Antimicrobial Resistance and Its Drivers—A Review
Abstract
:1. Introduction
2. Antimicrobial Resistance: A Worldwide Public Health Emergency
3. Antimicrobial Resistance: Potential Threats
4. Mechanisms and Drivers Contributing to the Spread of AMR
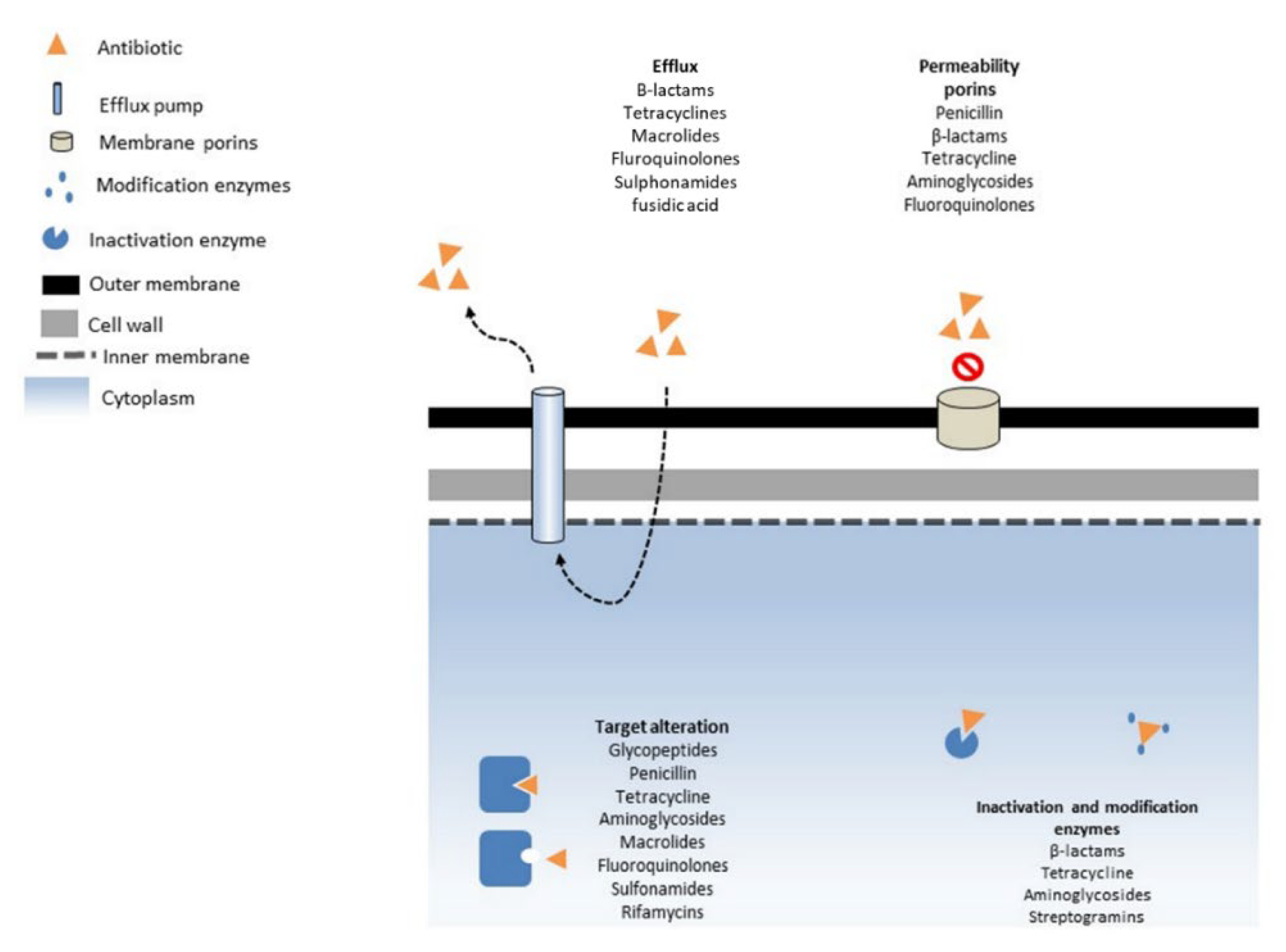
4.1. Mechanism of AMR
4.2. The Excessive Use of Antibiotics
4.3. Biocides
4.4. Metals
5. Antibiotic-Related Environmental Transmission Networks
5.1. Wastewater
5.2. Veterinary and Livestock
5.3. Manure and Sludge
6. Methods of Monitoring AMR
6.1. Culture-Based Methods
6.2. Molecular
6.3. Mass Spectrometry
7. Current Knowledge Gaps in Understanding AMR
8. Strategies and Action Plan to Combat Antimicrobial Resistance
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 12 March 2022).
