charge of β-Barrel Porins
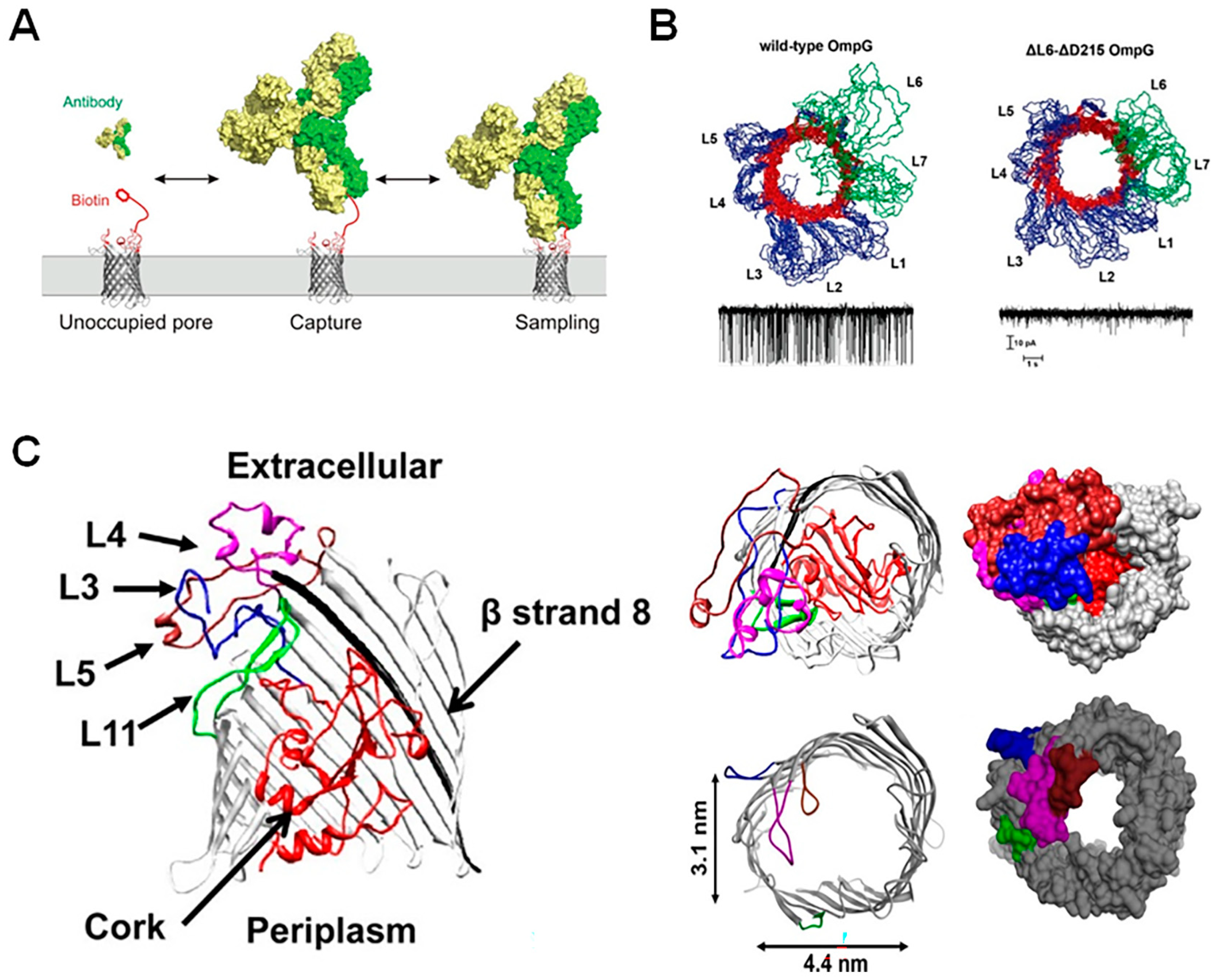
2.1. Modifying Pore Structure
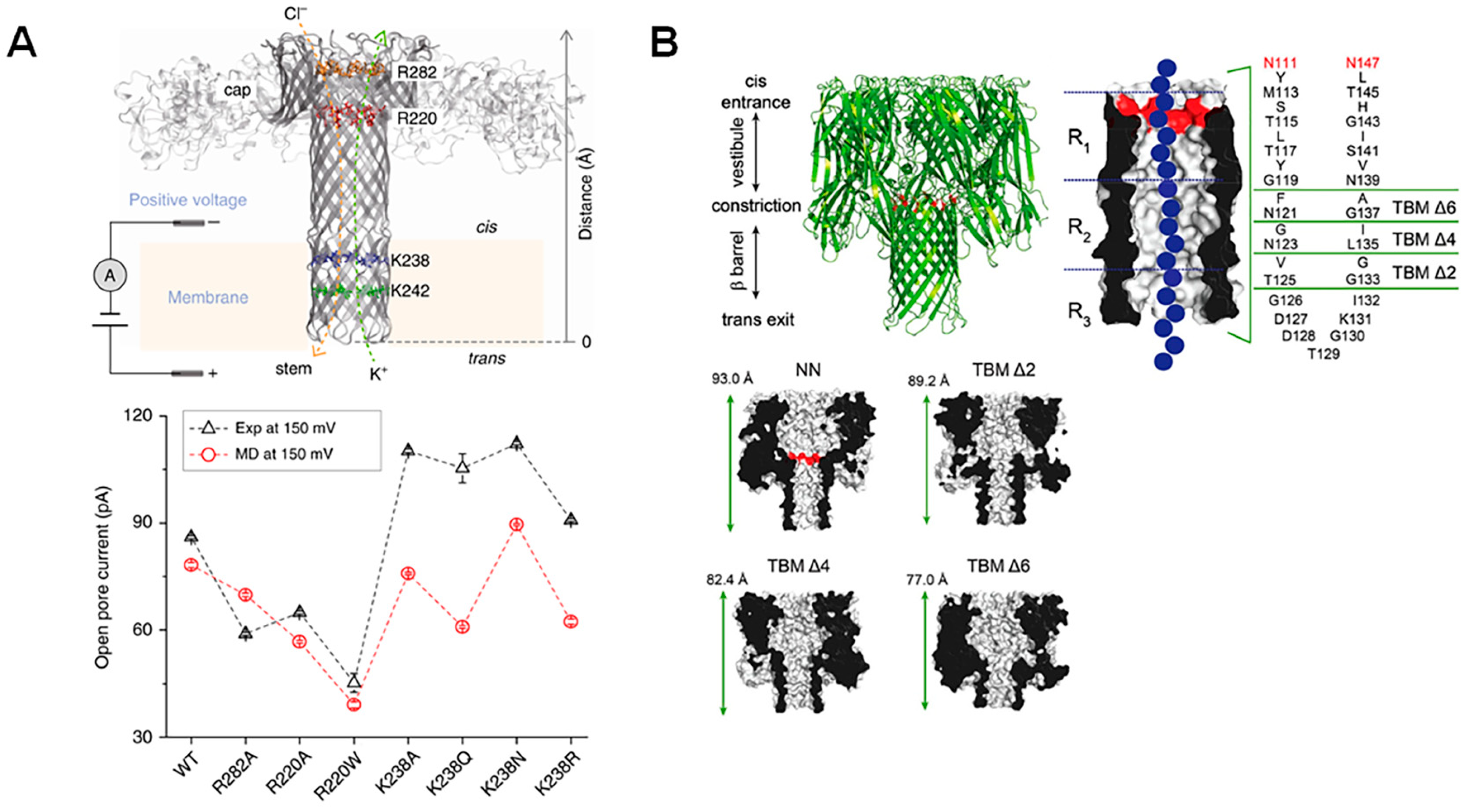
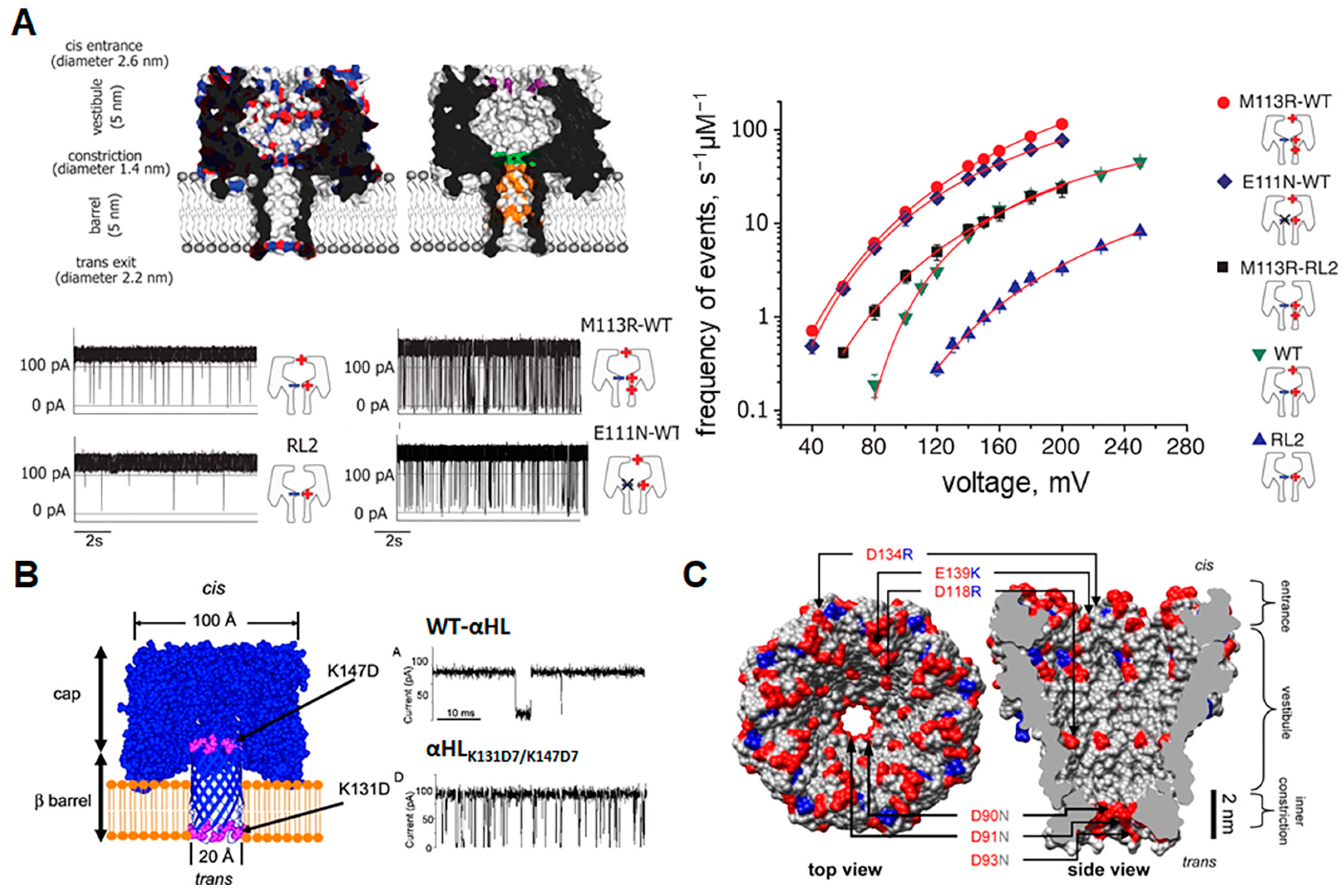
2.2. Modification of Pore Surface Charge
3. Functionalization of Nanopores with Enzymes
3.1. Choosing an Enzyme
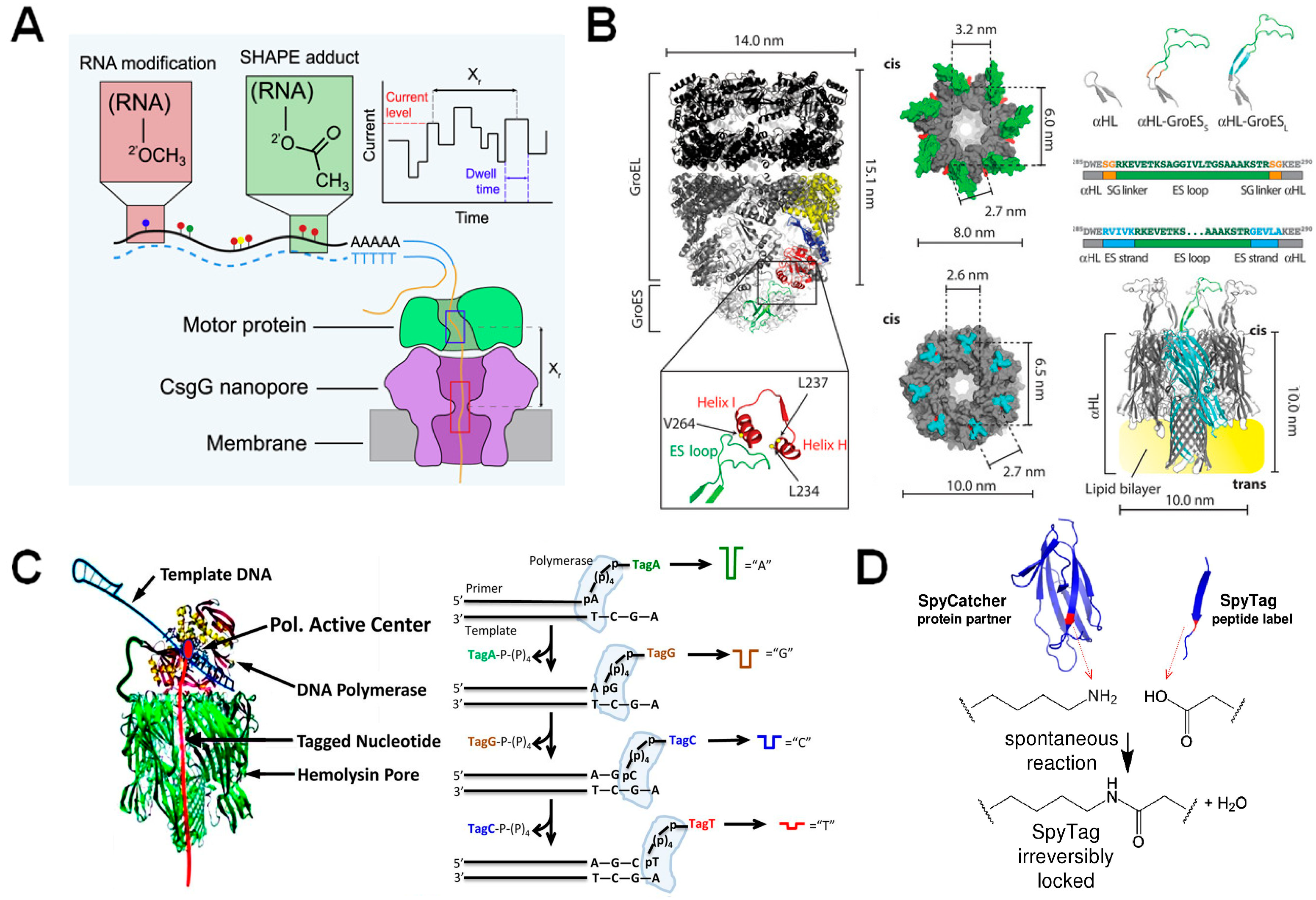
3.2. Functionalization of Protein Nanopores with Enzymes
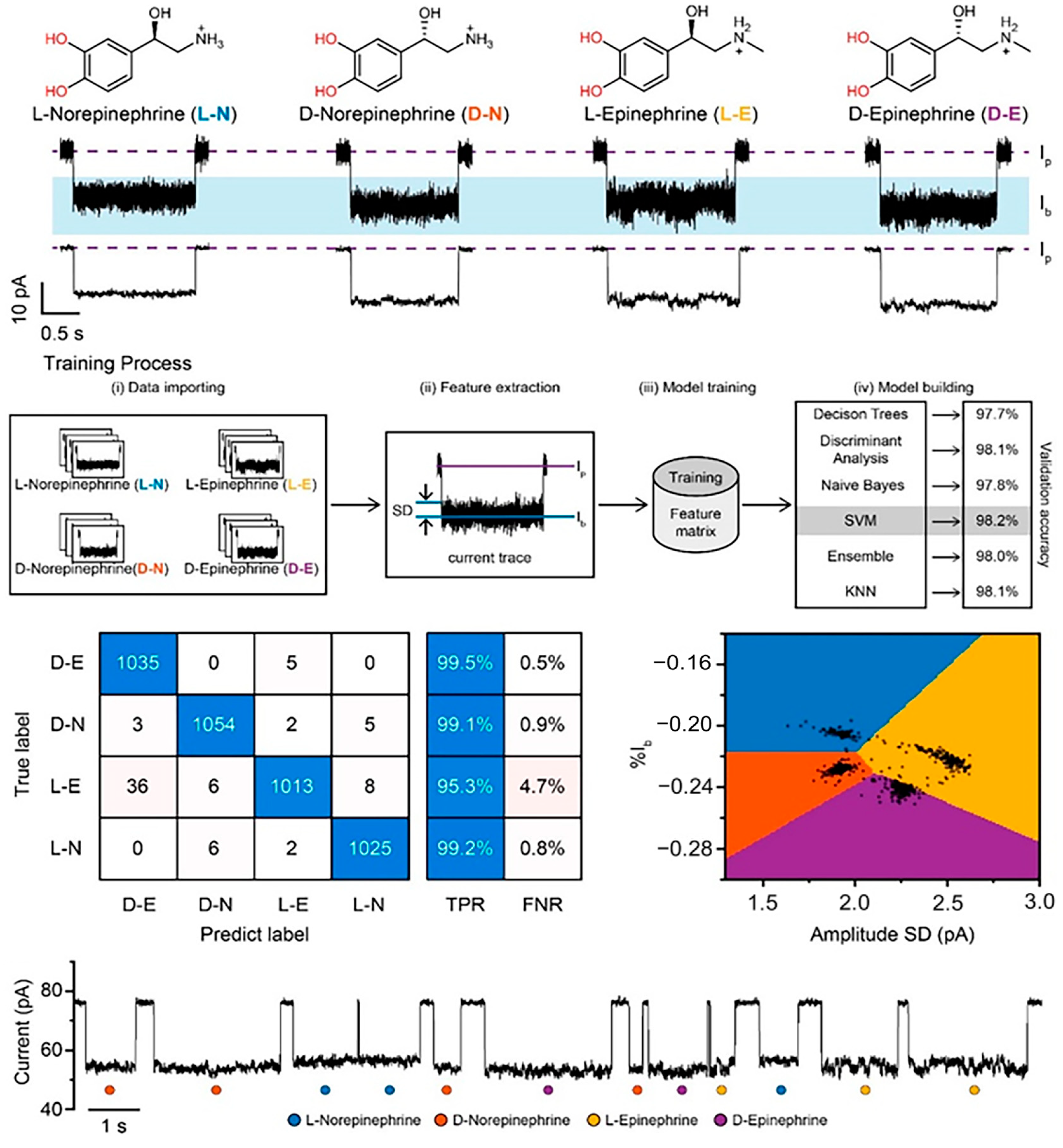
3.3. Diagnostic Applications
4. Modification of Nanopores with Aptamers
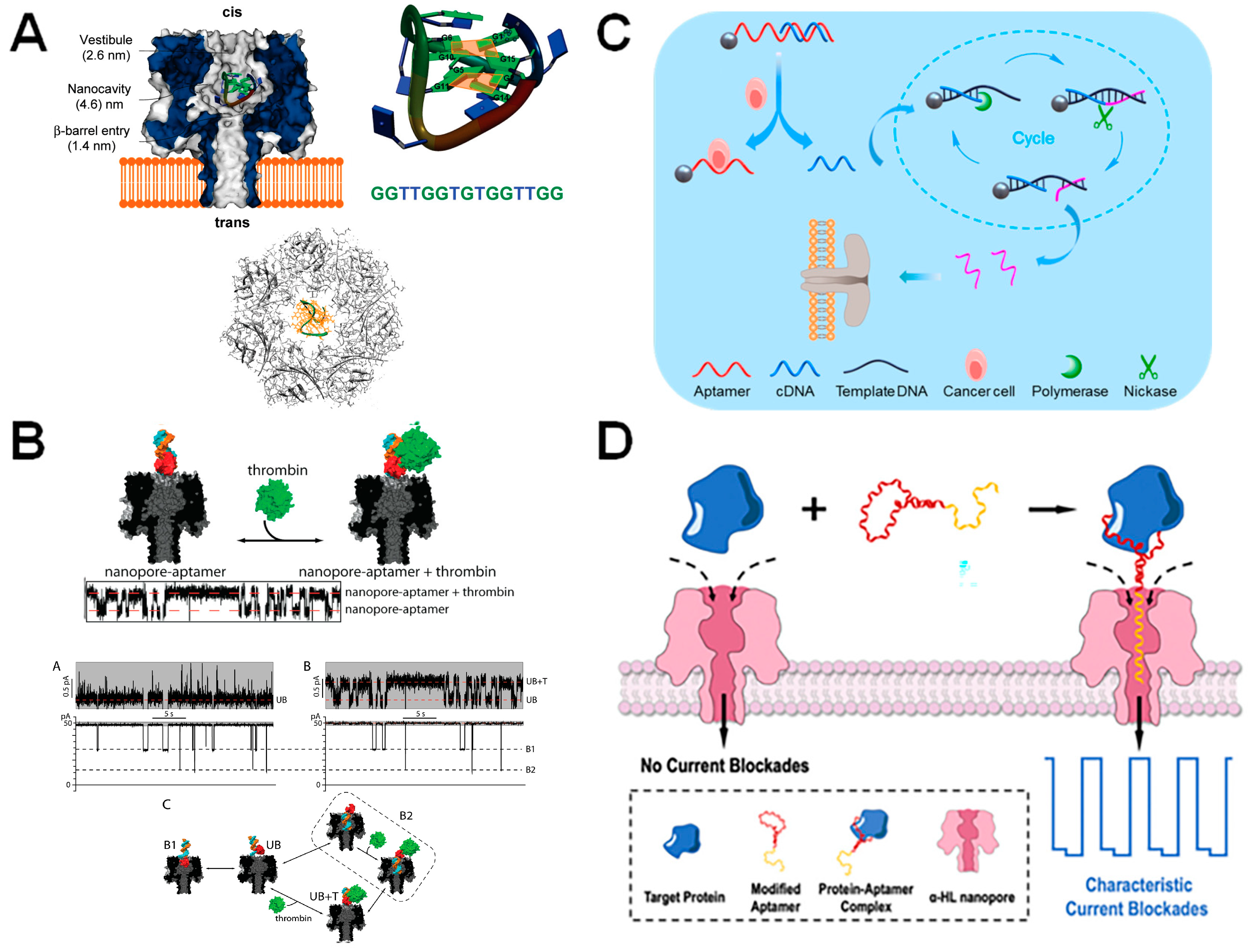
4.1. Choosing an Aptamer
4.2. Aptamer-Functionalized Nanopores
4.3. Diagnostic Applications
5. Modification of Nanopores with Protein Probes
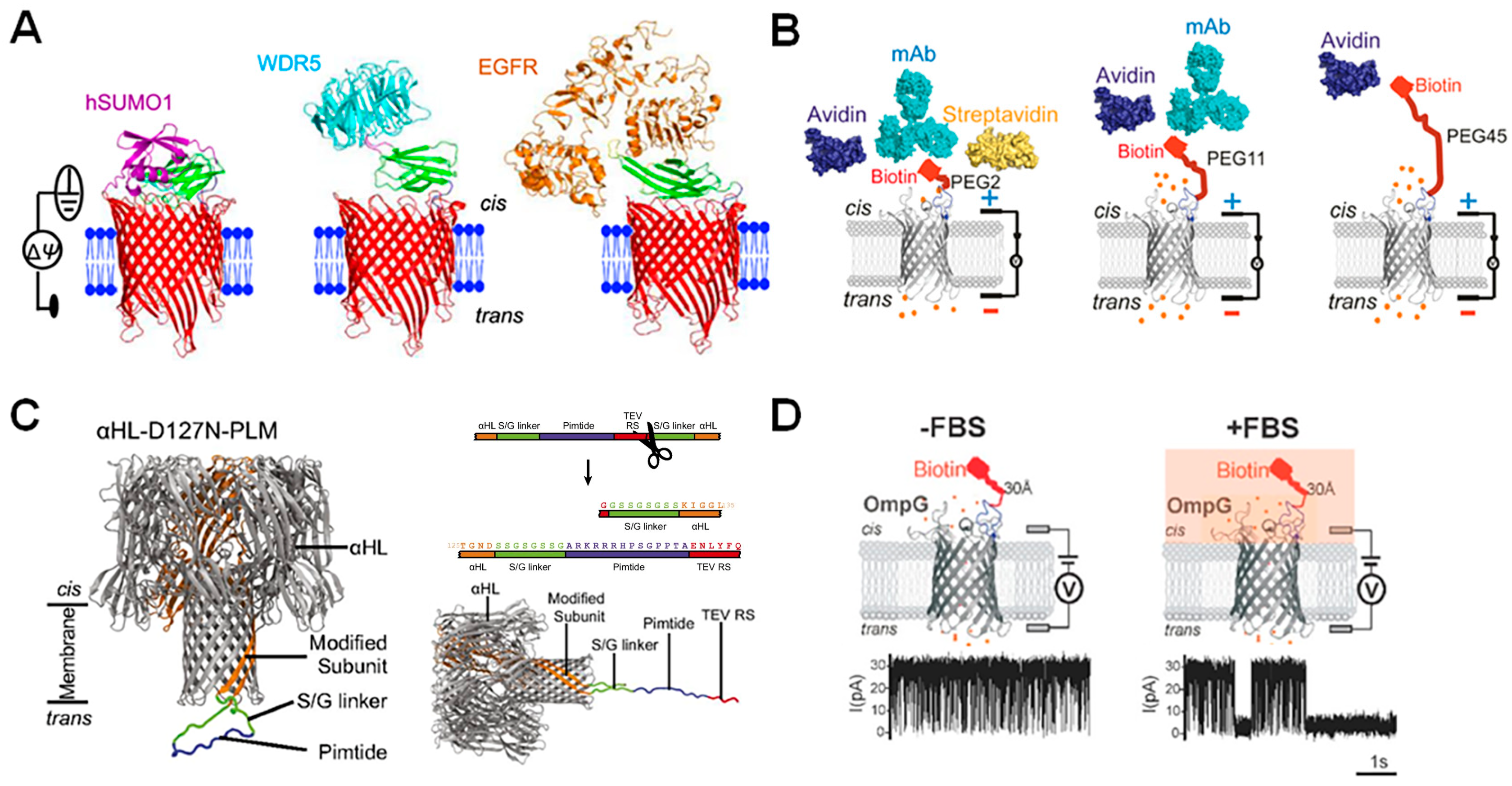
5.1. Choosing a Protein Probe
5.2. Protein-Probe Based Nanopores
5.3. Diagnostic Applications
6. Conclusions and Further Prospects in Pore Engineering for Diagnostics
Author Contributions
Funding
Conflicts of Interest
References
- Adashek, J.J.; Janku, F.; Kurzrock, R. Signed in Blood: Circulating Tumor DNA in Cancer Diagnosis, Treatment and Screening. Cancers 2021, 13, 3600. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, L.; Xie, Y.-H.; Wu, J. Advancements in detection of SARS-CoV-2 infection for confronting COVID-19 pandemics. Lab. Investig. 2022, 102, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Rentschler, S.; Kaiser, L.; Deigner, H.-P. Emerging Options for the Diagnosis of Bacterial Infections and the Characterization of Antimicrobial Resistance. Int. J. Mol. Sci. 2021, 22, 456. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, F. Application of Nanopore Sequencing in the Diagnosis and Treatment of Pulmonary Infections. Mol. Diagn. Ther. 2023, 27, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Pallerla, S.R.; Van Dong, D.; Linh, L.T.K.; Van Son, T.; Quyen, D.T.; Hoan, P.Q.; Trung, N.T.; The, N.T.; Rüter, J.; Boutin, S.; et al. Diagnosis of pathogens causing bacterial meningitis using Nanopore sequencing in a resource-limited setting. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Quick, J.; Loman, N.J.; Duraffour, S.; Simpson, J.T.; Severi, E.; Cowley, L.; Bore, J.A.; Koundouno, R.; Dudas, G.; Mikhail, A.; et al. Real-time, portable genome sequencing for Ebola surveillance. Nature 2016, 530, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, D.; Cui, W.; Zhang, H.; Pang, W.; Duan, X. Fabrications, Applications and Challenges of Solid-State Nanopores: A Mini Review. Nanomater. Nanotechnol. 2016, 6, 35. [Google Scholar] [CrossRef]
- Dekker, C. Solid-state nanopores. Nat. Nanotechnol. 2007, 2, 209–215. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tsutsui, M.; Zhou, Y.; Miao, X.-S. Solid-state nanopore systems: From materials to applications. NPG Asia Mater. 2021, 13, 48. [Google Scholar] [CrossRef]
- Xue, L.; Yamazaki, H.; Ren, R.; Wanunu, M.; Ivanov, A.P.; Edel, J.B. Solid-state nanopore sensors. Nat. Rev. Mater. 2020, 5, 931–951. [Google Scholar] [CrossRef]
- Peinetti, A.S.; Lake, R.J.; Cong, W.; Cooper, L.; Wu, Y.; Ma, Y.; Pawel, G.T.; Toimil-Molares, M.E.; Trautmann, C.; Rong, L.; et al. Direct detection of human adenovirus or SARS-CoV-2 with ability to inform infectivity using DNA aptamer-nanopore sensors. Sci. Adv. 2021, 7, eabh2848. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kondylis, P.; Haywood, D.G.; Harms, Z.D.; Lee, L.S.; Zlotnick, A.; Jacobson, S.C. Characterization of Virus Capsids and Their Assembly Intermediates by Multicycle Resistive-Pulse Sensing with Four Pores in Series. Anal. Chem. 2018, 90, 7267–7274. [Google Scholar] [CrossRef] [PubMed]
