In Silico Detection of Antimicrobial Resistance Integrons in Salmonella enterica Isolates from Countries of the Andean Community
Abstract
:1. Introduction
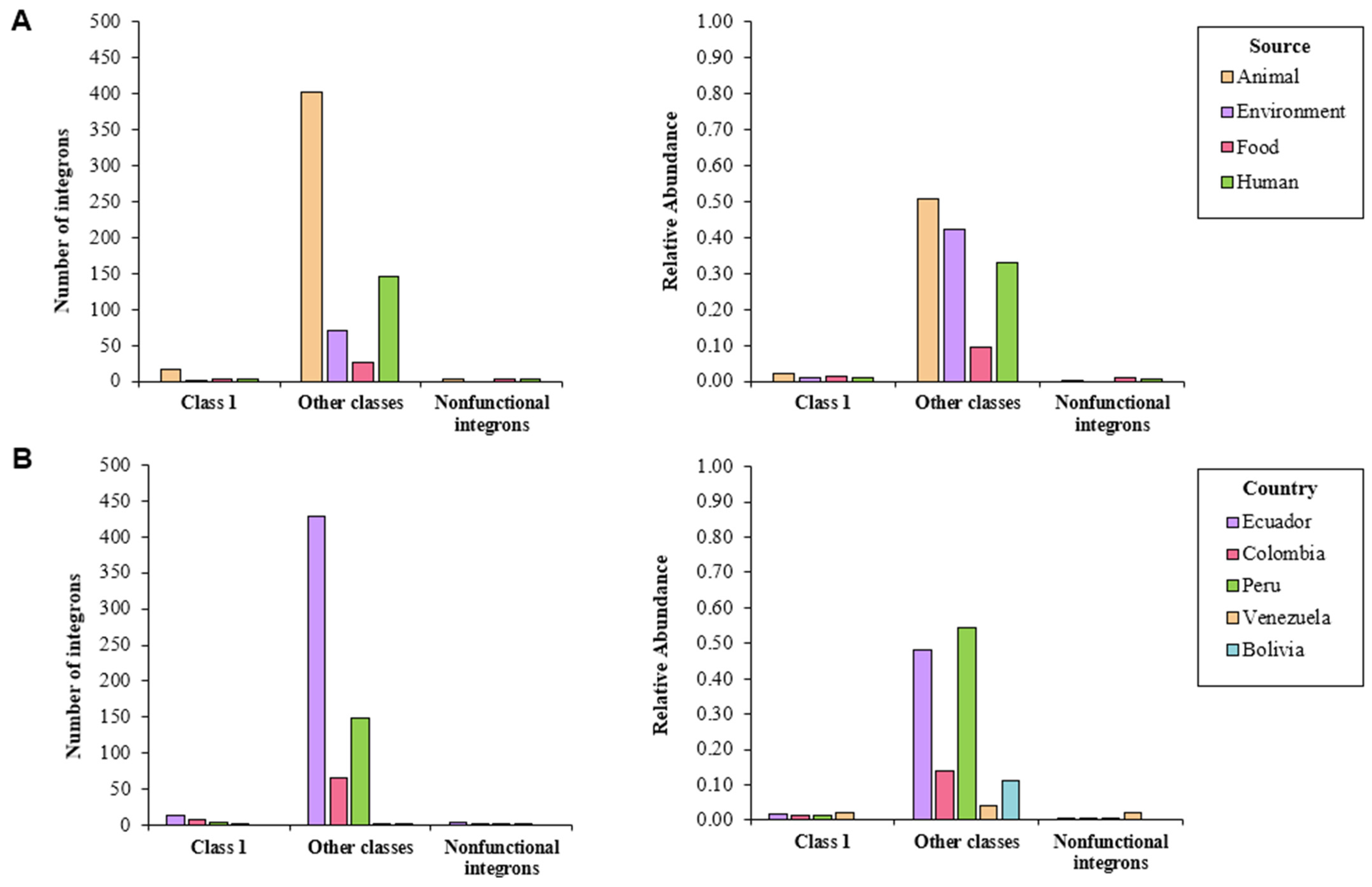
2. Results
2.1. Final Dataset
2.2. Characterization of Detected Integrons
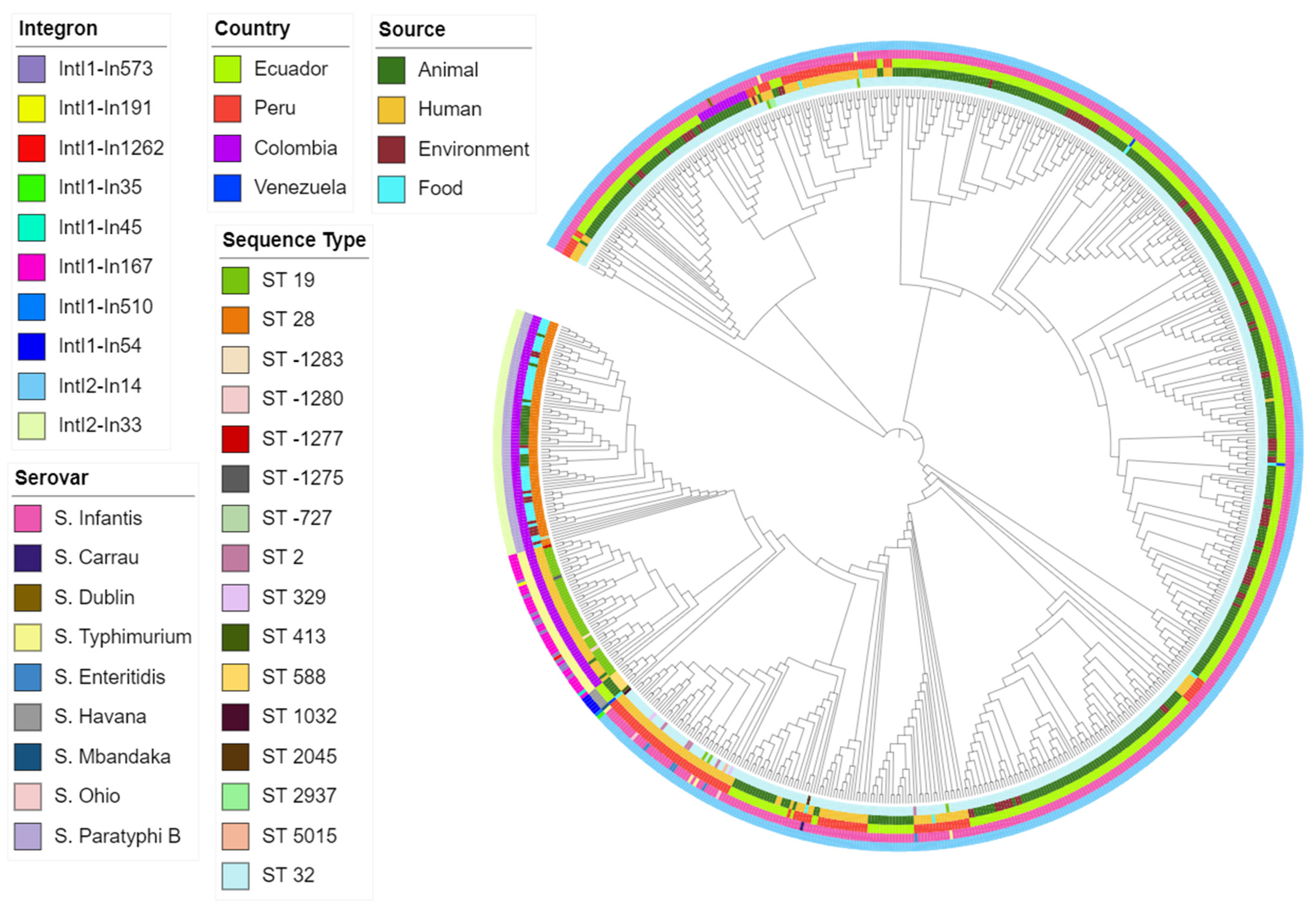
2.3. Differences between MLST Types and Serovars
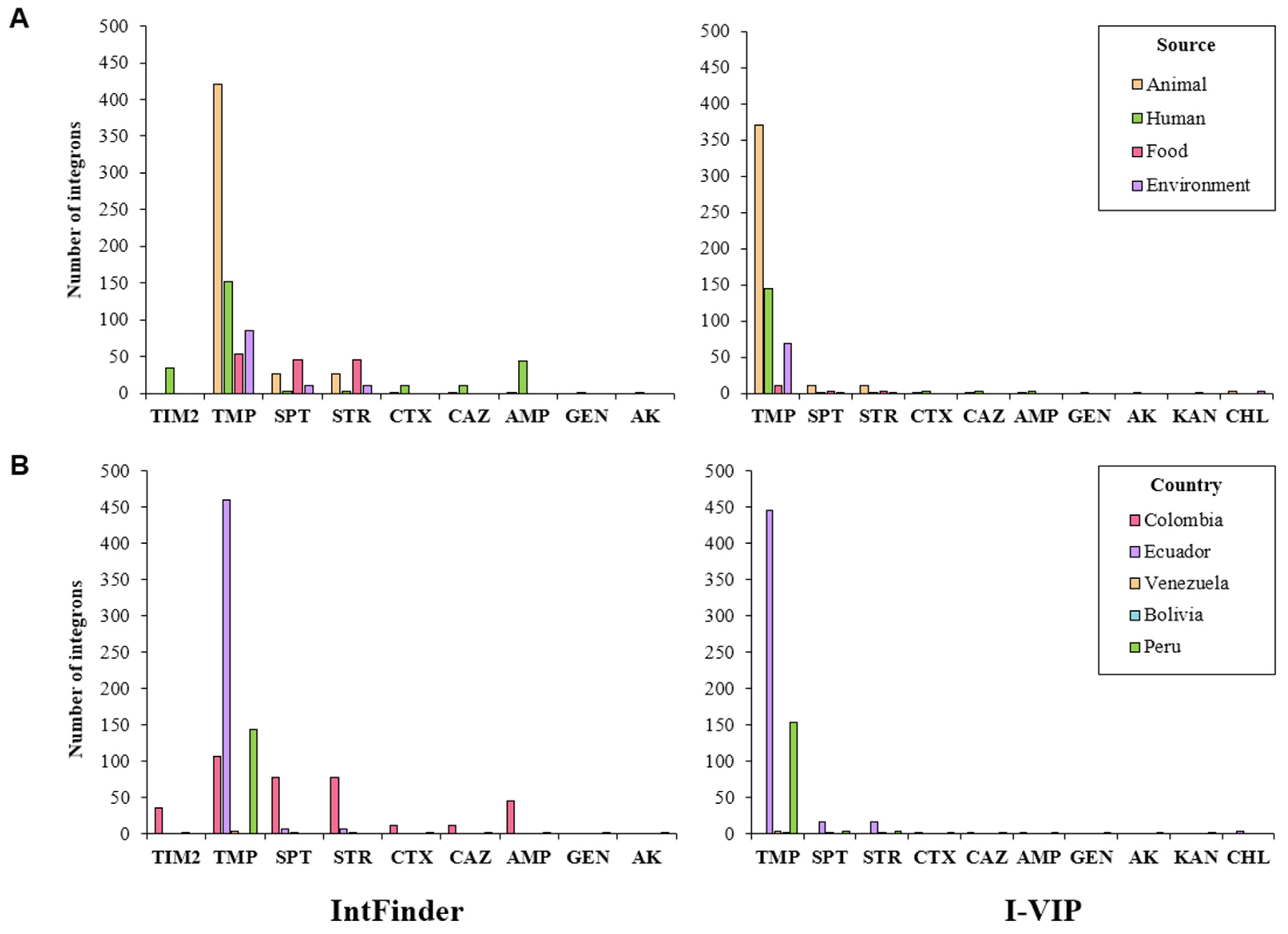
2.4. Association of Integrons and Antibiotic Resistance Genes
3. Discussion
4. Materials and Methods
4.1. Selection of Dataset
4.2. IntFinder 1.0
4.3. I-VIP v1.2
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parisi, A.; Crump, J.A.; Glass, K.; Howden, B.P.; Furuya-Kanamori, L.; Vilkins, S.; Gray, D.J.; Kirk, M.D. Health Outcomes from Multidrug-Resistant Salmonella Infections in High-Income Countries: A Systematic Review and Meta-Analysis. Foodborne Pathog. Dis. 2018, 15, 428–436. [Google Scholar] [CrossRef]
- Vinueza-Burgos, C.; Cevallos, M.; Ron-Garrido, L.; Bertrand, S.; De Zutter, L. Prevalence and Diversity of Salmonella Serotypes in Ecuadorian Broilers at Slaughter age. PLoS ONE 2016, 11, e0159567. [Google Scholar] [CrossRef]
- Ramirez-Hernandez, A.; Carrascal-Camacho, A.K.; Varón-García, A.; Brashears, M.M.; Sanchez-Plata, M.X. Genotypic characterization of antimicrobial resistant Salmonella spp. Strains from three poultry processing plants in Colombia. Foods 2021, 10, 491. [Google Scholar] [CrossRef] [PubMed]
- Vallejos-Sánchez, K.; Tataje-Lavanda, L.; Villanueva-Pérez, D.; Bendezú, J.; Montalván, Á.; Zimic-Peralta, M.; Fernández-Sánchez, M.; Fernández-Díaz, M. Whole-Genome Sequencing of a Salmonella enterica subsp. enterica Serovar Infantis Strain Isolated from Broiler Chicken in Peru. Microbiol. Resour. Announc. 2019, 8, e00826-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donado-Godoy, P.; Bernal, J.F.; Rodríguez, F.; Gomez, Y.; Agarwala, R.; Landsman, D.; Mariño-Ramírez, L. Genome sequences of multidrug-resistant Salmonella enterica serovar Paratyphi B (dT+) and Heidelberg strains from the Colombian poultry chain. Genome Announc. 2015, 3, e01265-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, J.; Fica, A.; Ebensperger, G.; Calfullan, H.; Prat, S.; Fernandez, A.; Alexandre, M.; Heitmann, I. Analysis of molecular epidemiology of Chilean Salmonella enterica serotype enteritidis isolates by pulsed-field gel electrophoresis and bacteriophage typing. J. Clin. Microbiol. 2003, 41, 1617–1622. [Google Scholar] [CrossRef] [Green Version]