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic Resistance Is Ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the Mechanisms and Drivers of Antimicrobial Resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Richardson, J.; Lockhart, C.; Pongolini, S.; Karesh, W.B.; Baylis, M.; Goldberg, T.; Slingenbergh, J.; Gale, P.; Venturini, T.; Catchpole, M.; et al. Drivers for Emerging Issues in Animal and Plant Health. EFSA J. 2016, 14, e00512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinsstag, J. Convergence of Ecohealth and One Health. EcoHealth 2012, 9, 371–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collignon, P.J.; McEwen, S.A. One Health—Its Importance in Helping to Better Control Antimicrobial Resistance. Trop. Med. Infect. Dis. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trotter, A.J.; Aydin, A.; Strinden, M.J.; O’Grady, J. Recent and Emerging Technologies for the Rapid Diagnosis of Infection and Antimicrobial Resistance. Curr. Opin. Microbiol. 2019, 51, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations/the Review on Antimicrobial Resistance Chaired by Jim O’Neill. Available online: https://wellcomecollection.org/works/rdpck35v (accessed on 11 March 2022).
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis. Available online: https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis (accessed on 6 May 2022).
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. MMBR 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meletis, G. Carbapenem Resistance: Overview of the Problem and Future Perspectives. Ther. Adv. Infect. Dis. 2016, 3, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Chen, L.; Tang, Y.-W.; Kreiswirth, B.N. Emergence of the Mcr-1 Colistin Resistance Gene in Carbapenem-Resistant Enterobacteriaceae. Lancet Infect. Dis. 2016, 16, 287–288. [Google Scholar] [CrossRef]
- Yao, X.; Doi, Y.; Zeng, L.; Lv, L.; Liu, J.-H. Carbapenem-Resistant and Colistin-Resistant Escherichia Coli Co-Producing NDM-9 and MCR-1. Lancet Infect. Dis. 2016, 16, 288–289. [Google Scholar] [CrossRef] [Green Version]
- Savin, M.; Bierbaum, G.; Blau, K.; Parcina, M.; Sib, E.; Smalla, K.; Schmithausen, R.; Heinemann, C.; Hammerl, J.A.; Kreyenschmidt, J. Colistin-Resistant Enterobacteriaceae Isolated From Process Waters and Wastewater From German Poultry and Pig Slaughterhouses. Front. Microbiol. 2020, 11, 575391. [Google Scholar] [CrossRef] [PubMed]
- Hassuna, N.A.; AbdelAziz, R.A.; Zakaria, A.; Abdelhakeem, M. Extensively-Drug Resistant Klebsiella Pneumoniae Recovered From Neonatal Sepsis Cases From a Major NICU in Egypt. Front. Microbiol. 2020, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Ashiru-Oredope, D.; Kessel, A.; Hopkins, S.; Ashiru-Oredope, D.; Brown, B.; Brown, N.; Carter, S.; Cichowka, A.; Susan Hopkins on behalf of the English Surveillance Programme for Antimicrobial Utilization and Resistance Oversight Group. Antimicrobial Stewardship: English Surveillance Programme for Antimicrobial Utilization and Resistance (ESPAUR). J. Antimicrob. Chemother. 2013, 68, 2421–2423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sipahi, O.R. Economics of Antibiotic Resistance. Expert Rev. Anti Infect. Ther. 2008, 6, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Hafner, M.; Yerushalmi, E.; Smith, R.; Bellasio, J.; Vardavas, R.; Bienkowska-Gibbs, T.; Rubin, J. Estimating the Economic Costs of Antimicrobial Resistance: Model and Results. Available online: https://www.rand.org/pubs/research_reports/RR911.html (accessed on 11 March 2022).
- Devasahayam, G.; Scheld, W.M.; Hoffman, P.S. Newer Antibacterial Drugs for a New Century. Expert Opin. Investig. Drugs 2010, 19, 215–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Migura, L.; Hendriksen, R.S.; Fraile, L.; Aarestrup, F.M. Antimicrobial Resistance of Zoonotic and Commensal Bacteria in Europe: The Missing Link between Consumption and Resistance in Veterinary Medicine. Vet. Microbiol. 2014, 170, 1–9. [Google Scholar] [CrossRef]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolhouse, M.; Ward, M.; van Bunnik, B.; Farrar, J. Antimicrobial Resistance in Humans, Livestock and the Wider Environment. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140083. [Google Scholar] [CrossRef] [PubMed]
- Finley, R.L.; Collignon, P.; Larsson, D.G.J.; McEwen, S.A.; Li, X.-Z.; Gaze, W.H.; Reid-Smith, R.; Timinouni, M.; Graham, D.W.; Topp, E. The Scourge of Antibiotic Resistance: The Important Role of the Environment. Clin. Infect. Dis. 2013, 57, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Tackling Drug-Resistant Infections Globally: An Overview of Our Work/the Review on Antimicrobial Resistance Chaired by Jim O’Neill. Available online: https://wellcomecollection.org/works/e8njjeed (accessed on 11 March 2022).
- Weir, M.; Rajić, A.; Dutil, L.; Uhland, C.; Bruneau, N. Zoonotic Bacteria and Antimicrobial Resistance in Aquaculture: Opportunities for Surveillance in Canada. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3354819/ (accessed on 31 January 2022).
