A Miniature Modular Fluorescence Flow Cytometry System
Abstract
1. Introduction
2. Materials and Methods
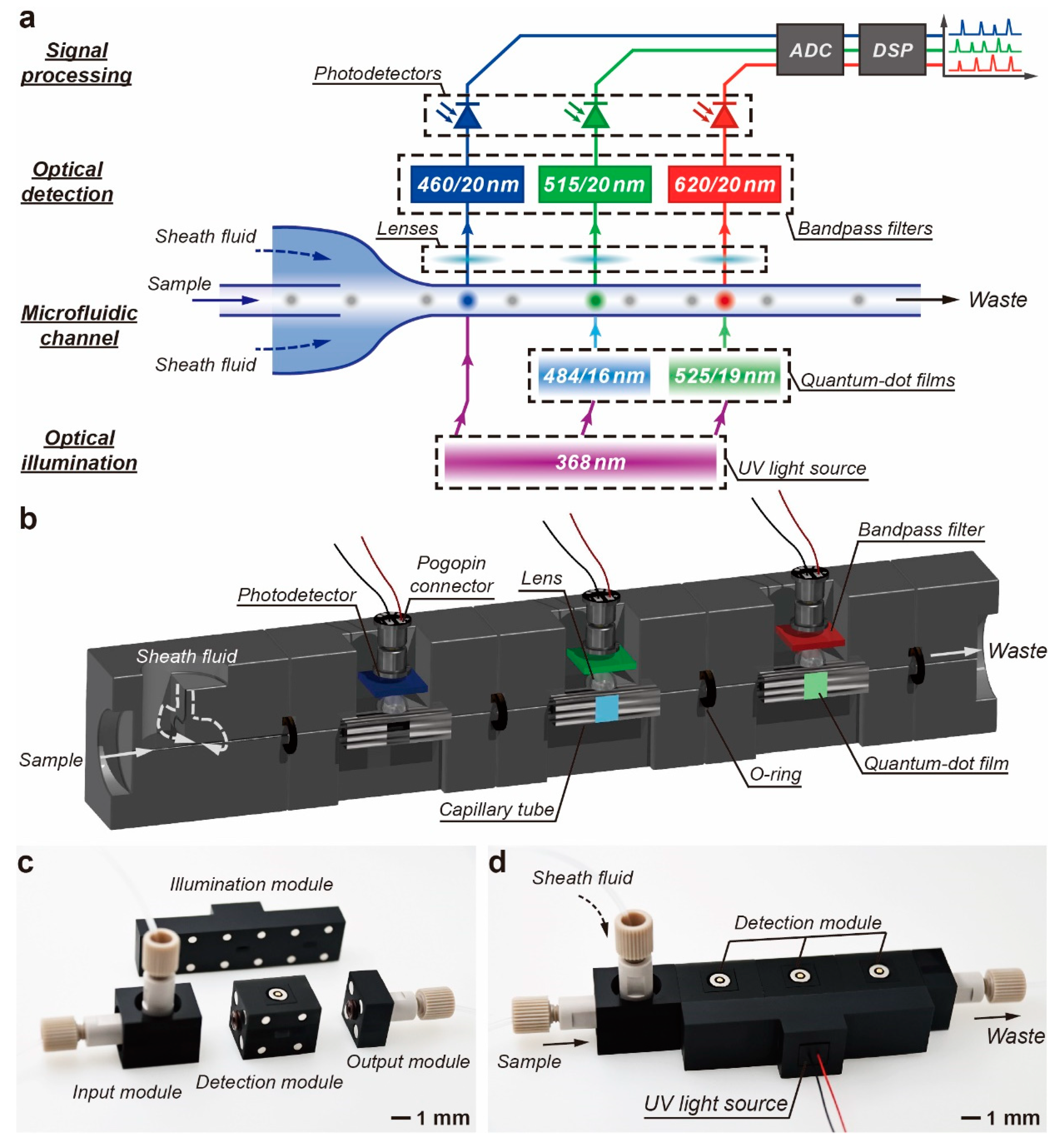
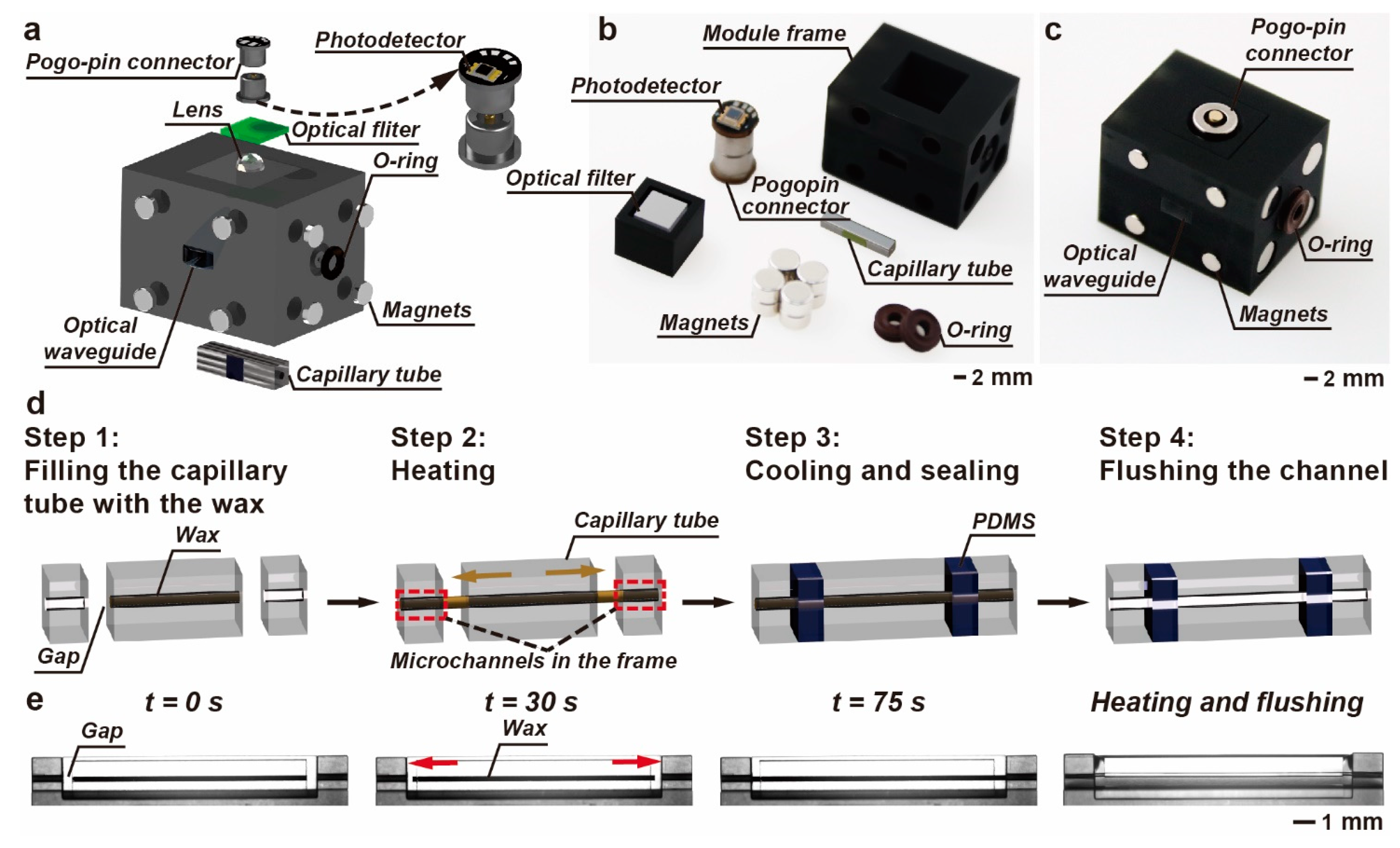
2.1. Design and Fabrication of the M-FCM
2.2. Characterization of Illumination Source
2.3. Data Acquisition and Processing
2.4. Cell Culture and Preparation
3. Results and Discussion
3.1. Enhancement of the Fluorescence Detection
3.2. Evaluation of the Optical Crosstalk
3.3. Integration of the Detection Module
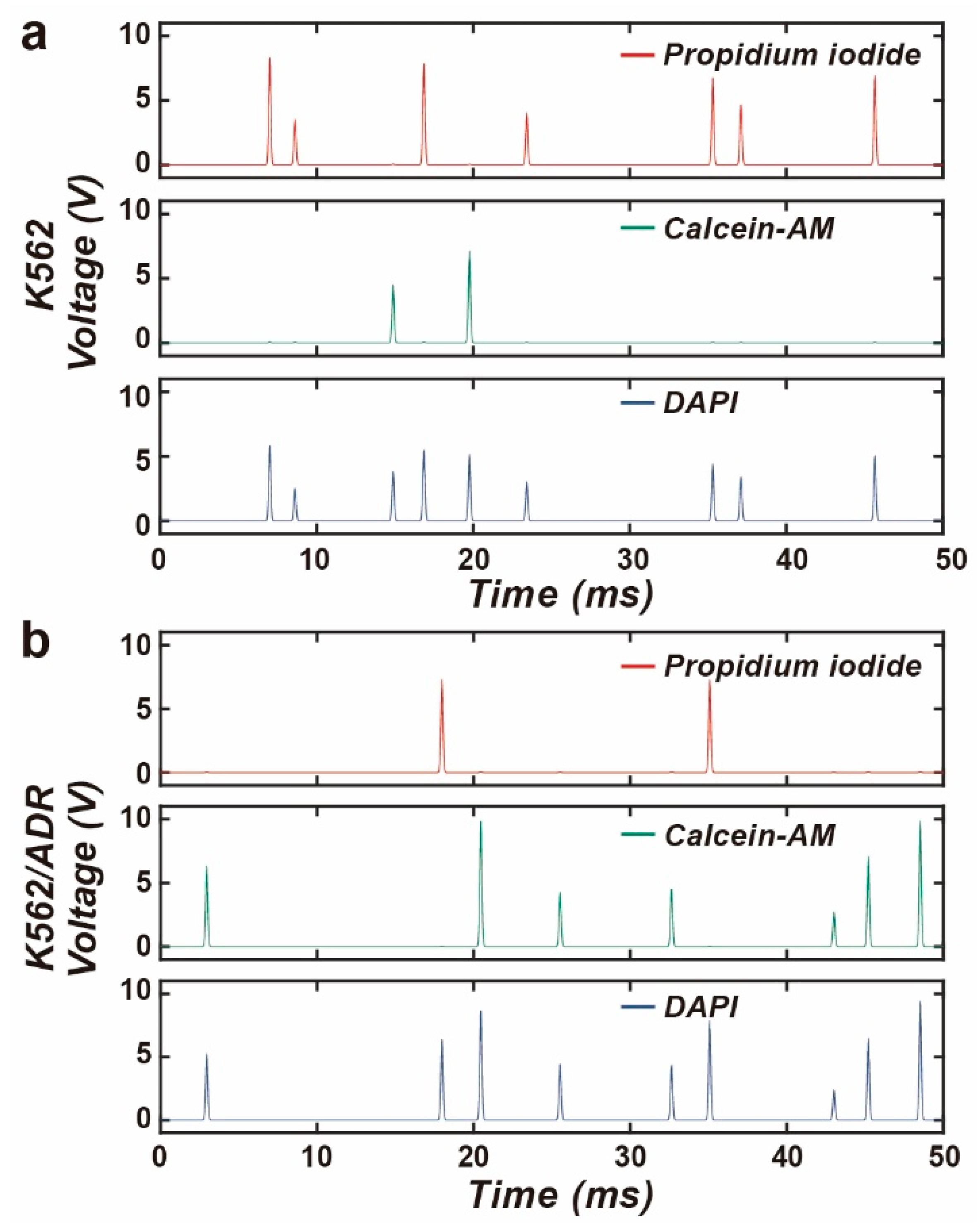
3.4. Drug Screening Using the M-FCM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwok, S.J.J.; Forward, S.; Fahlberg, M.D.; Assita, E.R.; Cosgriff, S.; Lee, S.H.; Abbott, G.R.; Zhu, H.; Minasian, N.H.; Vote, A.S.; et al. High-dimensional multi-pass flow cytometry via spectrally encoded cellular barcoding. Nat. Biomed. Eng. 2024, 8, 310–324. [Google Scholar] [CrossRef]
- Hua, X.; Han, K.; Mandracchia, B.; Radmand, A.; Liu, W.; Kim, H.; Yuan, Z.; Ehrlich, S.M.; Li, K.; Zheng, C.; et al. Light-field flow cytometry for high-resolution, volumetric and multiparametric 3D single-cell analysis. Nat. Commun. 2024, 15, 1975. [Google Scholar] [CrossRef] [PubMed]
- Mikami, H.; Kawaguchi, M.; Huang, C.-J.; Matsumura, H.; Sugimura, T.; Huang, K.; Lei, C.; Ueno, S.; Miura, T.; Ito, T.; et al. Virtual-freezing fluorescence imaging flow cytometry. Nat. Commun. 2020, 11, 1162. [Google Scholar] [CrossRef]
- Li, X.; Fan, B.; Cao, S.; Chen, D.; Zhao, X.; Men, D.; Yue, W.; Wang, J.; Chen, J. A microfluidic flow cytometer enabling absolute quantification of single-cell intracellular proteins. Lab Chip 2017, 17, 3129–3137. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-J.; Hsiao, Y.-C.; Weng, Y.-L.; Chen, Y.-H.; Chiou, P.-Y.; Sheen, H.-J. Development of a parallel three-dimensional microfluidic device for high- throughput cytometry. Sens. Actuators B Chem 2020, 320, 128255. [Google Scholar] [CrossRef]