- Quick, J.; Ashton, P.; Calus, S.; Chatt, C.; Gossain, S.; Hawker, J.; Nair, S.; Neal, K.; Nye, K.; Peters, T.; et al. Rapid draft sequencing and real-time nanopore sequencing in a hospital outbreak of Salmonella. Genome Biol. 2015, 16, 114. [Google Scholar] [CrossRef] [PubMed]
- Greninger, A.L.; Naccache, S.N.; Federman, S.; Yu, G.; Mbala, P.; Bres, V.; Stryke, D.; Bouquet, J.; Somasekar, S.; Linnen, J.M.; et al. Rapid metagenomic identification of viral pathogens in clinical samples by real-time nanopore sequencing analysis. Genome Med. 2015, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Meredith, L.W.; Hamilton, W.L.; Warne, B.; Houldcroft, C.J.; Hosmillo, M.; Jahun, A.S.; Curran, M.D.; Parmar, S.; Caller, L.G.; Caddy, S.L.; et al. Rapid implementation of SARS-CoV-2 sequencing to investigate cases of health-care associated COVID-19: A prospective genomic surveillance study. Lancet Infect. Dis. 2020, 20, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tang, R.; Li, Y.; Zhang, S.; Xi, D. Label-free Sensing of Main Protease Activity of SARS-CoV-2 with an Aerolysin Nanopore. Chem.—Asian J. 2022, 17, e202200747. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cao, C.; Long, Y.-T. Selective and Sensitive Detection of Methylcytosine by Aerolysin Nanopore under Serum Condition. Anal. Chem. 2017, 89, 11685–11689. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-J.; Kim, J.-S.; Lee, J.; Min, J.S.; Jeong, K.-B.; Kim, E.; Lee, M.-K.; Chi, S.-W. Single-Molecule Sensing of an Anticancer Therapeutic Protein–Protein Interaction Using the Chemically Modified OmpG Nanopore. Anal. Chem. 2022, 94, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Fahie, M.; Chisholm, C.; Chen, M. Resolved Single-Molecule Detection of Individual Species within a Mixture of anti-Biotin Antibodies Using an Engineered Monomeric Nanopore. ACS Nano 2015, 9, 1089–1098. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, D.; Tan, Q.; Wang, M.X.; Gu, L.Q. Nanopore-based detection of circulating microRNAs in lung cancer patients. Nat. Nanotechnol. 2011, 6, 668–674. [Google Scholar] [CrossRef]
- Ahmad, M.; Ha, J.-H.; Mayse, L.A.; Presti, M.F.; Wolfe, A.J.; Moody, K.J.; Loh, S.N.; Movileanu, L. A generalizable nanopore sensor for highly specific protein detection at single-molecule precision. Nat. Commun. 2023, 14, 1374. [Google Scholar] [CrossRef] [PubMed]
- Varongchayakul, N.; Song, J.; Meller, A.; Grinstaff, M.W. Single-molecule protein sensing in a nanopore: A tutorial. Chem. Soc. Rev. 2018, 47, 8512–8524. [Google Scholar] [CrossRef]
- Fragasso, A.; Schmid, S.; Dekker, C. Comparing Current Noise in Biological and Solid-State Nanopores. ACS Nano 2020, 14, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Kasianowicz, J.J.; Brandin, E.; Branton, D.; Deamer, D.W. Deamer Characterization of individual polynucleotide molecules using a membrane channel. Proc. Natl. Acad. Sci. USA 1996, 93, 13770–13773. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, D.; Heron, A.J.; Klingelhoefer, J.; Mikhailova, E.; Maglia, G.; Bayley, H. Nucleobase Recognition in ssDNA at the Central Constriction of the α-Hemolysin Pore. Nano Lett. 2010, 10, 3633–3637. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.M.; Movileanu, L. Excursion of a single polypeptide into a protein pore: Simple physics, but complicated biology. Eur. Biophys. J. 2008, 37, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.-Q.; Braha, O.; Conlan, S.; Cheley, S.; Bayley, H. Stochastic sensing of organic analytes by a pore-forming protein containing a molecular adapter. Nature 1999, 398, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Fleming, A.M.; Burrows, C.J.; White, H.S. Unzipping Kinetics of Duplex DNA Containing Oxidized Lesions in an α-Hemolysin Nanopore. J. Am. Chem. Soc. 2012, 134, 11006–11011. [Google Scholar] [CrossRef]