- Klous, G.; Huss, A.; Heederik, D.J.J.; Coutinho, R.A. Human–Livestock Contacts and Their Relationship to Transmission of Zoonotic Pathogens, a Systematic Review of Literature. One Health 2016, 2, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Starr, M.P.; Reynolds, D.M. Streptomycin Resistance of Coliform Bacteria from Turkeys Fed Streptomycin. Am. J. Public Health Nations Health 1951, 41, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism mcr-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Rhouma, M.; Beaudry, F.; Thériault, W.; Letellier, A. Colistin in Pig Production: Chemistry, Mechanism of Antibacterial Action, Microbial Resistance Emergence, and One Health Perspectives. Front. Microbiol. 2016, 7, 1789. [Google Scholar] [CrossRef] [Green Version]
- Baron, S.; Hadjadj, L.; Rolain, J.-M.; Olaitan, A.O. Molecular Mechanisms of Polymyxin Resistance: Knowns and Unknowns. Int. J. Antimicrob. Agents 2016, 48, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Karakonstantis, S.; Kritsotakis, E.I.; Gikas, A. Pandrug-Resistant Gram-Negative Bacteria: A Systematic Review of Current Epidemiology, Prognosis and Treatment Options. J. Antimicrob. Chemother. 2020, 75, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.; Loeffen, F.; Bakker, J.; Klaassen, C.; Wulf, M. Methicillin-Resistant Staphylococcus Aureus in Pig Farming. Emerg. Infect. Dis. 2005, 11, 1965–1966. [Google Scholar] [CrossRef] [PubMed]
- Wendlandt, S.; Kadlec, K.; Feßler, A.T.; Monecke, S.; Ehricht, R.; van de Giessen, A.W.; Hengeveld, P.D.; Huijsdens, X.; Schwarz, S.; van Duijkeren, E. Resistance Phenotypes and Genotypes of Methicillin-Resistant Staphylococcus Aureus Isolates from Broiler Chickens at Slaughter and Abattoir Workers. J. Antimicrob. Chemother. 2013, 68, 2458–2463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadlec, K.; Ehricht, R.; Monecke, S.; Steinacker, U.; Kaspar, H.; Mankertz, J.; Schwarz, S. Diversity of Antimicrobial Resistance Pheno- and Genotypes of Methicillin-Resistant Staphylococcus Aureus ST398 from Diseased Swine. J. Antimicrob. Chemother. 2009, 64, 1156–1164. [Google Scholar] [CrossRef] [Green Version]
- Köck, R.; Schaumburg, F.; Mellmann, A.; Köksal, M.; Jurke, A.; Becker, K.; Friedrich, A.W. Livestock-Associated Methicillin-Resistant Staphylococcus Aureus (MRSA) as Causes of Human Infection and Colonization in Germany. PLoS ONE 2013, 8, e55040. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melese, A.; Genet, C.; Andualem, T. Prevalence of Vancomycin Resistant Enterococci (VRE) in Ethiopia: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2020, 20, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, G.; Coque, T.M.; Hammerum, A.M.; Hope, R.; Hryniewicz, W.; Johnson, A.; Klare, I.; Kristinsson, K.G.; Leclercq, R.; Lester, C.H.; et al. Emergence and Spread of Vancomycin Resistance among Enterococci in Europe. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2008, 13, 19046. [Google Scholar] [CrossRef]
- Bourgeois-Nicolaos, N.; Moubareck, C.; Mangeney, N.; Butel, M.-J.; Doucet-Populaire, F. Comparative Study of VanA Gene Transfer from Enterococcus Faecium to Enterococcus Faecalis and to Enterococcus Faecium in the Intestine of Mice. FEMS Microbiol. Lett. 2006, 254, 27–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, C.A.; Murray, B.E. The Rise of the Enterococcus: Beyond Vancomycin Resistance. Nat. Rev. Microbiol. 2012, 10, 266–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadashi, M.; Sharifian, P.; Bostanshirin, N.; Hajikhani, B.; Bostanghadiri, N.; Khosravi-Dehaghi, N.; van Belkum, A.; Darban-Sarokhalil, D. The Global Prevalence of Daptomycin, Tigecycline, and Linezolid-Resistant Enterococcus Faecalis and Enterococcus Faecium Strains from Human Clinical Samples: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 720647. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, J.; Li, Q.; Tang, Y.; Wang, J.; Pan, Z.; Chen, X.; Jiao, X. First Detection of NDM-5-Positive Salmonella Enterica Serovar Typhimurium Isolated from Retail Pork in China. Microb. Drug Resist. Larchmt. N 2020, 26, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.Á.; Díaz, P.L.; Rodríguez, E.C.; Montaño, L.A.; Gartner, D.M.; Vernaza, M.E.; Eljach, V.; Realpe, M.E. A nalidixic acid-resistant Salmonella enteritidis outbreak in Popayán, Cauca, 2011. Biomed. Rev. Inst. Nac. Salud 2013, 33, 62–69. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, Y.H.; Wang, H. Residues of Veterinary Antibiotics in Manures from Feedlot Livestock in Eight Provinces of China. Sci. Total Environ. 