- Tian, Y.; Xue, C.; Zhang, W.; Chen, C.; Ma, L.; Niu, Q.; Wu, L.; Yan, X. Refractive Index Determination of Individual Viruses and Small Extracellular Vesicles in Aqueous Media Using Nano-Flow Cytometry. Anal. Chem. 2022, 94, 14299–14307. [Google Scholar] [CrossRef] [PubMed]
- Green, R.E.; Sosik, H.M.; Olson, R.J.; DuRand, M.D. Flow cytometric determination of size and complex refractive index for marine particles: Comparison with independent and bulk estimates. Appl. Opt. 2003, 42, 526–541. [Google Scholar] [CrossRef]
- Reale, R.; Peruzzi, G.; Ghoreishi, M.; Stabile, H.; Ruocco, G.; Leonetti, M. A low-cost, label-free microfluidic scanning flow cytometer for high-accuracy quantification of size and refractive index of particles. Lab Chip 2023, 23, 2039–2047. [Google Scholar] [CrossRef]
- Leblanc-Hotte, A.; Nkwe, N.S.; Chabot-Roy, G.; Affar, E.B.; Lesage, S.; Delisle, J.-S.; Peter, Y.-A. On-chip refractive index cytometry for whole-cell deformability discrimination. Lab Chip 2019, 19, 464–474. [Google Scholar] [CrossRef]
- Petchakup, C.; Hutchinson, P.E.; Tay, H.M.; Leong, S.Y.; Li, K.H.H.; Hou, H.W. Label-free quantitative lymphocyte activation profiling using microfluidic impedance cytometry. Sens. Actuators B Chem. 2021, 339, 129864. [Google Scholar] [CrossRef]
- Honrado, C.; Bisegna, P.; Swami, N.S.; Caselli, F. Single-cell microfluidic impedance cytometry: From raw signals to cell phenotypes using data analytics. Lab Chip 2021, 21, 22–54. [Google Scholar] [CrossRef]