- Zankari, E.; Allesøe, R.; Joensen, K.G.; Cavaco, L.M.; Lund, O.; Aarestrup, F.M. PointFinder: A novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J. Antimicrob. Chemother. 2017, 72, 2764–2768. [Google Scholar] [CrossRef] [Green Version]
- Huddleston, J.R. Horizontal gene transfer in the human gastrointestinal tract: Potential spread of antibiotic resistance genes. Infect. Drug Resist. 2014, 7, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 2018, 31, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Che, Y.; Yang, Y.; Xu, X.; Rinda, K.B.; Polz, M.F.; Hanage, W.P.; Zhang, T. Conjugative plasmids interact with insertion sequences to shape the horizontal transfer of antimicrobial resistance genes. Proc. Natl. Acad. Sci. USA 2021, 118, e2008731118. [Google Scholar] [CrossRef]
- Martínez, N.; Mendoza, C.M.; Rodríguez, I.; Soto, S.; Bances, M.; Rodicio, M.R. Detailed structure of integrons and transposons carried by large conjugative plasmids responsible for multidrug resistance in diverse genomic types of Salmonella enterica serovar Brandenburg. J. Antimicrob. Chemother. 2007, 60, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Escudero, J.A.; Loot, C.; Mazel, D. Integrons as Adaptive Devices. In Grand Challenges in Biology and Biotechnology; Springer Science and Business Media B.V.: Cham, Switzerland, 2018; pp. 199–239. [Google Scholar]
- Michael, C.A.; Labbate, M. Gene cassette transcription in a large integron-associated array. BMC Genet. 2010, 11, 82. [Google Scholar] [CrossRef] [Green Version]
- Jacquier, H.; Zaoui, C.; Sanson-Le Pors, M.J.; Mazel, D.; Berçot, B. Translation regulation of integrons gene cassette expression by the attC sites. Mol. Microbiol. 2009, 72, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Bao, X.; Ji, L.; Chen, L.; Liu, J.; Miao, J.; Chen, D.; Bian, H.; Li, Y.; Yu, G. Resistance integrons: Class 1, 2 and 3 integrons. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 45. [Google Scholar] [CrossRef] [Green Version]
- Doublet, B.; Zhao, H.; Chen, W.; Xu, X.; Zhou, X.; Shi, C. Transmissible ST3-IncHI2 Plasmids Are Predominant Carriers of Diverse Complex IS26-Class 1 Integron Arrangements in Multidrug-Resistant Salmonella. Front. Microbiol. 2018, 9, 2492. [Google Scholar] [CrossRef] [Green Version]
- Argüello, H.; Guerra, B.; Rodríguez, I.; Rubio, P.; Carvajal, A. Characterization of Antimicrobial Resistance Determinants and Class 1 and Class 2 Integrons in Salmonella enterica spp., Multidrug-Resistant Isolates from Pigs. Genes 2018, 9, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’mahony, R.; Quinn, T.; Drudy, D.; Walsh, C.; Whyte, P.; Mattar, S.; Fanning, S. Antimicrobial Resistance in Nontyphoidal Salmonella from Food Sources in Colombia: Evidence for an Unusual Plasmid-Localized Class 1 Integron in Serotypes Typhimurium and Anatum. Microb. DRUG Resist. 