Does Vancomycin Wrapping in Anterior Cruciate Ligament Reconstruction Affect Tenocyte Activity In Vitro?
Abstract
:1. Introduction
2. Results
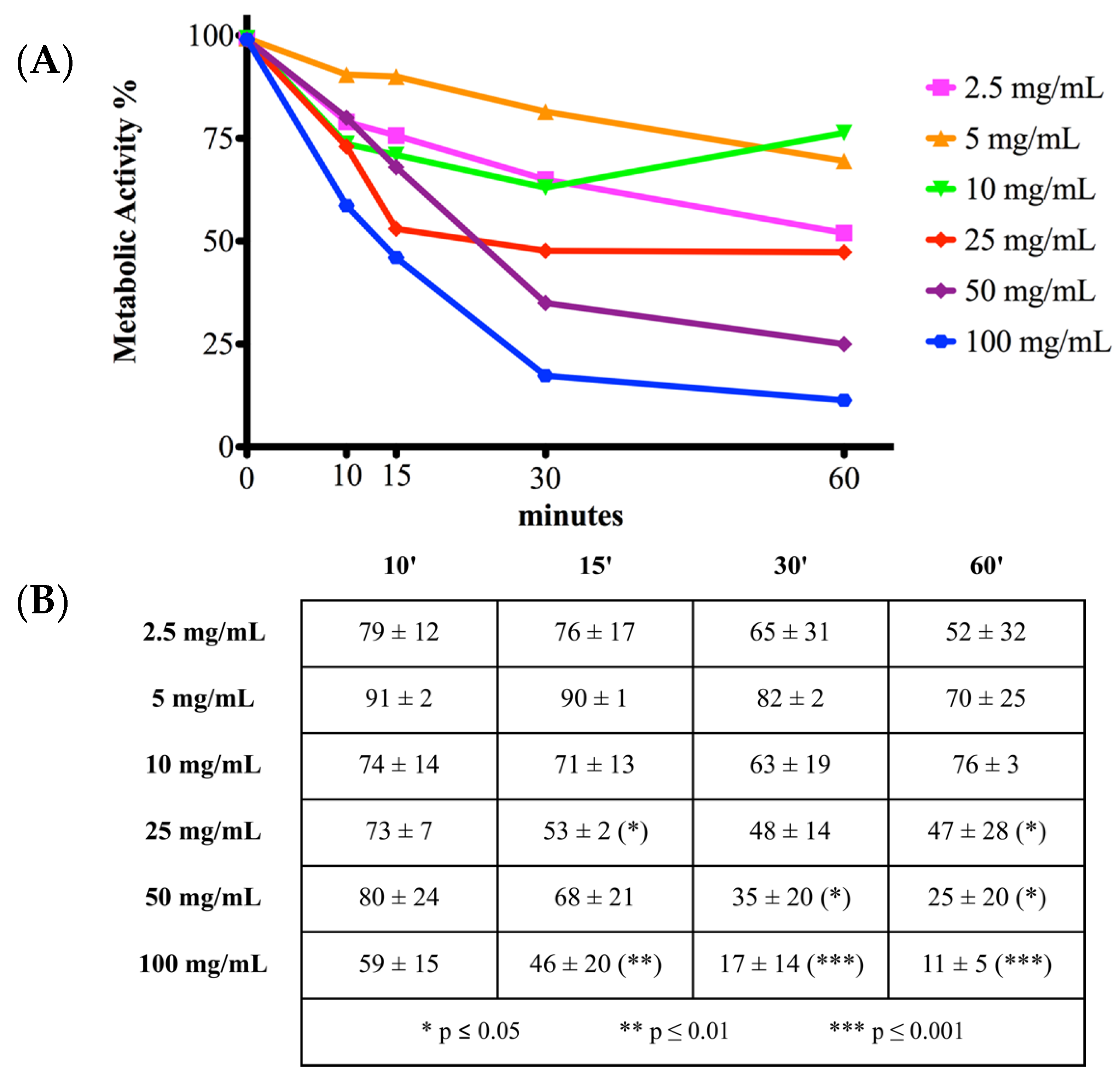
2.1. Cell Metabolic Activity
2.2. Cell Viability and Toxicity
2.3. Cell Apoptosis
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of Human Tendon-Derived Primary Cells
4.2. Vancomycin Treatment
4.3. Cell Activity
4.4. Cell Viability and Toxicity
4.5. Cell Apoptosis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, N. International Epidemiology of Anterior Cruciate Ligament Injuries. Orthop. Res. Online J. 2018, 1, 94–96. [Google Scholar] [CrossRef]
- Parada, S.A.; Grassbaugh, J.A.; Devine, J.G.; Arrington, E.D. Instrumentation-specific infection after anterior cruciate ligament reconstruction. Sports Health 2009, 1, 481–485. [Google Scholar] [CrossRef] [Green Version]
- Vadala, G.; Petrillo, S.; Buschini, F.; Papalia, R.; Denaro, V. Posterolateral bundle reconstruction of the anterior cruciate ligament to restore rotational stability of the knee. J. Biol. Regul. Homeost Agents 2017, 31, 153–158. [Google Scholar]
- Calvo, R.; Figueroa, D.; Anastasiadis, Z.; Vaisman, A.; Olid, A.; Gili, F.; Valderrama, J.J.; De La Fuente, P. Septic arthritis in ACL reconstruction surgery with hamstring autografts. Eleven years of experience. Knee 2014, 21, 717–720. [Google Scholar] [CrossRef]
- Papalia, R.; Moro, L.; Franceschi, F.; Albo, E.; D’Adamio, S.; Di Martino, A.; Vadala, G.; Faldini, C.; Denaro, V. Endothelial dysfunction and tendinopathy: How far have we come? Musculoskelet. Surg. 2013, 97, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Schuster, P.; Schulz, M.; Immendoerfer, M.; Mayer, P.; Schlumberger, M.; Richter, J. Septic Arthritis After Arthroscopic Anterior Cruciate Ligament Reconstruction: Evaluation of an Arthroscopic Graft-Retaining Treatment Protocol. Am. J. Sports Med. 2015, 43, 3005–3012. [Google Scholar] [CrossRef] [PubMed]
- Stucken, C.; Garras, D.N.; Shaner, J.L.; Cohen, S.B. Infections in anterior cruciate ligament reconstruction. Sports Health 2013, 5, 553–557. [Google Scholar] [CrossRef] [Green Version]
- Torres-Claramunt, R.; Pelfort, X.; Erquicia, J.; Gil-Gonzalez, S.; Gelber, P.E.; Puig, L.; Monllau, J.C. Knee joint infection after ACL reconstruction: Prevalence, management and functional outcomes. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 2844–2849. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lee, Y.H.; Siebold, R. Recommendations for the management of septic arthritis after ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- Plante, M.J.; Li, X.; Scully, G.; Brown, M.A.; Busconi, B.D.; DeAngelis, N.A. Evaluation of sterilization methods following contamination of hamstring autograft during anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Schollin-Borg, M.; Michaelsson, K.; Rahme, H. Presentation, outcome, and cause of septic arthritis after anterior cruciate ligament reconstruction: A case control study. Arthroscopy 2003, 19, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Schuster, P.; Schlumberger, M.; Mayer, P.; Eichinger, M.; Gesslein, M.; Richter, J. Soaking of autografts in vancomycin is highly effective in preventing postoperative septic arthritis after revision anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1154–1158. [Google Scholar] [CrossRef]
- Perez-Prieto, D.; Portillo, M.E.; Torres-Claramunt, R.; Pelfort, X.; Hinarejos, P.; Monllau, J.C. Contamination occurs during ACL graft harvesting and manipulation, but it can be easily eradicated. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 558–562. [Google Scholar] [CrossRef]
- Badran, M.A.; Moemen, D.M. Hamstring graft bacterial contamination during anterior cruciate ligament reconstruction: Clinical and microbiological study. Int. Orthop. 2016, 40, 1899–1903. [Google Scholar] [CrossRef]
- Vertullo, C.J.; Quick, M.; Jones, A.; Grayson, J.E. A surgical technique using presoaked vancomycin hamstring grafts to decrease the risk of infection after anterior cruciate ligament reconstruction. Arthroscopy 2012, 28, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, K.; Karlsson, J. Local vancomycin in ACL reconstruction: A modern rationale (2016) for morbidity prevention and patient safety. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2721–2723. [Google Scholar] [CrossRef]
- Phegan, M.; Grayson, J.E.; Vertullo, C.J. No infections in 1300 anterior cruciate ligament reconstructions with vancomycin pre-soaking of hamstring grafts. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2729–2735. [Google Scholar] [CrossRef] [PubMed]
- Perez-Prieto, D.; Torres-Claramunt, R.; Gelber, P.E.; Shehata, T.M.A.; Pelfort, X.; Monllau, J.C. Autograft soaking in vancomycin reduces the risk of infection after anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2724–2728. [Google Scholar] [CrossRef]
- Kirst, H.A.; Thompson, D.G.; Nicas, T.I. Historical yearly usage of vancomycin. Antimicrob. Agents Chemother. 1998, 42, 1303–1304. [Google Scholar] [CrossRef] [Green Version]
- Brophy, R.H.; Wright, R.W.; Huston, L.J.; Nwosu, S.K.; Group, M.K.; Spindler, K.P. Factors associated with infection following anterior cruciate ligament reconstruction. J. Bone Jt. Surg. Am. 2015, 97, 450–454. [Google Scholar] [CrossRef] [Green Version]
