The aadE*-sat4-aphA-3 Gene Cluster of Mycoplasma bovirhinis HAZ141_2 Undergoes Genomic Rearrangements Influencing the Primary Promoter Sequence
Abstract
:1. Introduction
2. Results
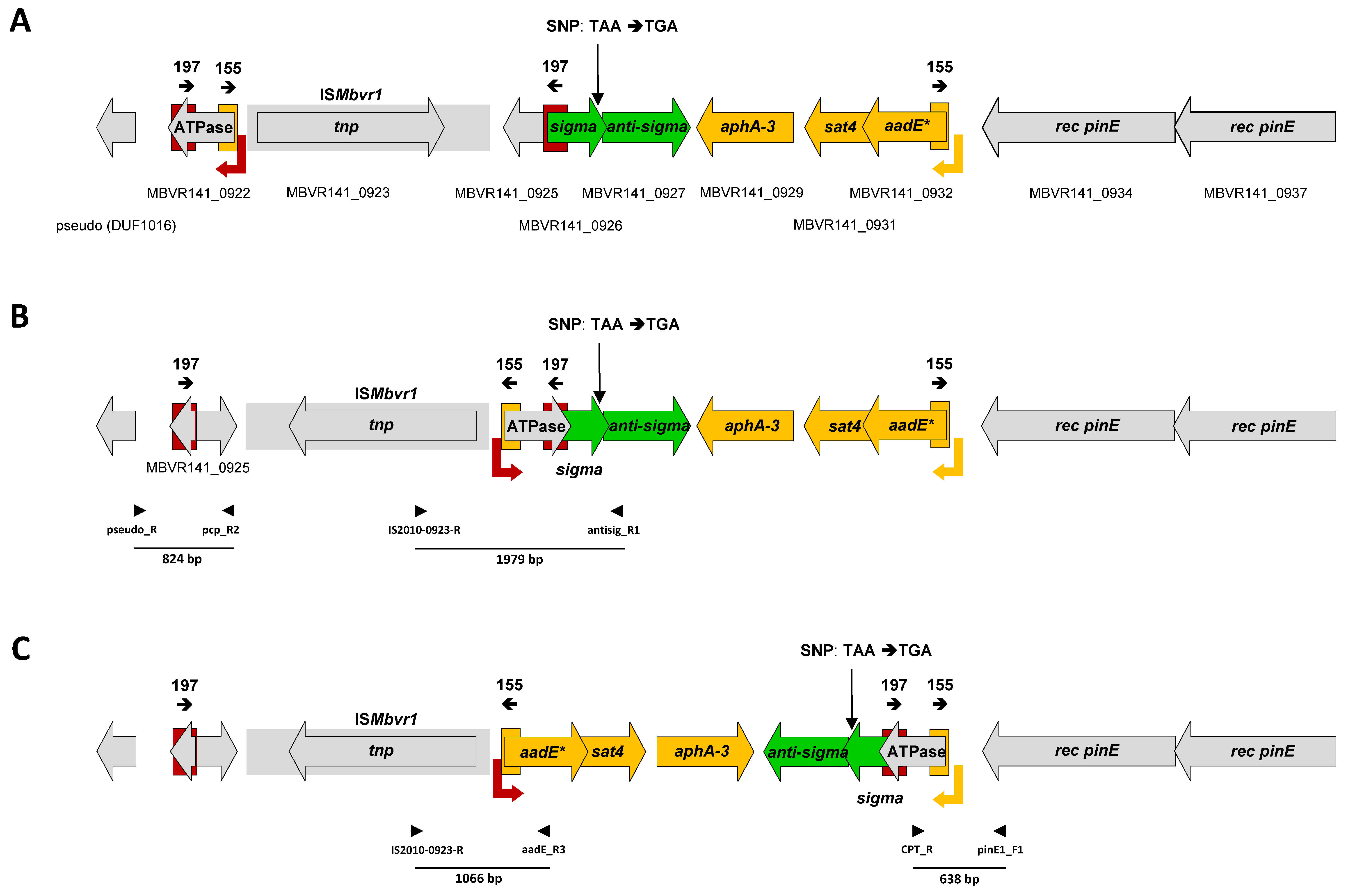
2.1. Different Types of Repetitive Sequences Flank and Overlap with the Primary P* Promoter of the aadE*-sat4-aphA-3 Gene Cluster
2.2. Recombination between IRs Results in Genomic Inversions of the aadE*-sat4-aphA-3 Gene-Containing Regions
2.3. In Vitro Validation of the aadE*-sat4-aphA-3 Expression from Different Promoters
2.4. Competency for Excision of the aadE*-sat4-aphA-3 Gene Cluster
2.5. Stability of M. bovirhinis HAZ141_2 Prophage as Well as aadE*-sat4-aphA-3 Gene Cluster under Non-Selective Conditions
3. Discussion
4. Materials and Methods
4.1. Mycoplasma bovirhinis Growth Condition
4.2. Genomic DNA Extraction and PCR Amplifications
4.3. Enzymes and Antibiotics
4.4. Construction of pACYC_∆tet∆P2vec∆P4vec Derivate Plasmid Vector
4.5. Construction of the P2-Promoterless aphA-3 Gene (∆P2_aphA3) to Test an Influence of P5 Promoter, Regulating Expression of the Cat Gene in pACYC_∆tet∆P2vec∆P4vec
4.6. Construction of the aadE*-sat4-aphA-3 Gene Cluster Derivatives under Different Promoters
4.7. MIC Experiments
4.8. Serial Passage of M. bovirhinis Strain HAZ141-2 under Nonselective Conditions
4.9. Computational Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hata, E.; Nagai, K.; Murakami, K. Complete genome sequence of Mycoplasma bovirhinis strain HAZ141_2 from bovine nasal discharge in Japan. Genome Announc. 2017, 5, e01000-17. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Hao, H.; Zhao, P.; Liu, Y.; Chu, Y. Genome-wide analysis of Mycoplasma bovirhinis GS01 reveals potential virulence factors and phylogenetic relationships. G3 (Bethesda) 2018, 8, 1417–1424. [Google Scholar] [CrossRef] [Green Version]
- Lysnyansky, I.; Borovok, I. A GC-rich prophage-like genomic region of Mycoplasma bovirhinis HAZ141_2 carries a gene cluster encoding resistance to kanamycin and neomycin. Antimicrob. Agents Chemother. 2021, 65, e01010-20. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, R.A.J.; Ayling, R.; McAuliffe, L. Mycoplasma Diseases of Ruminants; CABI: Wallingford, UK, 2009; p. 300. [Google Scholar]
- Vakulenko, S.B.; Mobashery, S. Versatility of aminoglycosides and prospects for their future. Clin. Microbiol. Rev. 2003, 16, 430–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, S.; Wang, Y.; Zhang, Q.; Chen, X.; Shen, Z.; Deng, F.; Wu, C.; Shen, J. Identification of a novel genomic island conferring resistance to multiple aminoglycoside antibiotics in Campylobacter coli. Antimicrob. Agents Chemother. 2012, 56, 5332–5339. [Google Scholar] [CrossRef] [Green Version]