2006, 12, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Puchol, S.; Riveros, M.; Ruidias, K.; Granda, A.; Ruiz-Roldán, L.; Zapata-Cachay, C.; Ochoa, T.J.; Pons, M.J.; Ruiz, J. Dissemination of a multidrug resistant CTX-M-65 producer Salmonella enterica serovar Infantis clone between marketed chicken meat and children. Int. J. Food Microbiol. 2021, 344, 109109. [Google Scholar] [CrossRef]
- Ricardo Castellanos, L.; van der Graaf-Van Bloois, L.; Donado-Godoy, P.; Veldman, K.; Duarte, F.; Acuña, M.T.; Jarquín, C.; Weill, F.X.; Mevius, D.J.; Wagenaar, J.A.; et al. Antimicrobial resistance in Salmonella enterica serovar paratyphi B variant Java in poultry from Europe and Latin America. Emerg. Infect. Dis. 2020, 26, 1164–1173. [Google Scholar] [CrossRef]
- Johansson, M.H.K.; Bortolaia, V.; Tansirichaiya, S.; Aarestrup, F.M.; Roberts, A.P.; Petersen, T.N. Detection of mobile genetic elements associated with antibiotic resistance in Salmonella enterica using a newly developed web tool: MobileElementFinder. J. Antimicrob. Chemother. 2021, 76, 101–109. [Google Scholar] [CrossRef]
- Singh, T.; Dar, S.A.; Singh, S.; Shekhar, C.; Wani, S.; Akhter, N.; Bashir, N.; Haque, S.; Ahmad, A.; Das, S. Integron mediated antimicrobial resistance in diarrheagenic Escherichia coli in children: In vitro and in silico analysis. Microb. Pathog. 2021, 150. [Google Scholar] [CrossRef] [PubMed]
- Loaiza, K. IntFinder Development and Validation. Available online: https://github.com/kalilamali/Integrons (accessed on 25 September 2021).
- Zhang, A.N.; Li, L.G.; Ma, L.; Gillings, M.R.; Tiedje, J.M.; Zhang, T. Conserved phylogenetic distribution and limited antibiotic resistance of class 1 integrons revealed by assessing the bacterial genome and plasmid collection. Microbiome 2018, 6, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, A.; Soares, M.; Pereira, C.; Leitão, N.; Henriques, I.; Correia, A. INTEGRALL: A database and search engine for integrons, integrases and gene cassettes. Bioinformatics 2009, 25, 1096–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cury, J.; Jové, T.; Touchon, M.; Néron, B.; Rocha, E.P. Identification and analysis of integrons and cassette arrays in bacterial genomes. Nucleic Acids Res. 2016, 44, 4539–4550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, E.; Tietze, E.; Helmuth, R.; Junker, E.; Prager, R.; Schroeter, A.; Rabsch, W.; Fruth, A.; Toboldt, A.; Malorny, B. Clonal