- Movileanu, L.; Howorka, S.; Braha, O.; Bayley, H. Detecting protein analytes that modulate transmembrane movement of a polymer chain within a single protein pore. Nat. Biotechnol. 2000, 18, 1091–1095. [Google Scholar] [CrossRef]
- Zuo, J.; Song, N.-N.; Wang, J.; Zhao, X.; Cheng, M.-Y.; Wang, Q.; Tang, W.; Yang, Z.; Qiu, K. Review—Single-Molecule Sensors Based on Protein Nanopores. J. Electrochem. Soc. 2021, 168, 126502. [Google Scholar] [CrossRef]
- Bayley, H.; Cremer, P.S. Stochastic sensors inspired by biology. Nature 2001, 413, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, D.; Jayawardhana, D.A.; Guan, X. Stochastic sensing of biomolecules in a nanopore sensor array. Nanotechnology 2008, 19, 505504. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, L.; Li, Y.; Xie, J.; Wu, H.C. A universal strategy for aptamer-based nanopore sensing through host-guest interactions inside alpha-hemolysin. Angew. Chem. Int. Ed. Engl. 2015, 54, 7568–7571. [Google Scholar] [CrossRef] [PubMed]
- Benner, S.; Chen, R.J.; Wilson, N.A.; Abu-Shumays, R.; Hurt, N.; Lieberman, K.R.; Deamer, D.W.; Dunbar, W.B.; Akeson, M. Sequence-specific detection of individual DNA polymerase complexes in real time using a nanopore. Nat. Nanotechnol. 2007, 2, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Mayse, L.A.; Movileanu, L. Gating of beta-Barrel Protein Pores, Porins, and Channels: An Old Problem with New Facets. Int. J. Mol. Sci. 2023, 24, 2095. [Google Scholar] [CrossRef] [PubMed]
- Lastra, L.S.; Sharma, V.; Farajpour, N.; Nguyen, M.; Freedman, K.J. Nanodiagnostics: A review of the medical capabilities of nanopores. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102425. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Hui, J.; Mao, H. Nanopore Technology and Its Applications in Gene Sequencing. Biosensors 2021, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Pu, M.; Sun, K.; Song, G.; Geng, J. Nanopore Electrochemistry for Pathogen Detection. Chem.—Asian J. 2022, 17, e202200774. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Bollas, A.; Wang, Y.; Au, K.F. Nanopore sequencing technology, bioinformatics and applications. Nat. Biotechnol. 2021, 39, 1348–1365. [Google Scholar] [CrossRef]
- Zheng, W.; Saliba, J.G.; Wei, X.; Shu, Q.; Pierson, L.M.; Mao, L.; Liu, C.; Lyon, C.J.; Li, C.-Z.; Wimley, W.C.; et al. Nanopore-based disease diagnosis using pathogen-derived tryptic peptides from serum. Nano Today 2022, 45, 101515. [Google Scholar] [CrossRef]
- Cao, C.; Cirauqui, N.; Marcaida, M.J.; Buglakova, E.; Duperrex, A.; Radenovic, A.; Dal Peraro, M. Single-molecule sensing of peptides and nucleic acids by engineered aerolysin nanopores. Nat. Commun. 2019, 10, 4918. [Google Scholar] [CrossRef]
- Ervin, E.N.; Barrall, G.A.; Pal, P.; Bean, M.K.; Schibel, A.E.P.; Hibbs, A.D. Creating a Single Sensing Zone Within an Alpha-Hemolysin Pore via Site-Directed Mutagenesis. BioNanoScience 2014, 4, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, D.; Ayub, M.; Höfler, L.; Raychaudhuri, P.; Klingelhoefer, J.W.; Maglia, G.; Heron, A.; Bayley, H. Functional truncated membrane pores. Proc. Natl. Acad. Sci. USA 2014, 111, 2425–2430. [Google Scholar] [CrossRef]
- Ayub, M.; Stoddart, D.; Bayley, H. Nucleobase Recognition by Truncated α-Hemolysin Pores. ACS Nano 2015, 9, 7895–7903. [Google Scholar] [CrossRef]