- Derbise, A.; Aubert, S.; El Solh, N. Mapping the regions carrying the three contiguous antibiotic resistance genes aadE, sat4, and aphA-3 in the genomes of staphylococci. Antimicrob. Agents Chemother. 1997, 41, 1024–1032. [Google Scholar] [CrossRef] [Green Version]
- Boerlin, P.; Burnens, A.P.; Frey, J.; Kuhnert, P.; Nicolet, J. Molecular epidemiology and genetic linkage of macrolide and aminoglycoside resistance in Staphylococcus intermedius of canine origin. Vet. Microbiol. 2001, 79, 155–169. [Google Scholar] [CrossRef]
- Werner, G.; Hildebrandt, B.; Witte, W. Aminoglycoside-streptothricin resistance gene cluster aadE-sat4-aphA-3 disseminated among multiresistant isolates of Enterococcus faecium. Antimicrob. Agents Chemother. 2001, 45, 3267–3269. [Google Scholar] [CrossRef] [Green Version]
- Nirdnoy, W.; Mason, C.J.; Guerry, P. Mosaic structure of a multiple-drug-resistant, conjugative plasmid from Campylobacter jejuni. Antimicrob. Agents Chemother. 2005, 49, 2454–2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caillaud, F.; Trieu-Cuot, P.; Carlier, C.; Courvalin, P. Nucleotide sequence of the kanamycin resistance determinant of the pneumococcal transposon Tn1545: Evolutionary relationships and transcriptional analysis of aphA-3 genes. Mol. Gen. Genet. 1987, 207, 509–513. [Google Scholar] [CrossRef]
- Trieu-Cuot, P.; Gerbaud, G.; Lambert, T.; Courvalin, P. In Vivo transfer of genetic information between gram-positive and gram-negative bacteria. EMBO J. 1985, 4, 3583–3587. [Google Scholar] [CrossRef]
- Trieu-Cuot, P.; Klier, A.; Courvalin, P. DNA sequences specifying the transcription of the streptococcal kanamycin resistance gene in Escherichia coli and Bacillus subtilis. Mol. Gen. Genet. 1985, 198, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Hecht, A.; Glasgow, J.; Jaschke, P.R.; Bawazer, L.A.; Munson, M.S.; Cochran, J.R.; Endy, D.; Salit, M. Measurements of translation initiation from all 64 codons in E. coli. Nucleic Acids Res. 2017, 45, 3615–3626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-P.; Chen, S.-J.; Lin, C.-H.; Wang, T.-L.; Wang, C.-C. A single sequence context cannot satisfy all non-AUG initiator codons in yeast. BMC Microbiol. 2010, 10, 188–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [Green Version]