dissemination of Salmonella enterica serovar Infantis in Germany. Foodborne Pathog. Dis. 2012, 9, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Doublet, B.; Praud, K.; Nguyen-Ho-Bao, T.; Argudín, M.A.; Bertrand, S.; Butaye, P.; Cloeckaert, A. Extended-spectrum β-lactamase-and ampc β-lactamase-producing D-tartrate-positive Salmonella enterica serovar Paratyphi B from broilers and human patients in Belgium, 2008–2010. J. Antimicrob. Chemother. 2014, 69, 1257–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahada, F.; Amamoto, A.; Chuma, T.; Shirai, A.; Okamoto, K. Antimicrobial susceptibility phenotypes, resistance determinants and DNA fingerprints of Salmonella enterica serotype Typhimurium isolated from bovine in Southern Japan. Int. J. Antimicrob. Agents 2007, 30, 150–156. [Google Scholar] [CrossRef]
- Krauland, M.G.; Marsh, J.W.; Paterson, D.L.; Harrison, L.H. Integron-mediated Multidrug Resistance in a Global Collection of Nontyphoidal Salmonella enterica Isolates. Emerg. Infect. Dis. 2009, 15, 388–396. [Google Scholar] [CrossRef]
- Marchello, C.S.; Carr, S.D.; Crump, J.A. A Systematic Review on Antimicrobial Resistance among Salmonella Typhi Worldwide. Am. J. Trop. Med. Hyg. 2020, 103, 2518–2527. [Google Scholar] [CrossRef]
- Cuong, N.V.; Padungtod, P.; Thwaites, G.; Carrique-Mas, J.J. Antimicrobial usage in animal production: A review of the literature with a focus on low-and middle-income countries. Antibiotics 2018, 7, 75. [Google Scholar] [CrossRef] [Green Version]
- Fuenmayor, Y.; Rodas-González, A.; Carruyo, G.; Hoet, A.E.; Wittum, T.; Narváez-Bravo, C. Salmonella Prevalence and Antimicrobial Drug Resistance in Dual-Purpose Cattle Operations in the Eastern Region of Zulia State, Venezuela. Foodborne Pathog. Dis. 2019, 16, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, S.M.; Medina-Pizzali, M.L.; Salmon-Mulanovich, G.; Larson, A.J.; Pinedo-Bardales, M.; Verastegui, H.; Riberos, M.; Mäusezahl, D. Antimicrobial resistance in humans, animals, water and household environs in rural andean peru: Exploring dissemination pathways through the one health lens. Int. J. Environ. Res. Public Health 2021, 18, 4604. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health (OIE). OEI Annual Report on Antimicrobial Agents Intended for Use in Animals; OIE: Paris, France, 2020. [Google Scholar]
- Sánchez-Osuna, M.; Cortés, P.; Llagostera, M.; Barbé, J.; Erill, I. Exploration into the origins and mobilization of di-hydrofolate reductase genes and the emergence of clinical resistance to trimethoprim. Microb. Genomics 2020, 6, e000440. [Google Scholar] [CrossRef]