- Fahie, M.A.; Yang, B.; Mullis, M.; Holden, M.A.; Chen, M. Selective Detection of Protein Homologues in Serum Using an OmpG Nanopore. Anal. Chem. 2015, 87, 11143–11149. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Foster, J.C.; Moore, M.D.; Chen, M. Improving Single-Molecule Antibody Detection Selectivity through Optimization of Peptide Epitope Presentation in OmpG Nanopore. ACS Sens. 2023, 8, 2673–2680. [Google Scholar] [CrossRef]
- Chen, M.; Khalid, S.; Sansom, M.S.P.; Bayley, H. Outer membrane protein G: Engineering a quiet pore for biosensing. Proc. Natl. Acad. Sci. USA 2008, 105, 6272–6277. [Google Scholar] [CrossRef]
- Mohammad, M.M.; Howard, K.R.; Movileanu, L. Redesign of a Plugged β-Barrel Membrane Protein. J. Biol. Chem. 2011, 286, 8000–8013. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, B. Engineering channels: Atomic biology. Proc. Natl. Acad. Sci. USA 2008, 105, 6211–6212. [Google Scholar] [CrossRef]
- Thakur, A.K.; Movileanu, L. Real-time measurement of protein–protein interactions at single-molecule resolution using a biological nanopore. Nat. Biotechnol. 2019, 37, 96–101. [Google Scholar] [CrossRef]
- Yildiz, O.; Vinothkumar, K.R.; Goswami, P.; Kuhlbrandt, W. Structure of the monomeric outer-membrane porin OmpG in the open and closed conformation. EMBO J. 2006, 25, 3702–3713. [Google Scholar] [CrossRef] [PubMed]
- Pham, B.; Chisholm, C.M.; Foster, J.; Friis, E.; Fahie, M.A.; Chen, M. A pH-independent quiet OmpG pore with enhanced electrostatic repulsion among the extracellular loops. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183485. [Google Scholar] [CrossRef] [PubMed]
- Sanganna Gari, R.R.; Seelheim, P.; Liang, B.; Tamm, L.K. Quiet Outer Membrane Protein G (OmpG) Nanopore for Biosensing. ACS Sens. 2019, 4, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Larimi, M.G.; Gooden, K.; Movileanu, L. Aberrantly Large Single-Channel Conductance of Polyhistidine Arm-Containing Protein Nanopores. Biochemistry 2017, 56, 4895–4905. [Google Scholar] [CrossRef] [PubMed]
- Maglia, G.; Restrepo, M.R.; Mikhailova, E.; Bayley, H. Enhanced translocation of single DNA molecules through α-hemolysin nanopores by manipulation of internal charge. Proc. Natl. Acad. Sci. USA 2008, 105, 19720–19725. [Google Scholar] [CrossRef] [PubMed]
- Jou, I.; Muthukumar, M. Effects of Nanopore Charge Decorations on the Translocation Dynamics of DNA. Biophys. J. 2017, 113, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Bikwemu, R.; Wolfe, A.J.; Xing, X.; Movileanu, L. Facilitated translocation of polypeptides through a single nanopore. J. Phys. Condens. Matter 2010, 22, 454117. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.J.; Mohammad; Cheley, S.; Bayley, H.; Movileanu, L. Catalyzing the Translocation of Polypeptides through Attractive Interactions. J. Am. Chem. Soc. 2007, 129, 14034–14041. [Google Scholar] [CrossRef]
- Howorka, S. Building membrane nanopores. Nat. Nanotechnol. 2017, 12, 619–630. [Google Scholar] [CrossRef]
- Manrao, E.A.; Derrington, I.M.; Laszlo, A.H.; Langford, K.W.; Hopper, M.K.; Gillgren, N.; Pavlenok, M.; Niederweis, M.; Gundlach, J.H. Reading DNA at single-nucleotide resolution with a mutant MspA nanopore and phi29 DNA polymerase. Nat. Biotechnol. 2012, 30, 349–353. [Google Scholar] [CrossRef]
- Laszlo, A.H.; Derrington, I.M.; Ross, B.C.; Brinkerhoff, H.; Adey, A.; Nova, I.C.; Craig, J.M.; Langford, K.W.; Samson, J.M.; Daza, R.; et al. Decoding long nanopore sequencing reads of natural DNA. Nat. Biotechnol. 2014, 32, 829–833. [Google Scholar] [CrossRef]