- Stuber, D.; Bujard, H. Organization of transcriptional signals in plasmids pBR322 and pACYC. Proc. Natl. Acad. Sci. USA 1981, 78, 167–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darmon, E.; Leach, D.R.F. Bacterial genome instability. Microbiol. Mol. Biol. Rev. 2014, 78, 1–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, J.R.; Benson, N.; Galitski, T.; Haack, K.; Lawrence, J.G.; Miesel, L. Rearrangements of the bacterial chromosome: Formation and application. In Escherichia coli and Salmonella: Cellular and Molecular Biology, 2nd ed.; Neidhardt, F.C., Curtiss, R.I., Ingraham, J.L., Lin, E.C.C., Low, K.B., Magasanik, B., Riley, M., Schaechter, M., Umbarger, H.E., Eds.; ASM Press: Washington, DC, USA, 1996. [Google Scholar]
- Zimmerman, C.U. Current insights into phase and antigenic variation in Mycoplasmas. In Mollicutes: Molecular Biology and Pathogenesis; Browning, G., Citti, C., Eds.; Caister Academic Press: Norfolk, UK, 2014; pp. 165–196. [Google Scholar]
- Tisza, M.J.; Buck, C.B. A catalog of tens of thousands of viruses from human metagenomes reveals hidden associations with chronic diseases. Proc. Natl. Acad. Sci. USA 2021, 118, e2023202118. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, M.J.; Wiedmann, M.; Boor, K.J. Alternative sigma factors and their roles in bacterial virulence. Microbiol. Mol. Biol. Rev. 2005, 69, 527–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgos, R.; Totten, P.A. MG428 is a novel positive regulator of recombination that triggers mgpB and mgpC gene variation in Mycoplasma genitalium. Mol. Microbiol. 2014, 94, 290–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Puig, S.; Broto, A.; Querol, E.; Piñol, J.; Pich, O.Q. A novel sigma factor reveals a unique regulon controlling cell-specific recombination in Mycoplasma genitalium. Nucleic Acids Res. 2015, 43, 4923–4936. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.M.; Freitag, C.S.; Clements, J.R.; Eisenstein, B.I. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc. Natl. Acad. Sci. USA 1985, 82, 5724–5727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krinos, C.M.; Coyne, M.J.; Weinacht, K.G.; Tzianabos, A.O.; Kasper, D.L.; Comstock, L.E. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature 2001, 414, 555–558. [Google Scholar] [CrossRef]
- Lysnyansky, I.; Ron, Y.; Yogev, D. Juxtraposition of an active promoter to vsp genes via site-specific DNA inversions generates antigenic variation in Mycoplasma bovis. J. Bacteriol. 2001, 183, 5698–5708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhugra, B.; Voelker, L.L.; Zou, N.; Yu, H.; Dybvig, K. Mechanism of antigenic variation in Mycoplasma pulmonis: Interwoven, site-specific DNA inversions. Mol. Microbiol. 1995, 18, 703–714. [Google Scholar] [CrossRef]