- Mejía, L.; Medina, J.L.; Bayas, R.; Salazar, C.S.; Villavicencio, F.; Zapata, S.; Matheu, J.; Wagenaar, J.A.; González-Candelas, F.; Vinueza-Burgos, C. Genomic Epidemiology of Salmonella Infantis in Ecuador: From Poultry Farms to Human Infections. Front. Vet. Sci. 2020, 7, 547891. [Google Scholar] [CrossRef] [PubMed]
- Šeputiene, V.; Povilonis, J.; Ružauskas, M.; Pavilonis, A.; Sužiedeliene, E. Prevalence of trimethoprim resistance genes in Escherichia coli isolates of human and animal origin in Lithuania. J. Med. Microbiol. 2010, 59, 315–322. [Google Scholar] [CrossRef]
- Rajaei, B.; Davar Siadat, S.; Sepehri Rad, N.; Badmasti, F.; Reza Razavi, M.; Reza Aghasadeghi, M.; Saboohi, R.; Rajaei, T.; Moshiri, A.; Nejati, M.; et al. Molecular Detection of Antimicrobial Resistance Gene Cassettes Associated with Class 2 Integron in Salmonella serovars Isolated in Iran. Microbiol. Res. J. 2014, 4, 132–141. [Google Scholar] [CrossRef]
- Stern, A.L.; Van der Verren, S.E.; Kanchugal, S.P.; Näsvall, J.; Gutiérrez-de-Terán, H.; Selmer, M. Structural mechanism of AadA, a dual-specificity aminoglycoside adenylyl transferase from Salmonella enterica. J. Biol. Chem. 2018, 293, 11481–11490. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Lai, J.; Wang, Y.; Liu, S.; Li, Y.; Liu, K.; Shen, J.; Wu, C. Prevalence and characterization of Salmonella species isolated from pigs, ducks and chickens in Sichuan Province, China. Int. J. Food Microbiol. 2013, 163, 14–18. [Google Scholar] [CrossRef]
- Molla, B.; Miko, A.; Pries, K.; Hildebrandt, G.; Kleer, J.; Schroeter, A.; Helmuth, R. Class 1 integrons and resistance gene cassettes among multidrug resistant Salmonella serovars isolated from slaughter animals and foods of animal origin in Ethiopia. Acta Trop. 2007, 103, 142–149. [Google Scholar] [CrossRef]
- Gassama-Sow, A.; Diallo, M.H.; Boye, C.S.; Garin, B.; Sire, J.M.; Sow, A.I.; Aïdara-Kane, A. Class 2 integron-associated antibiotic resistance in Shigella sonnei isolates in Dakar, Senegal. Int. J. Antimicrob. Agents 2006, 27, 267–270. [Google Scholar] [CrossRef]
- Opintan, J.A.; Newman, M.J.; Nsiah-Poodoh, O.A.; Okeke, I.N. Vibrio cholerae O1 from Accra, Ghana carrying a class 2 integron and the SXT element. J. Antimicrob. Chemother. 2008, 62, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, X.; Zhang, J.; Yang, S.; Zhang, S.; Chen, M.; Xue, L.; Ding, Y.; Zeng, H.; Gu, Q.; et al. Molecular characterisation of antimicrobial resistance determinants and class 1 integrons of Salmonella enterica subsp. enterica serotype Enteritidis strains from retail food in China. Food Control. 2021, 128, 108191. [Google Scholar] [CrossRef]