2010, 408, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.J.G.; Carson, C.A.; Finley, R.L.; Thomas, M.K.; Reid-Smith, R.J.; McEwen, S.A. Estimating the Number of Human Cases of Ceftiofur-Resistant Salmonella Enterica Serovar Heidelberg in Québec and Ontario, Canada. Clin. Infect. Dis. 2014, 59, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.E.; Besser, J.M.; Hedberg, C.W.; Leano, F.T.; Bender, J.B.; Wicklund, J.H.; Johnson, B.P.; Moore, K.A.; Osterholm, M.T. Quinolone-Resistant Campylobacter Jejuni Infections in Minnesota, 1992-1998. Investigation Team. N. Engl. J. Med. 1999, 340, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, C.; Volpi, A.; Maurici, M.; Lisena, F.P.; Visconti, G.; Panà, A. Healthcare-associated infections and antibiotic resistance: A global challenge for the 21st century. Ig. Sanita Pubblica 2013, 69, 657–691. [Google Scholar]
- Levy, S.B. The Challenge of Antibiotic Resistance. Sci. Am. 1998, 278, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Crofts, T.S.; Gasparrini, A.J.; Dantas, G. Next-Generation Approaches to Understand and Combat the Antibiotic Resistome. Nat. Rev. Microbiol. 2017, 15, 422–434. [Google Scholar] [CrossRef] [Green Version]
- Forsberg, K.J.; Patel, S.; Wencewicz, T.A.; Dantas, G. The Tetracycline Destructases: A Novel Family of Tetracycline-Inactivating Enzymes. Chem. Biol. 2015, 22, 888–897. [Google Scholar] [CrossRef] [Green Version]
- Shaw, W.V.; Packman, L.C.; Burleigh, B.D.; Dell, A.; Morris, H.R.; Hartley, B.S. Primary Structure of a Chloramphenicol Acetyltransferase Specified by R Plasmids. Nature 1979, 282, 870–872. [Google Scholar] [CrossRef]
- Yang, W.; Moore, I.F.; Koteva, K.P.; Bareich, D.C.; Hughes, D.W.; Wright, G.D. TetX Is a Flavin-Dependent Monooxygenase Conferring Resistance to Tetracycline Antibiotics. J. Biol. Chem. 2004, 279, 52346–52352. [Google Scholar] [CrossRef] [Green Version]
- Bush, K. Bench-to-Bedside Review: The Role of Beta-Lactamases in Antibiotic-Resistant Gram-Negative Infections. Crit. Care Lond. Engl. 2010, 14, 224. [Google Scholar] [CrossRef] [Green Version]
- Piddock, L.J.V. Clinically Relevant Chromosomally Encoded Multidrug Resistance Efflux Pumps in Bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.C. Tetracycline Resistance Determinants: Mechanisms of Action, Regulation of Expression, Genetic Mobility, and Distribution. FEMS Microbiol. Rev. 1996, 19, 1–24. [Google Scholar] [CrossRef]
- On the Mechanism of Solute Uptake in Pseudomonas—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/12700103/ (accessed on 22 September 2022).
- Babouee Flury, B.; Ellington, M.J.; Hopkins, K.L.; Turton, J.F.; Doumith, M.; Loy, R.; Staves, P.; Hinic, V.; Frei, R.; Woodford, N. Association of Novel Nonsynonymous Single Nucleotide Polymorphisms in AmpD with Cephalosporin Resistance and Phylogenetic Variations in AmpC, AmpR, OmpF, and OmpC in Enterobacter Cloacae Isolates That Are Highly Resistant to Carbapenems. Antimicrob. Agents Chemother. 2016, 60, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Understanding Antibiotic Resistance. Available online: https://www.open.edu/openlearn/mod/oucontent/science-maths-technology/understanding-antibiotic-resistance (accessed on 27 July 2022).
- D’Costa, V.M.; McGrann, K.M.; Hughes, D.W.; Wright, G.D. Sampling the Antibiotic Resistome. Science 2006, 311, 374–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vikesland, P.J.; Pruden, A.; Alvarez, P.J.J.; Aga, D.; Bürgmann, H.; Li, X.-D.; Manaia, C.M.; Nambi, I.; Wigginton, K.; Zhang, T.; et al. Toward a Comprehensive Strategy to Mitigate Dissemination of Environmental Sources of Antibiotic Resistance. Environ. Sci. Technol. 2017, 51, 13061–13069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vikesland, P.; Garner, E.; Gupta, S.; Kang, S.; Maile-Moskowitz, A.; Zhu, N. Differential Drivers of Antimicrobial Resistance across the World. Acc. Chem. Res. 2019, 52, 916–924. [Google Scholar] [CrossRef] [Green Version]
- Payne, D.J.; Miller, L.F.; Findlay, D.; Anderson, J.; Marks, L. Time for a Change: Addressing R&D and Commercialization Challenges for Antibacterials. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140086. [Google Scholar] [CrossRef] [Green Version]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global Increase and Geographic Convergence in Antibiotic Consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [Green Version]
- ResistanceMap—Antibiotic Use. Available online: https://resistancemap.cddep.org/ (accessed on 7 June 2022).