- Butler, T.Z.; Pavlenok, M.; Derrington, I.M.; Niederweis, M.; Gundlach, J.H. Single-molecule DNA detection with an engineered MspA protein nanopore. Proc. Natl. Acad. Sci. USA 2008, 105, 20647–20652. [Google Scholar] [CrossRef]
- Versloot, R.C.A.; Straathof, S.A.P.; Stouwie, G.; Tadema, M.J.; Maglia, G. beta-Barrel Nanopores with an Acidic-Aromatic Sensing Region Identify Proteinogenic Peptides at Low pH. ACS Nano 2022, 16, 7258–7268. [Google Scholar] [CrossRef]
- Huo, M.Z.; Hu, Z.L.; Ying, Y.L.; Long, Y.T. Enhanced identification of Tau acetylation and phosphorylation with an engineered aerolysin nanopore. Proteomics 2022, 22, e2100041. [Google Scholar] [CrossRef]
- Ho, C.-W.; Van Meervelt, V.; Tsai, K.-C.; De Temmerman, P.-J.; Mast, J.; Maglia, G. Engineering a nanopore with co-chaperonin function. Sci. Adv. 2015, 1, e1500905. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, G.; Versloot, R.C.A.; Bruininks, B.M.H.; De Souza, P.C.T.; Marrink, S.-J.; Maglia, G. Bottom-up fabrication of a proteasome–nanopore that unravels and processes single proteins. Nat. Chem. 2021, 13, 1192–1199. [Google Scholar] [CrossRef]
- Nivala, J.; Marks, D.B.; Akeson, M. Unfoldase-mediated protein translocation through an α-hemolysin nanopore. Nat. Biotechnol. 2013, 31, 247–250. [Google Scholar] [CrossRef]
- Stephenson, W.; Razaghi, R.; Busan, S.; Weeks, K.M.; Timp, W.; Smibert, P. Direct detection of RNA modifications and structure using single-molecule nanopore sequencing. Cell Genom. 2022, 2, 100097. [Google Scholar] [CrossRef]
- Olasagasti, F.; Lieberman, K.R.; Benner, S.; Cherf, G.M.; Dahl, J.M.; Deamer, D.W.; Akeson, M. Replication of individual DNA molecules under electronic control using a protein nanopore. Nat. Nanotechnol. 2010, 5, 798–806. [Google Scholar] [CrossRef]
- Lieberman, K.R.; Cherf, G.M.; Doody, M.J.; Olasagasti, F.; Kolodji, Y.; Akeson, M. Processive Replication of Single DNA Molecules in a Nanopore Catalyzed by phi29 DNA Polymerase. J. Am. Chem. Soc. 2010, 132, 17961–17972. [Google Scholar] [CrossRef]
- Fuller, C.W.; Kumar, S.; Porel, M.; Chien, M.; Bibillo, A.; Stranges, P.B.; Dorwart, M.; Tao, C.; Li, Z.; Guo, W.; et al. Real-time single-molecule electronic DNA sequencing by synthesis using polymer-tagged nucleotides on a nanopore array. Proc. Natl. Acad. Sci. USA 2016, 113, 5233–5238. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Natl. Acad. Sci. USA 2012, 109, E690–E697. [Google Scholar] [CrossRef] [PubMed]
- Branton, D.; Deamer, D.W.; Marziali, A.; Bayley, H.; Benner, S.A.; Butler, T.; Di Ventra, M.; Garaj, S.; Hibbs, A.; Huang, X.; et al. The potential and challenges of nanopore sequencing. Nat. Biotechnol. 2008, 26, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Huang, P.J.; Ding, J.; Liu, J. Aptamer-based biosensors for biomedical diagnostics. Analyst 2014, 139, 2627–2640. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.H.; Zhang, T.; Luo, H.; Yen, T.M.; Chen, P.W.; Han, Y.; Lo, Y.H. Nucleic Acid Aptamers: An Emerging Tool for Biotechnology and Biomedical Sensing. Sensors 2015, 15, 16281–16313. [Google Scholar] [CrossRef] [PubMed]
- Sefah, K.; Shangguan, D.; Xiong, X.; O’Donoghue, M.B.; Tan, W. Development of DNA aptamers using Cell-SELEX. Nat. Protoc. 2010, 5, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Long, S.B.; Long, M.B.; White, R.R.; Sullenger, B.A. Crystal structure of an RNA aptamer bound to thrombin. RNA 2008, 14, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.B.; Vu, D.; Cassiday, L.A.; Zimmerman, J.M.; Maher, L.J., 3rd; Ghosh, G. Crystal structure of NF-kappaB (p50)2 complexed to a high-affinity RNA aptamer. Proc. Natl. Acad. Sci. USA 2003, 100, 9268–9273. [Google Scholar] [CrossRef] [PubMed]