- Sitaraman, R.; Dybvig, K. The hsd loci of Mycoplasma pulmonis: Organization, rearrangements and expression of genes. Mol. Microbiol. 1997, 26, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Horino, A.; Sasaki, Y.; Sasaki, T.; Kenri, T. Multiple promoter inversions generate surface antigenic variation in Mycoplasma penetrans. J. Bacteriol. 2003, 185, 231–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Chen, K.; Chan, E.W.; Chen, S. Characterization of the stability and dynamics of Tn6330 in an Escherichia coli strain by nanopore long reads. J. Antimicrob. Chemother. 2019, 74, 1807–1811. [Google Scholar] [CrossRef]
- Johnsen, P.J.; Townsend, J.P.; Bohn, T.; Simonsen, G.S.; Sundsfjord, A.; Nielsen, K.M. Factors affecting the reversal of antimicrobial-drug resistance. Lancet Infect. Dis. 2009, 9, 357–364. [Google Scholar] [CrossRef]
- Li, R.; Chen, K.; Chan, E.W.-C.; Chen, S. Persistence of transferable extended-spectrum-b-lactamase resistance in the absence of antibiotic pressure. Antimicrob. Agents Chemother. 2012, 156, 4703–4706. [Google Scholar]
- Friis, N.F. Some recommendations concerning primary isolation of Mycoplasma suipneumoniae and Mycoplasma flocculare. Nord. Vet. 1975, 27, 333–339. [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Dieases (ESCMID). EUCAST Definitive Document E.DEF 3.1, June 2000: Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin. Microbiol. Infect. 2000, 6, 509–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrazek, J.; Xie, S. Pattern locator: A new tool for finding local sequence patterns in genomic DNA sequences. Bioinformatics 2006, 22, 3099–3100. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Plasmids | MIC (μg/mL) | ||
|---|---|---|---|
| Kn | Nm | NTC | |
| pACYC184 | 1 | 1 | 1 |
| pACYC_P* | 512–1024 | 1024 | 512 |
| pACYC_P*Der | 512–1024 | 1024 | 256 |
| pACYC_P* _∆aadE* | 512–1024 | 1024 | 512 |
| pACYC_P1″_∆P2_aphA3 | 512–1024 | 1024 | NA |
| pACYC_P2_aphA3 | 128 | 128 | NA |
| pACYC_∆P2_aphA3-17 | 256–512 | 256–512 | NA |
| pACYC_∆P2_aphA3-19 | 4 | 4 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lysnyansky, I.; Borovok, I. The aadE*-sat4-aphA-3 Gene Cluster of Mycoplasma bovirhinis HAZ141_2 Undergoes Genomic Rearrangements Influencing the Primary Promoter Sequence. Antibiotics 2021, 10, 1335. https://doi.org/10.3390/antibiotics10111335
Lysnyansky I, Borovok I. The aadE*-sat4-aphA-3 Gene Cluster of Mycoplasma bovirhinis HAZ141_2 Undergoes Genomic Rearrangements Influencing the Primary Promoter Sequence. Antibiotics. 2021; 10(11):1335. https://doi.org/10.3390/antibiotics10111335
Chicago/Turabian StyleLysnyansky, Inna, and Ilya Borovok. 2021. "The aadE*-sat4-aphA-3 Gene Cluster of Mycoplasma bovirhinis HAZ141_2 Undergoes Genomic Rearrangements Influencing the Primary Promoter Sequence" Antibiotics 10, no. 11: 1335. https://doi.org/10.3390/antibiotics10111335