- Lei, K.F.; Ho, Y.-C.; Huang, C.-H.; Huang, C.-H.; Pai, P.C. Characterization of stem cell-like property in cancer cells based on single-cell impedance measurement in a microfluidic platform. Talanta 2021, 229, 122259. [Google Scholar] [CrossRef]
- Rane, A.; Jarmoshti, J.; Siddique, A.-B.; Adair, S.; Torres-Castro, K.; Honrado, C.; Bauer, T.W.; Swami, N.S. Dielectrophoretic enrichment of live chemo- resistant circulating-like pancreatic cancer cells from media of drug-treated adherent cultures of solid tumors. Lab Chip 2024, 24, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, K.; Chen, D.; Fan, B.; Xu, Y.; Ye, Y.; Wang, J.; Chen, J.; Huang, C. Development of microfluidic impedance cytometry enabling the quantification of specific membrane capacitance and cytoplasm conductivity from 100,000 single cells. Biosens. Bioelectron. 2018, 111, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.; Rajagopal, V.; Stewart, A.; Collins, D.J. Critical review of single-cell mechanotyping approaches for biomedical applications. Lab Chip 2024, 24, 3036–3063. [Google Scholar] [CrossRef]
- Chen, Y.; Ni, C.; Zhang, X.; Ni, Z.; Xiang, N. High-Throughput Sorting and Single-Cell Mechanotyping by Hydrodynamic Sorting-Mechanotyping Cytometry. Small Methods 2024, 2301195. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Xu, H.; Ma, L.; Wang, Y.; Yu, L.; Wang, F.; Zhu, J.; Hu, X.; Yi, K.; et al. Touchable cell biophysics property recognition platforms enable multifunctional blood smart health care. Microsyst. Nanoeng. 2021, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Coker, Z.N.; Troyanova-Wood, M.; Steelman, Z.A.; Ibey, B.L.; Bixler, J.N.; Scully, M.O.; Yakovlev, V.V. Brillouin microscopy monitors rapid responses in subcellular compartments. PhotoniX 2024, 5, 9. [Google Scholar] [CrossRef]
