Distinct Neural Activities in Hippocampal Subregions Revealed Using a High-Performance Wireless Microsystem with PtNPs/PEDOT:PSS-Enhanced Microelectrode Arrays
Abstract
1. Introduction
2. Materials and Methods
2.1. Design, Fabrication, and Modification of MEA
2.2. Microsystem Architecture
2.3. FPGA Signal Processing
2.4. Wireless Communication
2.4.1. ESP32 Data Processing
2.4.2. NeuroWireless Development
2.5. In Vivo Experimental Setup
3. Results
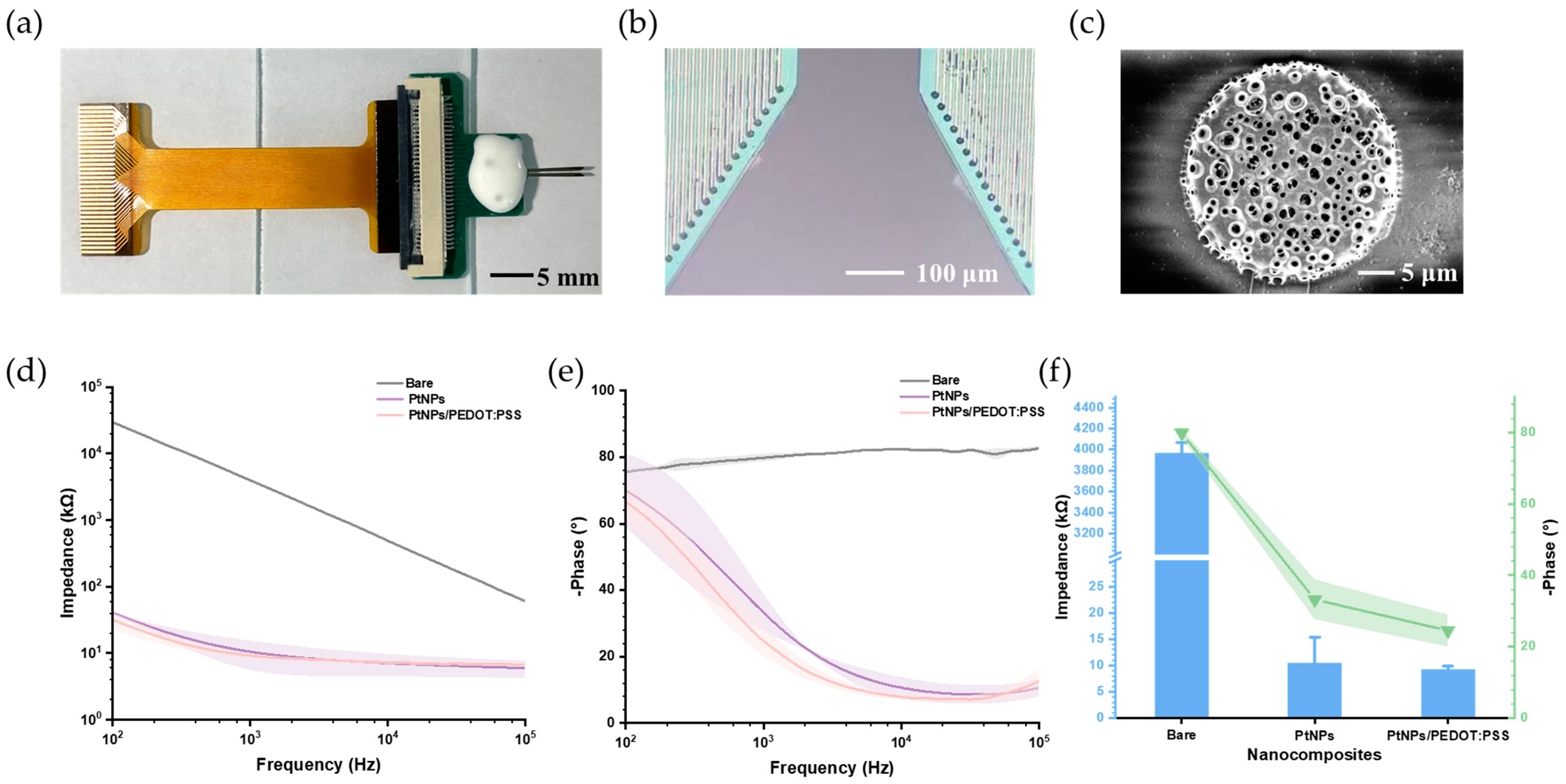
3.1. Characteristics of PtNPs and PtNPs/PEDOT:PSS
3.2. A Compact, High-Fidelity, and High-Throughput Wireless Microsystem
3.2.1. Compactness
3.2.2. High Fidelity and High-Throughput
3.2.3. Current Consumption
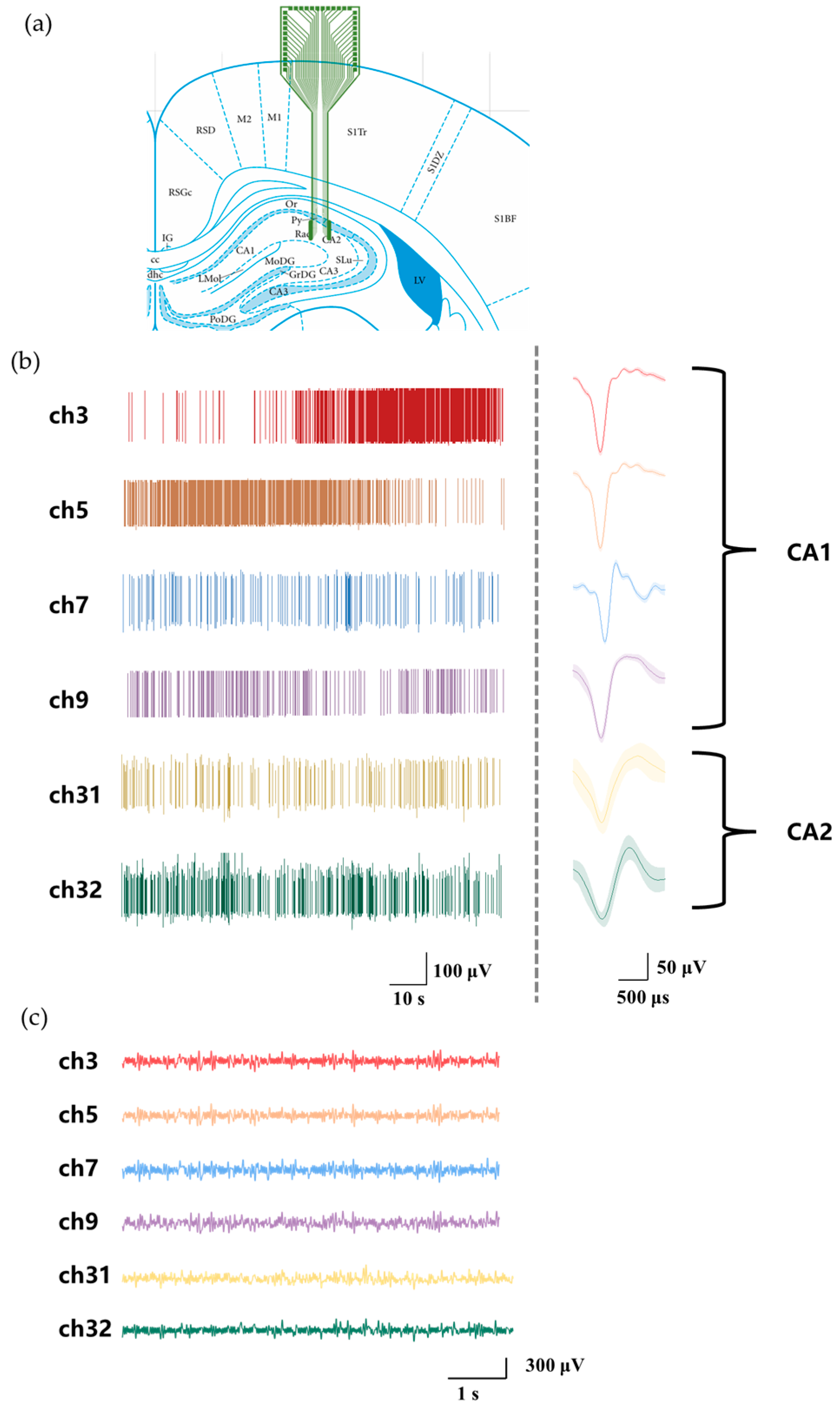
3.3. In Vivo Recording
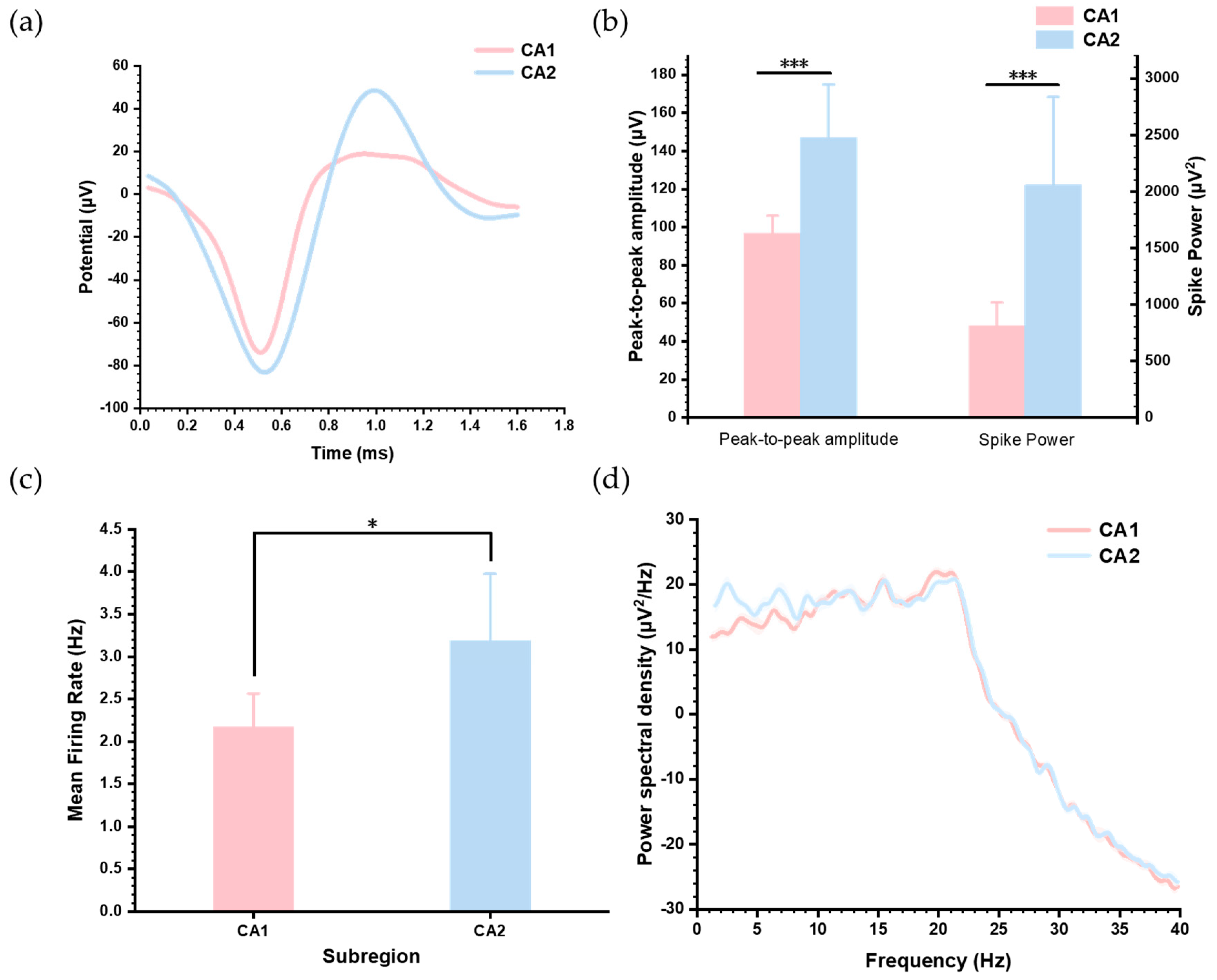
3.4. Distinct Neural Activity Patterns Between Hippocampus CA1 and CA2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buzsáki, G.; Anastassiou, C.A.; Koch, C. The Origin of Extracellular Fields and Currents—EEG, ECoG, LFP and Spikes. Nat. Rev. Neurosci. 2012, 13, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Brandman, D.M.; Cash, S.S.; Hochberg, L.R. Review: Human Intracortical Recording and Neural Decoding for Brain–Computer Interfaces. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1687–1696. [Google Scholar] [CrossRef]
- Jackson, A.; Hall, T.M. Decoding Local Field Potentials for Neural Interfaces. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1705–1714. [Google Scholar] [CrossRef]
- Khodagholy, D.; Gelinas, J.N.; Thesen, T.; Doyle, W.; Devinsky, O.; Malliaras, G.G.; Buzsáki, G. NeuroGrid: Recording Action Potentials from the Surface of the Brain. Nat. Neurosci. 2015, 18, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, Y.; Zhang, S.; Xiao, G.; Wang, M.; Song, Y.; Gao, F.; Li, Z.; Zhuang, P.; Chan, P.; et al. An Integrated System for Synchronous Detection of Neuron Spikes and Dopamine Activities in the Striatum of Parkinson Monkey Brain. J. Neurosci. Methods 2018, 304, 83–91. [Google Scholar] [CrossRef]