- Antoci, V., Jr.; Adams, C.S.; Hickok, N.J.; Shapiro, I.M.; Parvizi, J. Antibiotics for local delivery systems cause skeletal cell toxicity in vitro. Clin. Orthop. Relat. Res. 2007, 462, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Hantes, M.E.; Basdekis, G.K.; Varitimidis, S.E.; Giotikas, D.; Petinaki, E.; Malizos, K.N. Autograft contamination during preparation for anterior cruciate ligament reconstruction. J. Bone Jt. Surg. Am. 2008, 90, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Grayson, J.E.; Grant, G.D.; Dukie, S.; Vertullo, C.J. The in vitro elution characteristics of vancomycin from tendons. Clin. Orthop. Relat. Res. 2011, 469, 2948–2952. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Offerhaus, C.; Balke, M.; Hente, J.; Gehling, M.; Blendl, S.; Hoher, J. Vancomycin pre-soaking of the graft reduces postoperative infection rate without increasing risk of graft failure and arthrofibrosis in ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 3014–3021. [Google Scholar] [CrossRef] [PubMed]
- Naendrup, J.H.; Marche, B.; de Sa, D.; Koenen, P.; Otchwemah, R.; Wafaisade, A.; Pfeiffer, T.R. Vancomycin-soaking of the graft reduces the incidence of septic arthritis following ACL reconstruction: Results of a systematic review and meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1005–1013. [Google Scholar] [CrossRef]
- Aslantürk, O.S. In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages. Genotoxicity-A Predict. Risk Our Actual World 2018, 2, 64–80. [Google Scholar] [CrossRef] [Green Version]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Sittampalam, G.S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C.P., Baell, J., Bejcek, B., Caaveiro, J.M.M., Chung, T.D.Y., et al., Eds.; Bethesda: Bethesda, MD, USA, 2004. [Google Scholar]
- Shaw, K.A.; Eichinger, J.K.; Nadig, N.; Parada, S.A. In Vitro Effect of Vancomycin on the Viability of Articular Chondrocytes. J. Orthop. Trauma 2018, 32, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, H.R.; Jamei Moayedi, R.; Shokrgozar, M.A.; Dehghan, M.M.; Mokhtari, T. Evaluation of delayed effect of intra-articular injection of cefazolin, gentamicin and vancomycin on articular cartilage: An experimental study in rabbit. J. Res. Orthop. Sci. 2014, 1. [Google Scholar]
- Rathbone, C.R.; Cross, J.D.; Brown, K.V.; Murray, C.K.; Wenke, J.C. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J. Orthop. Res. 2011, 29, 1070–1074. [Google Scholar] [CrossRef]
- Edin, M.L.; Miclau, T.; Lester, G.E.; Lindsey, R.W.; Dahners, L.E. Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin. Orthop. Relat. Res. 1996, 333, 245–251. [Google Scholar] [CrossRef]