- Zhou, Z.; Ni, C.; Zhu, Z.; Chen, Y.; Ni, Z.; Xiang, N. High-throughput adjustable deformability cytometry utilizing elasto-inertial focusing and virtual fluidic channel. Lab chip 2023, 23, 4528–4539. [Google Scholar] [CrossRef]
- Urbanska, M.; Muñoz, H.E.; Bagnall, J.S.; Otto, O.; Manalis, S.R.; Carlo, D.D.; Guck, J. A comparison of microfluidic methods for high-throughput cell deformability measurements. Nat. Methods 2020, 17, 587–593. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Yan, R.; Tang, T.; Wei, S.; Li, R.; Hou, D.; Weng, Y.; Wang, D.; Shen, H. An optimized PDMS microfluidic device for ultra- fast and high-throughput imaging flow cytometry. Lab chip 2023, 23, 3571–3580. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cheng, Z.; Chai, H.; He, W.; Huang, L.; Wang, W. Neural network-enhanced real-time impedance flow cytometry for single-cell intrinsic characterization. Lab chip 2022, 22, 240–249. [Google Scholar] [CrossRef]
- Mäeots, M.-E.; Lee, B.; Nans, A.; Jeong, S.-G.; Esfahani, M.M.N.; Ding, S.; Smith, D.J.; Lee, C.-S.; Lee, S.S.; Peter, M. Modular microfluidics enables kinetic insight from time-resolved cryo-EM. Nat. Commun. 2020, 11, 3465. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.E.; Hart, A.J. High-precision modular microfluidics by micromilling of interlocking injection-molded blocks. Lab chip 2018, 18, 890–901. [Google Scholar] [CrossRef]
- Kanitthamniyom, P.; Zhou, A.; Feng, S.; Liu, A.; Vasoo, S.; Zhang, Y. A 3D-printed modular magnetic digital microfluidic architecture for on-demand bioanalysis. Microsyst. Nanoeng. 2020, 6, 48. [Google Scholar] [CrossRef]