- Topalovic, U.; Barclay, S.; Ling, C.; Alzuhair, A.; Yu, W.; Hokhikyan, V.; Chandrakumar, H.; Rozgic, D.; Jiang, W.; Basir-Kazeruni, S.; et al. A Wearable Platform for Closed-Loop Stimulation and Recording of Single-Neuron and Local Field Potential Activity in Freely Moving Humans. Nat. Neurosci. 2023, 26, 517–527. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Q.; Chen, K.; Zhang, H.; Yang, Y.; Zheng, N.; Hong, H. An Ultra-Low-Noise, Low Power and Miniaturized Dual-Channel Wireless Neural Recording Microsystem. Biosensors 2022, 12, 613. [Google Scholar] [CrossRef] [PubMed]
- Topalovic, U.; Aghajan, Z.M.; Villaroman, D.; Hiller, S.; Christov-Moore, L.; Wishard, T.J.; Stangl, M.; Hasulak, N.R.; Inman, C.S.; Fields, T.A.; et al. Wireless Programmable Recording and Stimulation of Deep Brain Activity in Freely Moving Humans. Neuron 2020, 108, 322–334.e9. [Google Scholar] [CrossRef]
- Kwon, Y.W.; Ahn, D.B.; Park, Y.-G.; Kim, E.; Lee, D.H.; Kim, S.-W.; Lee, K.-H.; Kim, W.-Y.; Hong, Y.-M.; Koh, C.S.; et al. Power-Integrated, Wireless Neural Recording Systems on the Cranium Using a Direct Printing Method for Deep-Brain Analysis. Sci. Adv. 2024, 10, eadn3784. [Google Scholar] [CrossRef]
- Ji, B.; Liang, Z.; Yuan, X.; Xu, H.; Wang, M.; Yin, E.; Guo, Z.; Wang, L.; Zhou, Y.; Feng, H.; et al. Recent Advances in Wireless Epicortical and Intracortical Neuronal Recording Systems. Sci. China Inf. Sci. 2022, 65, 140401. [Google Scholar] [CrossRef]
- Maharbiz, M.M.; Muller, R.; Alon, E.; Rabaey, J.M.; Carmena, J.M. Reliable Next-Generation Cortical Interfaces for Chronic Brain–Machine Interfaces and Neuroscience. Proc. IEEE 2017, 105, 73–82. [Google Scholar] [CrossRef]
- Musk, E. Neuralink An Integrated Brain-Machine Interface Platform With Thousands of Channels. J. Med. Internet Res. 2019, 21, e16194. [Google Scholar] [CrossRef]
- Wang, S.; Lopez, C.M.; Garakoui, S.K.; Chun, H.; Salinas, D.G.; Sijbers, W.; Putzeys, J.; Martens, E.; Craninckx, J.; Van Helleputte, N. A Compact Quad-Shank CMOS Neural Probe With 5,120 Addressable Recording Sites and 384 Fully Differential Parallel Channels. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1625–1634. [Google Scholar] [CrossRef]