- Gasson, J.; Blockman, M.; Willems, B. Antibiotic Prescribing Practice and Adherence to Guidelines in Primary Care in the Cape Town Metro District, South Africa. S. Afr. Med. J. 2018, 108, 304–310. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, M.R.; Saqib, A.; Iftikhar, S.; Sadiq, T. Antimicrobial Use by WHO Methodology at Primary Health Care Centers: A Cross Sectional Study in Punjab, Pakistan. BMC Infect. Dis. 2018, 18, 492. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, P.; Xinghe, W.; Zheng, Y.; Xiao, Y. Use and Prescription of Antibiotics in Primary Health Care Settings in China. JAMA Intern. Med. 2014, 174, 1914–1920. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, K.L.; Langford, B.J.; Daneman, N.; Chen, B.; Brown, K.A.; McIsaac, W.; Tu, K.; Candido, E.; Johnstone, J.; Leung, V.; et al. Unnecessary Antibiotic Prescribing in a Canadian Primary Care Setting: A Descriptive Analysis Using Routinely Collected Electronic Medical Record Data. CMAJ Open 2020, 8, E360. [Google Scholar] [CrossRef]
- Richard, V.M.; Jonathan, K.W.; Jonathan, E.; Jonathan, H.; Pedro, C.; Jefferson, B. Reducing Inappropriate Outpatient Antibiotic Prescribing: Normative Comparison Using Unblinded Provider Reports. BMJ Open Qual. 2019, 8, e000351. [Google Scholar] [CrossRef]
- Kariyawasam, R.M.; Julien, D.A.; Jelinski, D.C.; Larose, S.L.; Rennert-May, E.; Conly, J.M.; Dingle, T.C.; Chen, J.Z.; Tyrrell, G.J.; Ronksley, P.E.; et al. Antimicrobial Resistance (AMR) in COVID-19 Patients: A Systematic Review and Meta-Analysis (November 2019–June 2021). Antimicrob. Resist. Infect. Control 2022, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 22 July 2022).
- National Research Council (US); Committee to Study the Human Health Effects of Subtherapeutic Antibiotic Use in Animal Feeds. The Effects on Human Health of Subtherapeutic Use of Antimicrobials in Animal Feeds; National Academies Press: Washington, DC, USA, 1980. [Google Scholar]
- Fifth OIE Annual Report on Antimicrobial Agents Intended for Use in Animals. Available online: https://www.woah.org/en/document/fifth-oie-annual-report-on-antimicrobial-agents-intended-for-use-in-animals/ (accessed on 10 June 2022).
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- Regulation (EC) No 1831/2003 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/eli/reg/2003/1831/oj/eng (accessed on 23 September 2022).
- U.S. Food and Drug Administration. Food Code 2013. Available online: https://www.fda.gov/food/fda-food-code/food-code-2013 (accessed on 12 March 2022).
- Ejo, M.; Garedew, L.; Alebachew, Z.; Worku, W. Prevalence and Antimicrobial Resistance of Salmonella Isolated from Animal-Origin Food Items in Gondar, Ethiopia. BioMed Res. Int. 2016, 2016, 4290506. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, M.U.; Thajuddin, N.; Ahamed, P.; Teklemariam, Z.; Jamil, K. Antimicrobial Drug Resistance in Strains of Escherichia Coli Isolated from Food Sources. Rev. Inst. Med. Trop. São Paulo 2014, 56, 341–346. [Google Scholar] [CrossRef]
- Kahrilas, G.A.; Blotevogel, J.; Stewart, P.S.; Borch, T. Biocides in Hydraulic Fracturing Fluids: A Critical Review of Their Usage, Mobility, Degradation, and Toxicity. Environ. Sci. Technol. 2015, 49, 16–32. [Google Scholar] [CrossRef]
- Buffet-Bataillon, S.; Tattevin, P.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Emergence of Resistance to Antibacterial Agents: The Role of Quaternary Ammonium Compounds—A Critical Review. Int. J. Antimicrob. Agents 2012, 39, 381–389. [Google Scholar] [CrossRef]
- Poole, K. Mechanisms of Bacterial Biocide and Antibiotic Resistance. J. Appl. Microbiol. 2002, 92 (Suppl. S1), 55S–64S. [Google Scholar] [CrossRef]
- Hugo, W.B.; Longworth, A.R. Effect of Chlorhexidine Diacetate on “Protoplasts” and Spheroplasts of Escherichia Coli, Protoplasts of Bacillus Megaterium and the Gram Staining Reaction of Staphylococcus Aureus. J. Pharm. Pharmacol. 1964, 16, 751–758. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [Green Version]
- Russell, A.D. Mechanisms of Bacterial Resistance to Biocides. Int. Biodeterior. Biodegrad. 1995, 36, 247–265. [Google Scholar] [CrossRef]
- Brown, M.R.; Gilbert, P. Sensitivity of Biofilms to Antimicrobial Agents. J. Appl. Bacteriol. 1993, 74 (Suppl. S22), 87S–97S. [Google Scholar] [CrossRef] [PubMed]
- McMurry, L.M.; McDermott, P.F.; Levy, S.B. Genetic Evidence That InhA of Mycobacterium Smegmatis Is a Target for Triclosan. Antimicrob. Agents Chemother. 1999, 43, 711–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasatsu, M.; Shirai, Y.; Hase, M.; Noguchi, N.; Kono, M.; Behr, H.; Freney, J.; Arai, T. The Origin of the Antiseptic-Resistance Gene Ebr in Staphylococcus Aureus. Microbios 1995, 84, 161–169. [Google Scholar]
- Russell, A.D. Plasmids and Bacterial Resistance to Biocides. J. Appl. Microbiol. 1997, 83, 155–165. [Google Scholar] [CrossRef]
- Prince, H.N.; Nonemaker, W.S.; Norgard, R.C.; Prince, D.L. Drug Resistance Studies with Topical Antiseptics. J. Pharm. Sci. 1978, 67, 1629–1631. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Davies, A.; Russell, A.d. Sensitivity and Resistance of Escherichia Coli and Staphylococcus Aureus to Chlorhexidine. Lett. Appl. Microbiol. 1992, 14, 33–36. [Google Scholar] [CrossRef]
- Silver, S.; Misra, T.K. Plasmid-Mediated Heavy Metal Resistances. Annu. Rev. Microbiol. 1988, 42, 717–743. [Google Scholar] [CrossRef]
- Vijayakumar, R.; Sandle, T. A Review on Biocide Reduced Susceptibility Due to Plasmid-Borne Antiseptic-Resistant Genes—Special Notes on Pharmaceutical Environmental Isolates. J. Appl. Microbiol. 2019, 126, 1011–1022. [Google Scholar] [CrossRef] [Green Version]
- Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 Concerning the Making. Market and Use of Biocidal Products Text with EEA Relevance. 2012. L 167/1, pp. 1–123. Available online: https://www.legislation.gov.uk/eur/2012/528/contents (accessed on 28 July 2022).