- Madec, J.Y.; Doublet, B.; Ponsin, C.; Cloeckaert, A.; Haenni, M. Extended-spectrum β-lactamase blaCTX-M-1 gene carried on an IncI1 plasmid in multidrug-resistant Salmonella enterica serovar Typhimurium DT104 in cattle in France. J. Antimicrob. Chemother. 2011, 66, 942–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danel, F.; Hall, L.M.C.; Gur, D.; Livermore, D.M. OXA-15, an Extended-Spectrum Variant of OXA-2-Lactamase, Isolated from a Pseudomonas aeruginosa Strain. Antimicrob. Agents Chemother. 1997, 41, 785–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monte, D.F.M.; Sellera, F.P.; Lopes, R.; Keelara, S.; Landgraf, M.; Greene, S.; Fedorka-Cray, P.J.; Thakur, S. Class 1 integron-borne cassettes harboring blaCARB-2 gene in multidrug-resistant and virulent Salmonella typhimurium ST19 strains recovered from clinical human stool samples, United States. PLoS ONE 2020, 15, e0240978. [Google Scholar] [CrossRef]
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, B.M.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [Green Version]
- Redding, L.E.; Cubas-Delgado, F.; Sammel, M.D.; Smith, G.; Galligan, D.T.; Levy, M.Z.; Hennessy, S. The use of antibiotics on small dairy farms in rural Peru. Prev. Vet. Med. 2014, 113, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Arenas, N.E.; Abril, D.A.; Valencia, P.; Khandige, S.; Soto, C.Y.; Moreno-Melo, V. Screening food-borne and zoonotic pathogens associated with livestock practices in the Sumapaz region, Cundinamarca, Colombia. Trop. Anim. Health Prod. 2017, 49, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Moser, K.A.; Zhang, L.; Spicknall, I.; Braykov, N.P.; Levy, K.; Marrs, C.F.; Foxman, B.; Trueba, G.; Cevallos, W.; Goldstick, J.; et al. The Role of Mobile Genetic Elements in the Spread of Antimicrobial-Resistant Escherichia coli from Chickens to Humans in Small-Scale Production Poultry Operations in Rural Ecuador. Am. J. Epidemiol. 2018, 187, 558–567. [Google Scholar] [CrossRef]
- Donado-Godoy, P.; Clavijo, V.; León, M.; Arevalo, A.; Castellanos, R.; Bernal, J.; Tafur, M.A.; Ovalle, M.; Alali, W.Q.; Hume, M.; et al. Counts, serovars, and antimicrobial resistance phenotypes of Salmonella on raw chicken meat at retail in Colombia. J. Food Prot. 2014, 77, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Abbasoglu, D.; Akcelık, M. Phenotypic and genetic characterization of multidrug-resistant Salmonella Infantis strains isolated from broiler chicken meats in Turkey. Biologia 2011, 66, 406–410. [Google Scholar] [CrossRef]