- Delgado-Restituto, M.; Rodriguez-Perez, A.; Darie, A.; Soto-Sanchez, C.; Fernandez-Jover, E.; Rodriguez-Vazquez, A. System-Level Design of a 64-Channel Low Power Neural Spike Recording Sensor. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Hong, D.; Jang, J.-W.; Lee, K.Y.; Park, B.; Kim, J.-H.; Kim, J.S.; Kim, S. A Wireless ECoG Recording System to Detect Brain Responses to Tactile Stimulation. IEEE Sens. J. 2023, 23, 13692–13701. [Google Scholar] [CrossRef]
- Shin, H.; Byun, J.; Roh, D.; Choi, N.; Shin, H.-S.; Cho, I.-J. Interference-Free, Lightweight Wireless Neural Probe System for Investigating Brain Activity during Natural Competition. Biosens. Bioelectron. 2022, 195, 113665. [Google Scholar] [CrossRef]
- Bilodeau, G.; Gagnon-Turcotte, G.; Gagnon, L.L.; Keramidis, I.; Timofeev, I.; De Koninck, Y.; Ethier, C.; Gosselin, B. A Wireless Electro-Optic Platform for Multimodal Electrophysiology and Optogenetics in Freely Moving Rodents. Front. Neurosci. 2021, 15, 718478. [Google Scholar] [CrossRef]
- Erofeev, A.; Antifeev, I.; Vinokurov, E.; Bezprozvanny, I.; Vlasova, O. An Open-Source Wireless Electrophysiology System for In Vivo Neuronal Activity Recording in the Rodent Brain: 2.0. Sensors 2023, 23, 9735. [Google Scholar] [CrossRef]
- Idogawa, S.; Yamashita, K.; Sanda, R.; Numano, R.; Koida, K.; Kawano, T. A Lightweight, Wireless Bluetooth-Low-Energy Neuronal Recording System for Mice. Sens. Actuators B 2021, 331, 129423. [Google Scholar] [CrossRef]
- Shupe, L.E.; Miles, F.P.; Jones, G.; Yun, R.; Mishler, J.; Rembado, I.; Murphy, R.L.; Perlmutter, S.I.; Fetz, E.E. Neurochip3: An Autonomous Multichannel Bidirectional Brain-Computer Interface for Closed-Loop Activity-Dependent Stimulation. Front. Neurosci. 2021, 15, 718465. [Google Scholar] [CrossRef]