- Lai, X.; Shi, Z.; Pu, Z.; Zhang, P.; Zhang, X.; Yu, H.; Li, D. A Rubik’s microfluidic cube. Microsyst. Nanoeng. 2020, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wu, J.; Zheng, L.; Long, Y.; Chen, J.; Li, J.; Dai, B.; Lin, F.; Zhuang, S.; Zhang, D. 3D free-assembly modular microfluidics inspired by movable type printing. Microsyst. Nanoeng. 2023, 9, 111. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Xu, J.; Ma, B. One-step liquid molding based modular microfluidic circuits. Analyst 2020, 145, 6813–6820. [Google Scholar] [CrossRef]
- Bédarad, A.-C.; Adamo, A.; Aroh, K.C.; Russell, M.G.; Bedermann, A.A.; Torosian, J.; Yue, B.; Jensen, K.F.; Jamison, T.F. Reconfigurable system for automated optimization of diverse chemical reactions. Science 2018, 361, 1220–1225. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, B.; Oh, I.; Choi, S. Optofluidic Modular Blocks for On-Demand and Open-Source Prototyping of Microfluidic Systems. Small 2018, 14, 1802769. [Google Scholar] [CrossRef]
- Vollertsen, A.R.; Boer, D.; Dekker, S.; Wesselink, B.A.M.; Haverkate, R.; Rho, H.S.; Boom, R.J.; Skolimowski, M.; Blom, M.; Passier, R. Modular operation of microfluidic chips for highly parallelized cell culture and liquid dosing via a fluidic circuit board. Microsyst. Nanoeng. 2020, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Zhao, D.; Phan, D.T.T.; Wang, X.; Park, J.J.; Biviji, Z.; Hughes, C.C.W.; Lee, A.P. A modular microfluidic system based on a multilayered configuration to generate large-scale perfusable microvascular networks. Microsyst. Nanoeng. 2021, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Volk, A.A.; Epps, R.W.; Abolhasani, M. Accelerated Development of Colloidal Nanomaterials Enabled by Modular Microfluidic Reactors: Toward Autonomous Robotic Experimentation. Adv. Mater. 2020, 33, 2004495. [Google Scholar] [CrossRef] [PubMed]