- Sattar, S.A.; Tetro, J.A.; Springthorpe, V.S. Effects of Environmental Chemicals and the Host-Pathogen Relationship: Are There Any Negative Consequences for Human Health? In New Biocides Development; ACS Symposium Series; American Chemical Society: New York, NY, USA, 2007; Volume 967, pp. 2–30. ISBN 978-0-8412-7405-1. [Google Scholar]
- Karvelas, M.; Katsoyiannis, A.; Samara, C. Occurrence and Fate of Heavy Metals in the Wastewater Treatment Process. Chemosphere 2003, 53, 1201–1210. [Google Scholar] [CrossRef]
- Nicholson, F.A.; Smith, S.R.; Alloway, B.J.; Carlton-Smith, C.; Chambers, B.J. An Inventory of Heavy Metals Inputs to Agricultural Soils in England and Wales. Sci. Total Environ. 2003, 311, 205–219. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Wright, M.S.; Stepanauskas, R.; McArthur, J.V. Co-Selection of Antibiotic and Metal Resistance. Trends Microbiol. 2006, 14, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Nies, D.H. Efflux-Mediated Heavy Metal Resistance in Prokaryotes. FEMS Microbiol. Rev. 2003, 27, 313–339. [Google Scholar] [CrossRef]
- Ardestani, M.M.; van Straalen, N.M.; van Gestel, C.A.M. Biotic Ligand Modeling Approach: Synthesis of the Effect of Major Cations on the Toxicity of Metals to Soil and Aquatic Organisms. Environ. Toxicol. Chem. 2015, 34, 2194–2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olaniran, A.O.; Balgobind, A.; Pillay, B. Bioavailability of Heavy Metals in Soil: Impact on Microbial Biodegradation of Organic Compounds and Possible Improvement Strategies. Int. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tipping, E.; Lofts, S. Metal Mixture Toxicity to Aquatic Biota in Laboratory Experiments: Application of the WHAM-FTOX Model. Aquat. Toxicol. 2013, 142–143, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of Antimicrobial Resistance in the Environment and Its Relevance to Environmental Regulators. Front. Microbiol. 2016, 7, 1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giller, K.E.; Witter, E.; Mcgrath, S.P. Toxicity of Heavy Metals to Microorganisms and Microbial Processes in Agricultural Soils: A Review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]
- Smith, S.R. A Critical Review of the Bioavailability and Impacts of Heavy Metals in Municipal Solid Waste Composts Compared to Sewage Sludge. Environ. Int. 2009, 35, 142–156. [Google Scholar] [CrossRef]
- Braam, F.; Klapwijk, A. Effect of Copper on Nitrification in Activated Sludge. Water Res. 1981, 15, 1093–1098. [Google Scholar] [CrossRef]
- Waara, K.-O. Effects of Copper, Cadmium, Lead and Zinc on Nitrate Reduction in a Synthetic Water Medium and Lake Water from Northern Sweden. Water Res. 1992, 26, 355–364. [Google Scholar] [CrossRef]
- Ajmal, M.; Ahmad, A.; Nomani, A.A. Influence of Toxic Metals on the Repression of Carbonaceous Oxygen Demand. Water Res. 1983, 17, 799–802. [Google Scholar] [CrossRef]
- Chipasa, K.B. Accumulation and Fate of Selected Heavy Metals in a Biological Wastewater Treatment System. Waste Manag. 2003, 23, 135–143. [Google Scholar] [CrossRef]
- Zhang, C.; Nie, S.; Liang, J.; Zeng, G.; Wu, H.; Hua, S.; Liu, J.; Yuan, Y.; Xiao, H.; Deng, L.; et al. Effects of Heavy Metals and Soil Physicochemical Properties on Wetland Soil Microbial Biomass and Bacterial Community Structure. Sci. Total Environ. 2016, 557–558, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Fan, J.; Zhu, W.; Amombo, E.; Lou, Y.; Chen, L.; Fu, J. Effect of Heavy Metals Pollution on Soil Microbial Diversity and Bermudagrass Genetic Variation. Front. Plant Sci. 2016, 7, 755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological Approaches to Tackle Heavy Metal Pollution: A Survey of Literature. J. Environ. Manage. 2018, 217, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Das, K.R.; Naik, M.M. Co-Selection of Multi-Antibiotic Resistance in Bacterial Pathogens in Metal and Microplastic Contaminated Environments: An Emerging Health Threat. Chemosphere 2019, 215, 846–857. [Google Scholar] [CrossRef]