- Walker, J.D.; Pirschel, F.; Sundiang, M.; Niekrasz, M.; MacLean, J.N.; Hatsopoulos, N.G. Chronic Wireless Neural Population Recordings with Common Marmosets. Cell Rep. 2021, 36, 109379. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Santacruz, S.R.; Johnson, B.C.; Alexandrov, G.; Moin, A.; Burghardt, F.L.; Rabaey, J.M.; Carmena, J.M.; Muller, R. A Wireless and Artefact-Free 128-Channel Neuromodulation Device for Closed-Loop Stimulation and Recording in Non-Human Primates. Nat. Biomed. Eng. 2019, 3, 15–26. [Google Scholar] [CrossRef]
- Su, Y.; Routhu, S.; Moon, K.; Lee, S.; Youm, W.; Ozturk, Y. A Wireless 32-Channel Implantable Bidirectional Brain Machine Interface. Sensors 2016, 16, 1582. [Google Scholar] [CrossRef]
- Rajangam, S.; Tseng, P.-H.; Yin, A.; Lehew, G.; Schwarz, D.; Lebedev, M.A.; Nicolelis, M.A.L. Wireless Cortical Brain-Machine Interface for Whole-Body Navigation in Primates. Sci. Rep. 2016, 6, 22170. [Google Scholar] [CrossRef]
- Schwarz, D.A.; Lebedev, M.A.; Hanson, T.L.; Dimitrov, D.F.; Lehew, G.; Meloy, J.; Rajangam, S.; Subramanian, V.; Ifft, P.J.; Li, Z.; et al. Chronic, Wireless Recordings of Large-Scale Brain Activity in Freely Moving Rhesus Monkeys. Nat. Methods 2014, 11, 670–676. [Google Scholar] [CrossRef]
- Simeral, J.D.; Hosman, T.; Saab, J.; Flesher, S.N.; Vilela, M.; Franco, B.; Kelemen, J.N.; Brandman, D.M.; Ciancibello, J.G.; Rezaii, P.G.; et al. Home Use of a Percutaneous Wireless Intracortical Brain-Computer Interface by Individuals With Tetraplegia. IEEE Trans. Biomed. Eng. 2021, 68, 2313–2325. [Google Scholar] [CrossRef]
- Matsushita, K.; Hirata, M.; Suzuki, T.; Ando, H.; Yoshida, T.; Ota, Y.; Sato, F.; Morris, S.; Sugata, H.; Goto, T.; et al. A Fully Implantable Wireless ECoG 128-Channel Recording Device for Human Brain–Machine Interfaces: W-HERBS. Front. Neurosci. 2018, 12, 511. [Google Scholar] [CrossRef] [PubMed]
- Mestais, C.S.; Charvet, G.; Sauter-Starace, F.; Foerster, M.; Ratel, D.; Benabid, A.L. WIMAGINE: Wireless 64-Channel ECoG Recording Implant for Long Term Clinical Applications. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Keramatzadeh, K.; Kiakojouri, A.; Nahvi, M.S.; Khazaei, Y.; Feizi-nejad, A.; Maghami, M.H.; Mohammadi, R.; Sharifshazileh, M.; Nasiri, S.; Akbari Boroumand, F.; et al. Wireless, Miniaturized, Semi-Implantable Electrocorticography Microsystem Validated in Vivo. Sci. Rep. 2020, 10, 21261. [Google Scholar] [CrossRef]