- Adan, A.; Alizada, G.; Kiraz, Y.; Baran, Y.; Nalbant, A. Flow cytometry: Basic principles and applications. Crit. Rev. Biotechnol. 2017, 37, 163–176. [Google Scholar] [CrossRef]
- Panwar, J.; Merten, C.A. Fluorescence crosstalk reduction by modulated excitation-synchronous acquisition for multispectral analysis in high-throughput droplet microfluidics. Lab Chip 2023, 23, 2514–2520. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.L.; Roseiro, J.C.; Reis, A. Applications and perspectives of multi-parameter flow cytometry to microbial biofuels production processes. Trends Biotechnol. 2012, 30, 225–232. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Li, J.; Wei, L.; Zheng, L.; Shi, Z.; Guo, S.; Dai, B.; Zhang, D.; Zhuang, S. A Miniature Modular Fluorescence Flow Cytometry System. Biosensors 2024, 14, 395. https://doi.org/10.3390/bios14080395
Huang S, Li J, Wei L, Zheng L, Shi Z, Guo S, Dai B, Zhang D, Zhuang S. A Miniature Modular Fluorescence Flow Cytometry System. Biosensors. 2024; 14(8):395. https://doi.org/10.3390/bios14080395
Chicago/Turabian StyleHuang, Shaoqi, Jiale Li, Li Wei, Lulu Zheng, Zheng Shi, Shiwei Guo, Bo Dai, Dawei Zhang, and Songlin Zhuang. 2024. "A Miniature Modular Fluorescence Flow Cytometry System" Biosensors 14, no. 8: 395. https://doi.org/10.3390/bios14080395
APA StyleHuang, S., Li, J., Wei, L., Zheng, L., Shi, Z., Guo, S., Dai, B., Zhang, D., & Zhuang, S. (2024). A Miniature Modular Fluorescence Flow Cytometry System. Biosensors, 14(8), 395. https://doi.org/10.3390/bios14080395