- Verlicchi, P.; Zambello, E. Predicted and Measured Concentrations of Pharmaceuticals in Hospital Effluents. Examination of the Strengths and Weaknesses of the Two Approaches through the Analysis of a Case Study. Sci. Total Environ. 2016, 565, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-E.; Zhang, H.; Ying, G.-G.; Zhou, L.-J.; Jones, K.C. Passive Sampling: A Cost-Effective Method for Understanding Antibiotic Fate, Behaviour and Impact. Environ. Int. 2015, 85, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive Removal of Antibiotics from Water and Wastewater: Progress and Challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Sun, Y.; Ding, X.; Wang, M.; Zeng, Z. Selective Pressure of Antibiotics on ARGs and Bacterial Communities in Manure-Polluted Freshwater-Sediment Microcosms. Front. Microbiol. 2015, 6, 194. [Google Scholar] [CrossRef]
- Gardner, M.; Jones, V.; Comber, S.; Scrimshaw, M.D.; Coello-Garcia, T.; Cartmell, E.; Lester, J.; Ellor, B. Performance of UK Wastewater Treatment Works with Respect to Trace Contaminants. Sci. Total Environ. 2013, 456–457, 359–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.-F.; Lin, C.-F.; Wu, C.-J.; Ng, K.-K.; Yu-Chen Lin, A.; Andy Hong, P.-K. Fate of Sulfonamide Antibiotics in Contact with Activated Sludge—Sorption and Biodegradation. Water Res. 2012, 46, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Polesel, F.; Lehnberg, K.; Dott, W.; Trapp, S.; Thomas, K.V.; Plósz, B.G. Factors Influencing Sorption of Ciprofloxacin onto Activated Sludge: Experimental Assessment and Modelling Implications. Chemosphere 2015, 119, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, F.; Udikovic-Kolic, N.; Andrew, S.; Handelsman, J. Diverse Antibiotic Resistance Genes in Dairy Cow Manure. mBio 2014, 5, e01017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berendsen, B.J.A.; Wegh, R.S.; Memelink, J.; Zuidema, T.; Stolker, L.A.M. The Analysis of Animal Faeces as a Tool to Monitor Antibiotic Usage. Talanta 2015, 132, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Bloom of Resident Antibiotic-Resistant Bacteria in Soil Following Manure Fertilization|PNAS. Available online: https://www.pnas.org/content/111/42/15202 (accessed on 31 January 2022).
- Clarke, B.O.; Smith, S.R. Review of ‘Emerging’ Organic Contaminants in Biosolids and Assessment of International Research Priorities for the Agricultural Use of Biosolids. Environ. Int. 2011, 37, 226–247. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, Y.; Gao, L.; Liu, J.; Cai, Y. Occurrence, Distribution and Potential Affecting Factors of Antibiotics in Sewage Sludge of Wastewater Treatment Plants in China. Sci. Total Environ. 2013, 445–446, 306–313. [Google Scholar] [CrossRef]
- McClellan, K.; Halden, R.U. Pharmaceuticals and Personal Care Products in Archived U.S. Biosolids from the 2001 EPA National Sewage Sludge Survey. Water Res. 2010, 44, 658–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Zhang, T. Biodegradation and Adsorption of Antibiotics in the Activated Sludge Process. Environ. Sci. Technol. 2010, 44, 3468–3473. [Google Scholar] [CrossRef]
- VITEK® 2: Healthcare. Available online: https://www.biomerieux-usa.com/vitek-2 (accessed on 28 July 2022).
- MicroScan WalkAway plus Microbiology System. Available online: httpss://www.beckmancoulter.com/products/microbiology/microscan-walkaway-plus-system (accessed on 28 July 2022).
- BD PhoenixTM Automated Identification and Susceptibility Testing System. Available online: https://www.bd.com/en-ca/offerings/capabilities/microbiology-solutions/identification-and-susceptibility-testing/bd-phoenix-automated-identification-and-susceptibility-testing-system (accessed on 28 July 2022).
- Biolog—Microbial Identification & Characterization—Biolog—World Leader in Cell Based Technology and Assays for Microbiology & Cell Biology Using Phenotype Microarray Technology. Available online: https://www.biolog.com/ (accessed on 20 September 2022).