- Jia, Q.; Liu, Y.; Lv, S.; Wang, Y.; Jiao, P.; Xu, W.; Xu, Z.; Wang, M.; Cai, X. Wireless Closed-Loop Deep Brain Stimulation Using Microelectrode Array Probes. J. Zhejiang Univ. Sci. B 2024, 25, 803–823. [Google Scholar] [CrossRef]
- Liu, F.; Wu, Y.; Almarri, N.; Habibollahi, M.; Lancashire, H.T.; Bryson, B.; Greensmith, L.; Jiang, D.; Demosthenous, A. A Fully Implantable Opto-Electro Closed-Loop Neural Interface for Motor Neuron Disease Studies. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 752–765. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, M.; Subei, B.; Richardson, A.G.; Lucas, T.H.; Van der Spiegel, J. The PennBMBI: Design of a General Purpose Wireless Brain-Machine-Brain Interface System. IEEE Trans. Biomed. Circuits Syst. 2015, 9, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Hitti, F.L.; Siegelbaum, S.A. The Hippocampal CA2 Region Is Essential for Social Memory. Nature 2014, 508, 88–92. [Google Scholar] [CrossRef]
- Nasrallah, K.; Therreau, L.; Robert, V.; Huang, A.J.Y.; McHugh, T.J.; Piskorowski, R.A.; Chevaleyre, V. Routing Hippocampal Information Flow through Parvalbumin Interneuron Plasticity in Area CA2. Cell Rep. 2019, 27, 86–98.e3. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Duan, Y.; Liu, Y.; Liu, J.; Luo, J.; Song, Y.; Xu, Z.; Zhang, K.; Shan, J.; Mo, F.; et al. High-Performance Bidirectional Microelectrode Array for Assessing Sevoflurane Anesthesia Effects and In Situ Electrical Stimulation in Deep Brain Regions. ACS Sens. 2024, 9, 2877–2887. [Google Scholar] [CrossRef]