- Paula Herrera-Sánchez, M.; Rodríguez-Hernández, R.; Schroniltgen Rondón-Barragán, I. Molecular characterization of antimicrobial resistance and enterobacterial repetitive intergenic consensus-PCR as a molecular typing tool for Salmonella spp. isolated from poultry and humans. Vet. World 2020, 13, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.R.; Kingsley, R.A. Evolution of Salmonella within Hosts. Trends Microbiol. 2018, 26, 986–998. [Google Scholar] [CrossRef] [Green Version]
- Novais, Â.; Baquero, F.; Machado, E.; Cantón, R.; Peixe, L.; Coque, T.M. International spread and persistence of TEM-24 is caused by the confluence of highly penetrating Enterobacteriaceae clones and an IncA/C2 plasmid containing Tn1696::Tn1 and IS5075-Tn21. Antimicrob. Agents Chemother. 2010, 54, 825–834. [Google Scholar] [CrossRef] [Green Version]
- Franco, A.; Leekitcharoenphon, P.; Feltrin, F.; Alba, P.; Cordaro, G.; Iurescia, M.; Tolli, R.; D’Incau, M.; Staffolani, M.; Di Giannatale, E.; et al. Emergence of a Clonal Lineage of Multidrug-Resistant ESBL-Producing Salmonella Infantis Transmitted from Broilers and Broiler Meat to Humans in Italy between 2011 and 2014. PLoS ONE 2015, 10, e0144802. [Google Scholar] [CrossRef] [Green Version]
- Bogomazova, A.N.; Gordeeva, V.D.; Krylova, E.V.; Soltynskaya, I.V.; Davydova, E.E.; Ivanova, O.E.; Komarov, A.A. Mega-plasmid found worldwide confers multiple antimicrobial resistance in Salmonella Infantis of broiler origin in Russia. Int. J. Food Microbiol. 2020, 319, 108497. [Google Scholar] [CrossRef]
- Silva, C.; Betancor, L.; García, C.; Astocondor, L.; Hinostroza, N.; Bisio, J.; Rivera, J.; Perezgasga, L.; Escanda, V.P.; Yim, L.; et al. Characterization of Salmonella enterica isolates causing bacteremia in Lima, Peru, using multiple typing methods. PLoS ONE 2017, 12, e0189946. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Clausen, P.T.L.C.; Aarestrup, F.M.; Lund, O. Rapid and precise alignment of raw reads against redundant databases with KMA. BMC Bioinform. 2018, 19. [Google Scholar] [CrossRef]
- Nawrocki, E.P.; Eddy, S.R. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics 2013, 29, 2933–2935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, R.M.; Brookes, D.E.; Stokes, H.W. Site-specific insertion of genes into integrons: Role of the 59~base element and determination of the recombination cross-over point. Mol. Microbiol. 1991, 5, 1941–1959. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.G.; Xia, Y.; Zhang, T. Co-occurrence of antibiotic and metal resistance genes revealed in complete genome collection. ISME J. 2017, 11, 651–662. [Google Scholar] [CrossRef] [PubMed]