- Horn, W.T.; Convery, M.A.; Stonehouse, N.J.; Adams, C.J.; Liljas, L.; Phillips, S.E.; Stockley, P.G. The crystal structure of a high affinity RNA stem-loop complexed with the bacteriophage MS2 capsid: Further challenges in the modeling of ligand-RNA interactions. RNA 2004, 10, 1776–1782. [Google Scholar] [CrossRef]
- Thomson, K.; Amin, I.; Morales, E.; Winters-Hilt, S. Preliminary nanopore cheminformatics analysis of aptamer-target binding strength. BMC Bioinform. 2007, 8 (Suppl. 7), S11. [Google Scholar] [CrossRef]
- Shim, J.W.; Gu, L.Q. Encapsulating a single G-quadruplex aptamer in a protein nanocavity. J. Phys. Chem. B 2008, 112, 8354–8360. [Google Scholar] [CrossRef] [PubMed]
- Rotem, D.; Jayasinghe, L.; Salichou, M.; Bayley, H. Protein detection by nanopores equipped with aptamers. J. Am. Chem. Soc. 2012, 134, 2781–2787. [Google Scholar] [CrossRef]
- Howorka, S.; Cheley, S.; Bayley, H. Sequence-specific detection of individual DNA strands using engineered nanopores. Nat. Biotechnol. 2001, 19, 636–639. [Google Scholar] [CrossRef]
- Howorka, S.; Movileanu, L.; Braha, O.; Bayley, H. Kinetics of duplex formation for individual DNA strands within a single protein nanopore. Proc. Natl. Acad. Sci. USA 2001, 98, 12996–13001. [Google Scholar] [CrossRef] [PubMed]
- Healey, M.J.; Sivakumaran, M.; Platt, M. Rapid quantification of prion proteins using resistive pulse sensing. Analyst 2020, 145, 2595–2601. [Google Scholar] [CrossRef]
- Tang, H.; Wang, H.; Yang, C.; Zhao, D.; Qian, Y.; Li, Y. Nanopore-based Strategy for Selective Detection of Single Carcinoembryonic Antigen (CEA) Molecules. Anal. Chem. 2020, 92, 3042–3049. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, L.; Wang, Y.; Xi, D.; Zhang, S. Unambiguous Discrimination of Multiple Protein Biomarkers by Nanopore Sensing with Double-Stranded DNA-Based Probes. Anal. Chem. 2020, 92, 1730–1737. [Google Scholar] [CrossRef]
- Xi, D.; Li, Z.; Liu, L.; Ai, S.; Zhang, S. Ultrasensitive Detection of Cancer Cells Combining Enzymatic Signal Amplification with an Aerolysin Nanopore. Anal. Chem. 2018, 90, 1029–1034. [Google Scholar] [CrossRef]
- Soskine, M.; Biesemans, A.; Moeyaert, B.; Cheley, S.; Bayley, H.; Maglia, G. An engineered ClyA nanopore detects folded target proteins by selective external association and pore entry. Nano Lett. 2012, 12, 4895–4900. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, S.; Wang, Y.; Zheng, L.; Guan, S.; Wang, D.; Wang, L.; Guan, X. Nanopore Single-molecule Analysis of Biomarkers: Providing Possible Clues to Disease Diagnosis. TrAC Trends Anal. Chem. 2023, 162, 117060. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Tang, P.; Wang, L.; Xie, W.; Chen, X.; Wang, Y.; Weng, T.; Tian, R.; Zhou, S.; Wang, Z.; et al. An aptamer-assisted nanopore strategy with a salt gradient for direct protein sensing. J. Mater. Chem. B 2023, 11, 11064–11072. [Google Scholar] [CrossRef] [PubMed]
- Fahie, M.A.; Yang, B.; Pham, B.; Chen, M. Tuning the Selectivity and Sensitivity of an OmpG Nanopore Sensor by Adjusting Ligand Tether Length. ACS Sens. 2016, 1, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.C.; Pham, B.; Pham, R.; Kim, M.; Moore, M.D.; Chen, M. An Engineered OmpG Nanopore with Displayed Peptide Motifs for Single-Molecule Multiplex Protein Detection. Angew. Chem. Int. Ed. 2023, 62, e202214566. [Google Scholar] [CrossRef] [PubMed]
- Fahie, M.A.V.; Yang, B.; Chisholm, C.M.; Chen, M. Protein Analyte Sensing with an Outer Membrane Protein G (OmpG) Nanopore; Springer: New York, NY, USA, 2021; pp. 77–94. [Google Scholar]