- Tien, Y.-C.; Li, B.; Zhang, T.; Scott, A.; Murray, R.; Sabourin, L.; Marti, R.; Topp, E. Impact of Dairy Manure Pre-Application Treatment on Manure Composition, Soil Dynamics of Antibiotic Resistance Genes, and Abundance of Antibiotic-Resistance Genes on Vegetables at Harvest. Sci. Total Environ. 2017, 581–582, 32–39. [Google Scholar] [CrossRef]
- Spencer, S.J.; Tamminen, M.V.; Preheim, S.P.; Guo, M.T.; Briggs, A.W.; Brito, I.L.; Weitz, D.A.; Pitkänen, L.K.; Vigneault, F.; Virta, M.P.; et al. Massively Parallel Sequencing of Single Cells by EpicPCR Links Functional Genes with Phylogenetic Markers. ISME J. 2016, 10, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Li, J.; Chen, H.; Bond, P.L.; Yuan, Z. Metagenomic Analysis Reveals Wastewater Treatment Plants as Hotspots of Antibiotic Resistance Genes and Mobile Genetic Elements. Water Res. 2017, 123, 468–478. [Google Scholar] [CrossRef]
- Munk, P.; Andersen, V.D.; de Knegt, L.; Jensen, M.S.; Knudsen, B.E.; Lukjancenko, O.; Mordhorst, H.; Clasen, J.; Agersø, Y.; Folkesson, A.; et al. A Sampling and Metagenomic Sequencing-Based Methodology for Monitoring Antimicrobial Resistance in Swine Herds. J. Antimicrob. Chemother. 2017, 72, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.; Webb, M.; Ghimire, S.; Blair, A.; Olson, K.; Fenske, G.J.; Fonder, A.T.; Christopher-Hennings, J.; Brake, D.; Scaria, J. Metagenomic Characterization of the Effect of Feed Additives on the Gut Microbiome and Antibiotic Resistome of Feedlot Cattle. Sci. Rep. 2017, 7, 12257. [Google Scholar] [CrossRef] [Green Version]
- Luby, E.; Ibekwe, A.M.; Zilles, J.; Pruden, A. Molecular Methods for Assessment of Antibiotic Resistance in Agricultural Ecosystems: Prospects and Challenges. J. Environ. Qual. 2016, 45, 441–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- M100Ed32|Performance Standards for Antimicrobial Susceptibility Testing, 32nd Edition. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 12 March 2022).
- ISO—ISO 20776-1:2006—Clinical Laboratory Testing and in Vitro Diagnostic Test Systems—Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Reference Method for Testing the in Vitro Activity of Antimicrobial Agents against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases. Available online: https://www.iso.org/standard/41630.html (accessed on 1 February 2022).
- Cepheid|Enhanced Antibiotic Stewardship. Available online: https://www.cepheid.com/en/impact/enhanced-antibiotic-stewardship (accessed on 28 July 2022).
- Molecular Diagnostics. Available online: https://diagnostics.roche.com/global/en/products/product-category/molecular-diagnostics.html (accessed on 28 July 2022).
- Sequencing|Key Methods and Uses. Available online: https://www.illumina.com/techniques/sequencing.html (accessed on 28 July 2022).
- MinION. Available online: http://nanoporetech.com/products/minion (accessed on 28 July 2022).
- How HiFi Sequencing Works. Available online: https://www.pacb.com/technology/hifi-sequencing/how-it-works/ (accessed on 28 July 2022).
- Microbial Identification. Available online: https://www.bruker.com/en/products-and-solutions/microbiology-and-diagnostics/microbial-identification.html (accessed on 28 July 2022).
- VITEK®, MS. Available online: https://www.biomerieux-diagnostics.com/Massspectrometrymicrobialidentificationsystem (accessed on 28 July 2022).
- Home—Carb-X. Available online: https://carb-x.org/ (accessed on 20 September 2022).
- Global Action Plan on Antimicrobial Resistance. Available online: https://www.who.int/publications-detail-redirect/9789241509763 (accessed on 11 March 2022).
- Huang, Q.M.; Horn, M.A.; Ruan, S.G. Modeling the Effect of Antibiotic Exposure on the Transmission of Methicillin-Resistant Staphylococcus Aureus in Hospitals with Environmental Contamination. Math. Biosci. Eng. MBE 2019, 16, 3641–3673. [Google Scholar] [CrossRef] [PubMed]
- Cantón, R.; Akóva, M.; Carmeli, Y.; Giske, C.G.; Glupczynski, Y.; Gniadkowski, M.; Livermore, D.M.; Miriagou, V.; Naas, T.; Rossolini, G.M.; et al. Rapid Evolution and Spread of Carbapenemases among Enterobacteriaceae in Europe. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012, 18, 413–431. [Google Scholar] [CrossRef] [Green Version]
- Bigdeli, M.; Jacobs, B.; Tomson, G.; Laing, R.; Ghaffar, A.; Dujardin, B.; Van Damme, W. Access to Medicines from a Health System Perspective. Health Policy Plan. 2013, 28, 692–704. [Google Scholar] [CrossRef] [Green Version]
- Fischbach, M.A. Combination Therapies for Combating Antimicrobial Resistance. Curr. Opin. Microbiol. 2011, 14, 519–523. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irfan, M.; Almotiri, A.; AlZeyadi, Z.A. Antimicrobial Resistance and Its Drivers—A Review. Antibiotics 2022, 11, 1362. https://doi.org/10.3390/antibiotics11101362
Irfan M, Almotiri A, AlZeyadi ZA. Antimicrobial Resistance and Its Drivers—A Review. Antibiotics. 2022; 11(10):1362. https://doi.org/10.3390/antibiotics11101362
Chicago/Turabian StyleIrfan, Mohammad, Alhomidi Almotiri, and Zeyad Abdullah AlZeyadi. 2022. "Antimicrobial Resistance and Its Drivers—A Review" Antibiotics 11, no. 10: 1362. https://doi.org/10.3390/antibiotics11101362
APA StyleIrfan, M., Almotiri, A., & AlZeyadi, Z. A. (2022). Antimicrobial Resistance and Its Drivers—A Review. Antibiotics, 11(10), 1362. https://doi.org/10.3390/antibiotics11101362