- Shan, J.; Song, Y.; Wang, Y.; Fan, P.; Lu, B.; Luo, J.; Xu, W.; Jing, L.; Mo, F.; Hu, R.; et al. Highly Activated Neuronal Firings Monitored by Implantable Microelectrode Array in the Paraventricular Thalamus of Insomnia Rats. Sensors 2023, 23, 4629. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Y.; Xu, Z.; Zhang, X.; Ruan, W.; Mo, F.; Lu, B.; Fan, P.; Dai, Y.; He, E.; et al. PtNPs/PEDOT:PSS-Modified Microelectrode Arrays for Detection of the Discharge of Head Direction Cells in the Retrosplenial Cortex of Rats under Dissociation between Visual and Vestibular Inputs. Biosensors 2023, 13, 496. [Google Scholar] [CrossRef]
- Prasad, A.; Sanchez, J.C. Quantifying Long-Term Microelectrode Array Functionality Using Chronic in Vivo Impedance Testing. J. Neural Eng. 2012, 9, 026028. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Cai, X.; Xie, Y.; Wang, T.; Cheng, D.; Li, L.; Li, R.; Deng, Y.; Ding, H.; et al. A Wireless, Implantable Optoelectrochemical Probe for Optogenetic Stimulation and Dopamine Detection. Microsyst. Nanoeng. 2020, 6, 64. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, S.; Ren, M.; Zhai, H.-J. Dispersed Carbon Nanotube Paper Tape Loaded Poly(3,4-Ethylenedioxythiophene) for Flexible Supercapacitors with Significantly Enhanced Energy Storage Performance and Deformation Durability. J. Power Sources 2025, 625, 235684. [Google Scholar] [CrossRef]
- Li, G.; Liu, Y.; Chen, Y.; Li, M.; Song, J.; Li, K.; Zhang, Y.; Hu, L.; Qi, X.; Wan, X.; et al. Polyvinyl Alcohol/Polyacrylamide Double-Network Hydrogel-Based Semi-Dry Electrodes for Robust Electroencephalography Recording at Hairy Scalp for Noninvasive Brain–Computer Interfaces. J. Neural Eng. 2023, 20, 026017. [Google Scholar] [CrossRef] [PubMed]




| Performance Parameters | Zhou (WAND) (2019) [22] | Keramatzadeh (2020) [29] | Bilodeau (2021) [17] | Shupe (NC3) (2021) [20] | Shin (2022) [16] | Lee (2023) [15] | This Work |
|---|---|---|---|---|---|---|---|
| Signal | Raw data | ECoG | LFP/spike | Spike | LFP/spike | ECoG | LFP/spike |
| Channels | 96 | 8/36/72 | 32 | 16/32 | 16 | 9/15/24/32 | 32 |
| Sampling rate (kHz) | 1 | 1 | 20 | 5/10/20 | 8 | 30 | 30 |
| Resolution (bits) | 15 | 10 | 16 | 16 | 16 | 16 | 16 |
| Size (mm3) | 36 × 33 × 15 | 29 × 29 × 25 | 28 × 15 × 11 | 56 × 40 × 50 | 24 × 20 × 2 | 18 × 15 × 10 | 24 × 22 × 11 |