- Harrington, L.; Alexander, L.T.; Knapp, S.; Bayley, H. Pim Kinase Inhibitors Evaluated with a Single-Molecule Engineered Nanopore Sensor. Angew. Chem. 2015, 127, 8272–8277. [Google Scholar] [CrossRef]
- Cheley, S.; Xie, H.; Bayley, H. A Genetically Encoded Pore for the Stochastic Detection of a Protein Kinase. ChemBioChem 2006, 7, 1923–1927. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Movileanu, L. Single-Molecule Protein Detection in a Biofluid Using a Quantitative Nanopore Sensor. ACS Sens. 2019, 4, 2320–2326. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.; Cheley, S.; Alexander, L.T.; Knapp, S.; Bayley, H. Stochastic detection of Pim protein kinases reveals electrostatically enhanced association of a peptide substrate. Proc. Natl. Acad. Sci. USA 2013, 110, E4417–E4426. [Google Scholar] [CrossRef] [PubMed]
- Pham, B.; Eron, S.J.; Hill, M.E.; Li, X.; Fahie, M.A.; Hardy, J.A.; Chen, M. A Nanopore Approach for Analysis of Caspase-7 Activity in Cell Lysates. Biophys. J. 2019, 117, 844–855. [Google Scholar] [CrossRef]
- Cao, C.; Magalhaes, P.; Krapp, L.F.; Bada Juarez, J.F.; Mayer, S.F.; Rukes, V.; Chiki, A.; Lashuel, H.A.; Dal Peraro, M. Deep Learning-Assisted Single-Molecule Detection of Protein Post-translational Modifications with a Biological Nanopore. ACS Nano 2024, 18, 1504–1515. [Google Scholar] [CrossRef]
- Jia, W.; Hu, C.; Wang, Y.; Liu, Y.; Wang, L.; Zhang, S.; Zhu, Q.; Gu, Y.; Zhang, P.; Ma, J.; et al. Identification of Single-Molecule Catecholamine Enantiomers Using a Programmable Nanopore. ACS Nano 2022, 16, 6615–6624. [Google Scholar] [CrossRef]
- Sen, P.; Hoi, H.; Gupta, M. Low Noise Hybrid Nanopore with Engineered OmpG and Bilayer MoS2. ACS Appl. Bio Mater. 2021, 4, 5416–5424. [Google Scholar] [CrossRef]
- Kahlstatt, J.; Reiss, P.; Halbritter, T.; Essen, L.O.; Koert, U.; Heckel, A. A light-triggered transmembrane porin. Chem. Commun. 2018, 54, 9623–9626. [Google Scholar] [CrossRef]
- Jia, W.; Hu, C.; Wang, Y.; Gu, Y.; Qian, G.; Du, X.; Wang, L.; Liu, Y.; Cao, J.; Zhang, S.; et al. Programmable nano-reactors for stochastic sensing. Nat. Commun. 2021, 12, 5811. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, K.; Wang, Y.; Wang, L.; Yan, S.; Du, X.; Zhang, P.; Chen, H.Y.; Huang, S. Machine Learning Assisted Simultaneous Structural Profiling of Differently Charged Proteins in a Mycobacterium smegmatis Porin A (MspA) Electroosmotic Trap. J. Am. Chem. Soc. 2022, 144, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, S.; Wang, Y.; Wang, L.; Cao, Z.; Sun, W.; Fan, P.; Zhang, P.; Chen, H.Y.; Huang, S. Nanopore Identification of Alditol Epimers and Their Application in Rapid Analysis of Alditol-Containing Drinks and Healthcare Products. J. Am. Chem. Soc. 2022, 144, 13717–13728. [Google Scholar] [CrossRef]
- Guan, X.; Wang, Y.; Zhang, J.; Shao, W.; Huang, S.; Zhang, D. Unsupervised deep learning for identifying the O (6)-carboxymethyl guanine by nanopore sequencing. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2022, 39, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, Y.; Chen, C.; Zhu, Y.; Miao, Z.; Mou, X.; Yuan, W.; Zhang, Z.; Li, K.; Chen, M.; et al. Direct and Continuous Monitoring of Multicomponent Antibiotic Gentamicin in Blood at Single-Molecule Resolution. ACS Nano 2024, 18, 9137–9149. [Google Scholar] [CrossRef]