- Chiu, C.H.; Lei, K.F.; Chan, Y.S.; Ueng, S.W.N.; Chen, A.C. Real-time detection of antibiotics cytotoxicity in rabbit periosteal cells using microfluidic devices with comparison to conventional culture assays. BMC Musculoskelet. Disord. 2019, 20, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.X.; Bravo, D.; Buza, J.; Kirsch, T.; Kennedy, O.; Rokito, A.; Zuckerman, J.D.; Virk, M.S. Topical vancomycin and its effect on survival and migration of osteoblasts, fibroblasts, and myoblasts: An in vitro study. J. Orthop. 2018, 15, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Schuttler, K.F.; Scharm, A.; Stein, T.; Heyse, T.J.; Lohoff, M.; Sommer, F.; Spiess-Naumann, A.; Efe, T. Biomechanical and microbiological effects of local vancomycin in anterior cruciate ligament (ACL) reconstruction: A porcine tendon model. Arch. Orthop. Trauma Surg. 2019, 139, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Pouzaud, F.; Bernard-Beaubois, K.; Thevenin, M.; Warnet, J.M.; Hayem, G.; Rat, P. In vitro discrimination of fluoroquinolones toxicity on tendon cells: Involvement of oxidative stress. J. Pharmacol. Exp. Ther. 2004, 308, 394–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Patient | Sex | Age | Comorbidities |
|---|---|---|---|
| 1 | M | 38 | None |
| 2 | M | 19 | None |
| 3 | M | 37 | None |
| 4 | F | 51 | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papalia, R.; Cicione, C.; Russo, F.; Ambrosio, L.; Di Giacomo, G.; Vadalà, G.; Denaro, V. Does Vancomycin Wrapping in Anterior Cruciate Ligament Reconstruction Affect Tenocyte Activity In Vitro? Antibiotics 2021, 10, 1087. https://doi.org/10.3390/antibiotics10091087
Papalia R, Cicione C, Russo F, Ambrosio L, Di Giacomo G, Vadalà G, Denaro V. Does Vancomycin Wrapping in Anterior Cruciate Ligament Reconstruction Affect Tenocyte Activity In Vitro? Antibiotics. 2021; 10(9):1087. https://doi.org/10.3390/antibiotics10091087
Chicago/Turabian StylePapalia, Rocco, Claudia Cicione, Fabrizio Russo, Luca Ambrosio, Giuseppina Di Giacomo, Gianluca Vadalà, and Vincenzo Denaro. 2021. "Does Vancomycin Wrapping in Anterior Cruciate Ligament Reconstruction Affect Tenocyte Activity In Vitro?" Antibiotics 10, no. 9: 1087. https://doi.org/10.3390/antibiotics10091087
APA StylePapalia, R., Cicione, C., Russo, F., Ambrosio, L., Di Giacomo, G., Vadalà, G., & Denaro, V. (2021). Does Vancomycin Wrapping in Anterior Cruciate Ligament Reconstruction Affect Tenocyte Activity In Vitro? Antibiotics, 10(9), 1087. https://doi.org/10.3390/antibiotics10091087