| Weight (g) | 7.4 | 27 | 1.7 | 40 | 2.44 | 2.1 | 8.68 |
| Transmit distance | - | - | - | 1 m | ~50 m | - | ~30 m |
| Accuracy | - | - | - | - | 95% (50 m) | 97% | 99.99% |
| Throughput | 250.88 kB/s | <256 kB/s | 179.2 kB/s | - | <128 kB/s | 1.92 MB/s (high mode) | 370 kB/s |
| Consumption | 172 mW | 209 mW | 37 mA | 420/630 mW | 74 mW | 205.4 mA (high mode) | 350 mA |
| Electrode | Commercially available | Gold | Commercially available | - | Pt black-modified | Gold | PtNPs/PEDOT:PSS-enhanced |
| Software | Python | - | - | MATLAB | - | Java | Python, .NEV available |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, P.; Jia, Q.; Li, S.; Shan, J.; Xu, W.; Wang, Y.; Liu, Y.; Wang, M.; Song, Y.; Zhang, Y.; et al. Distinct Neural Activities in Hippocampal Subregions Revealed Using a High-Performance Wireless Microsystem with PtNPs/PEDOT:PSS-Enhanced Microelectrode Arrays. Biosensors 2025, 15, 262. https://doi.org/10.3390/bios15040262
Jiao P, Jia Q, Li S, Shan J, Xu W, Wang Y, Liu Y, Wang M, Song Y, Zhang Y, et al. Distinct Neural Activities in Hippocampal Subregions Revealed Using a High-Performance Wireless Microsystem with PtNPs/PEDOT:PSS-Enhanced Microelectrode Arrays. Biosensors. 2025; 15(4):262. https://doi.org/10.3390/bios15040262
Chicago/Turabian StyleJiao, Peiyao, Qianli Jia, Shuqi Li, Jin Shan, Wei Xu, Yu Wang, Yu Liu, Mingchuan Wang, Yilin Song, Yulian Zhang, and et al. 2025. "Distinct Neural Activities in Hippocampal Subregions Revealed Using a High-Performance Wireless Microsystem with PtNPs/PEDOT:PSS-Enhanced Microelectrode Arrays" Biosensors 15, no. 4: 262. https://doi.org/10.3390/bios15040262
APA StyleJiao, P., Jia, Q., Li, S., Shan, J., Xu, W., Wang, Y., Liu, Y., Wang, M., Song, Y., Zhang, Y., Yu, Y., Wang, M., & Cai, X. (2025). Distinct Neural Activities in Hippocampal Subregions Revealed Using a High-Performance Wireless Microsystem with PtNPs/PEDOT:PSS-Enhanced Microelectrode Arrays. Biosensors, 15(4), 262. https://doi.org/10.3390/bios15040262