- Greive, S.J.; Bacri, L.; Cressiot, B.; Pelta, J. Identification of Conformational Variants for Bradykinin Biomarker Peptides from a Biofluid Using a Nanopore and Machine Learning. ACS Nano 2024, 18, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Jia, W.; Fan, P.; Wang, L.; Li, X.; Chen, J.; Cao, Z.; Du, X.; Liu, Y.; et al. Identification of nucleoside monophosphates and their epigenetic modifications using an engineered nanopore. Nat. Nanotechnol. 2022, 17, 976–983. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Wan, Y.J.; Ying, Y.L.; Yu, R.J.; Long, Y.T. SmartImage: A Machine Learning Method for Nanopore Identifying Chemical Modifications on RNA. Chem.—Asian J. 2023, 18, e202201144. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Penkauskas, T.; Reiner, J.E.; Kennard, C.; Uline, M.J.; Wang, Q.; Li, S.; Aksimentiev, A.; Robertson, J.W.F.; Liu, C. Engineering Biological Nanopore Approaches toward Protein Sequencing. ACS Nano 2023, 17, 16369–16395. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.-X.; Ying, Y.-L.; Li, M.-Y.; Yang, J.; Zhou, J.-L.; Wang, H.-F.; Yan, B.-Y.; Long, Y.-T. Learning Shapelets for Improving Single-Molecule Nanopore Sensing. Anal. Chem. 2019, 91, 10033–10039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Ying, Y.-L.; Gu, Z.; Meng, F.-N.; Long, Y.-T. High-bandwidth nanopore data analysis by using a modified hidden Markov model. Nanoscale 2017, 9, 3458–3465. [Google Scholar] [CrossRef] [PubMed]
- Winters-Hilt, S.; Vercoutere, W.; Deguzman, V.S.; Deamer, D.; Akeson, M.; Haussler, D. Highly Accurate Classification of Watson-Crick Basepairs on Termini of Single DNA Molecules. Biophys. J. 2003, 84, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dou, L.; Zhang, M.; Wang, Y.; Jiang, X.; Li, X.; Wei, L.; Chen, Y.; Zhou, C.; Geng, J. Real-time sensing of neurotransmitters by functionalized nanopores embedded in a single live cell. Mol. Biomed. 2021, 2, 6. [Google Scholar] [CrossRef]
- Chen, X.; Lu, L.; Xiong, X.; Xiong, X.; Liu, Y. Development of a real-time PCR assay for the identification and quantification of bovine ingredient in processed meat products. Sci. Rep. 2020, 10, 2052. [Google Scholar] [CrossRef]
- Garzarelli, V.; Chiriacò, M.S.; Cereda, M.; Gigli, G.; Ferrara, F. Ultrasensitive qPCR platform for rapid detection of bacterial contamination of raw biological samples at the point of care. Heliyon 2023, 9, e16229. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Yobas, L. Label-Free Multiplexed Electrical Detection of Cancer Markers on a Microchip Featuring an Integrated Fluidic Diode Nanopore Array. ACS Nano 2018, 12, 7892–7900. [Google Scholar] [CrossRef]
- Player, R.; Verratti, K.; Staab, A.; Bradburne, C.; Grady, S.; Goodwin, B.; Sozhamannan, S. Comparison of the performance of an amplicon sequencing assay based on Oxford Nanopore technology to real-time PCR assays for detecting bacterial biodefense pathogens. BMC Genom. 2020, 21, 166. [Google Scholar] [CrossRef]
- Yao, F.; Peng, X.; Su, Z.; Tian, L.; Guo, Y.; Kang, X.-F. Crowding-Induced DNA Translocation through a Protein Nanopore. Anal. Chem. 2020, 92, 3827–3833. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiswedel, R.; Bui, A.T.N.; Kim, J.; Lee, M.-K. Beta-Barrel Nanopores as Diagnostic Sensors: An Engineering Perspective. Biosensors 2024, 14, 345. https://doi.org/10.3390/bios14070345
Wiswedel R, Bui ATN, Kim J, Lee M-K. Beta-Barrel Nanopores as Diagnostic Sensors: An Engineering Perspective. Biosensors. 2024; 14(7):345. https://doi.org/10.3390/bios14070345
Chicago/Turabian StyleWiswedel, Rani, Anh Thi Ngoc Bui, Jinhyung Kim, and Mi-Kyung Lee. 2024. "Beta-Barrel Nanopores as Diagnostic Sensors: An Engineering Perspective" Biosensors 14, no. 7: 345. https://doi.org/10.3390/bios14070345