| Integrase | Gene Cassettes in the Variable Region | Associated Resistance Pattern | Name | Length (bp) | Accession Number |
|---|---|---|---|---|---|
| IntI1 | dfrA17, aadA5 | TMP–SPT–STR | In54 | 474–789 | AF220757 |
| IntI1 | blaCARB-2 | AMP–TIM2 | In167 | 866 | AF221899 |
| IntI1 | dfrA29, blaOXA-2 | TMP–AMP–CTX–CAZ | In573 | 472–828 | AM237806 |
| IntI1 | dfrA12-aadA2 | TMP–SPT–STR | In1262 | 497–791 | KX710093 |
| IntI1 | dfrA14 | TMP | In191 | 473 | HF545433 |
| IntI1 | aac(6’)-Ib-cr-aac(6’)-Ib3-blaOXA-2-blaOXA-34 | GEN–AK–AMP–CTX–CAZ | In35 | 587–554–827–770 | DQ139276 |
| IntI1 | aadA12 | TMP | In45 | 791 | FJ460240 |
| IntI1 | dfrA12-aadA2 | TMP–SPT–STR | In510 | 498–792 | EU853659 |
| IntI2 | dfrA14 | TMP | In14 | 483 | EU780012 |
| IntI2 | dfrA1, aadA1 | TMP–SPT–STR | In33 | 475 | FJ914220 |
| Integrase | Gene Cassettes in the Variable Region | Associated Resistance Pattern | Type |
|---|---|---|---|
| IntI1 | aac(6)-I-OXA-2 | GEN–AK–AMP–CTX–CAZ | Class 1 |
| IntI1 | OXA-2 | AMP–CTX–CAZ | Class 1 |
| IntI1 | aph(3)-I-aph(6)-I-dfrA1-aadA | KAN–TMP–SPT–STR | Class 1 |
| IntI1 | dfrA12-aadA | TMP–SPT–STR | Class 1 |
| IntI1 | dfrA17-aadA | TMP–SPT–STR | Class 1 |
| IntI1 | dfrA12-aadA-cmlA-aadA | TMP–SPT–STR–CHL | Class 1 |
| IntI1 | aadA | SPT–STR | Class 1 |
| Other | dfrA14 | TMP | Other integrases |
| Other | dfrA14-dfrA14 | TMP | Other integrases |
| No integrase | aadA | SPT–STR | Nonfunctional |
| No integrase | dfrA1-aadA | TMP–SPT–STR | Nonfunctional |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Elizalde, L.; Ortega-Paredes, D.; Loaiza, K.; Fernández-Moreira, E.; Larrea-Álvarez, M. In Silico Detection of Antimicrobial Resistance Integrons in Salmonella enterica Isolates from Countries of the Andean Community. Antibiotics 2021, 10, 1388. https://doi.org/10.3390/antibiotics10111388
Torres-Elizalde L, Ortega-Paredes D, Loaiza K, Fernández-Moreira E, Larrea-Álvarez M. In Silico Detection of Antimicrobial Resistance Integrons in Salmonella enterica Isolates from Countries of the Andean Community. Antibiotics. 2021; 10(11):1388. https://doi.org/10.3390/antibiotics10111388
Chicago/Turabian StyleTorres-Elizalde, Lilibeth, David Ortega-Paredes, Karen Loaiza, Esteban Fernández-Moreira, and Marco Larrea-Álvarez. 2021. "In Silico Detection of Antimicrobial Resistance Integrons in Salmonella enterica Isolates from Countries of the Andean Community" Antibiotics 10, no. 11: 1388. https://doi.org/10.3390/antibiotics10111388






