Hydrophilic Composites of Chitosan with Almond Gum: Characterization and Mechanical, and Antimicrobial Activity for Compostable Food Packaging
Abstract
:1. Introduction
2. Results and Discussion
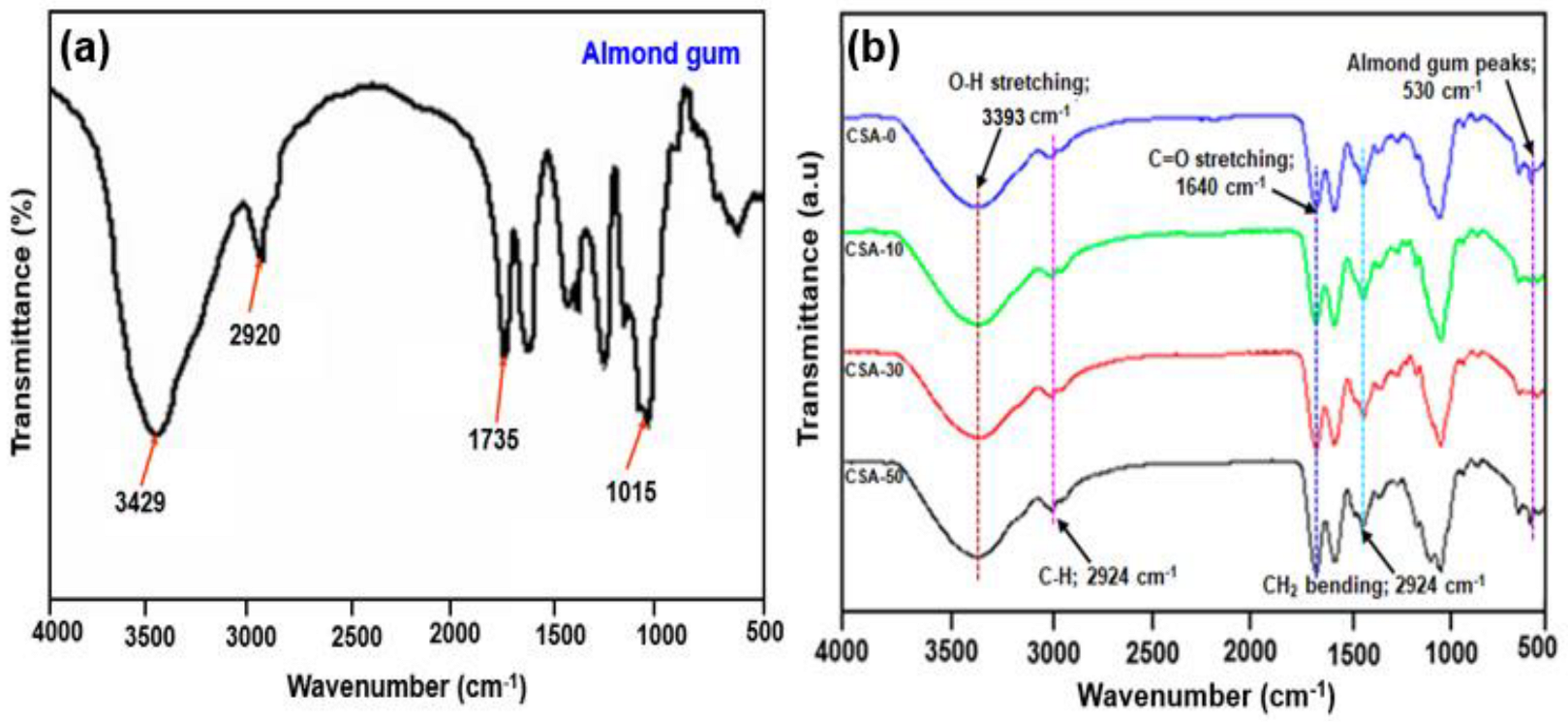
2.1. FTIR Analysis
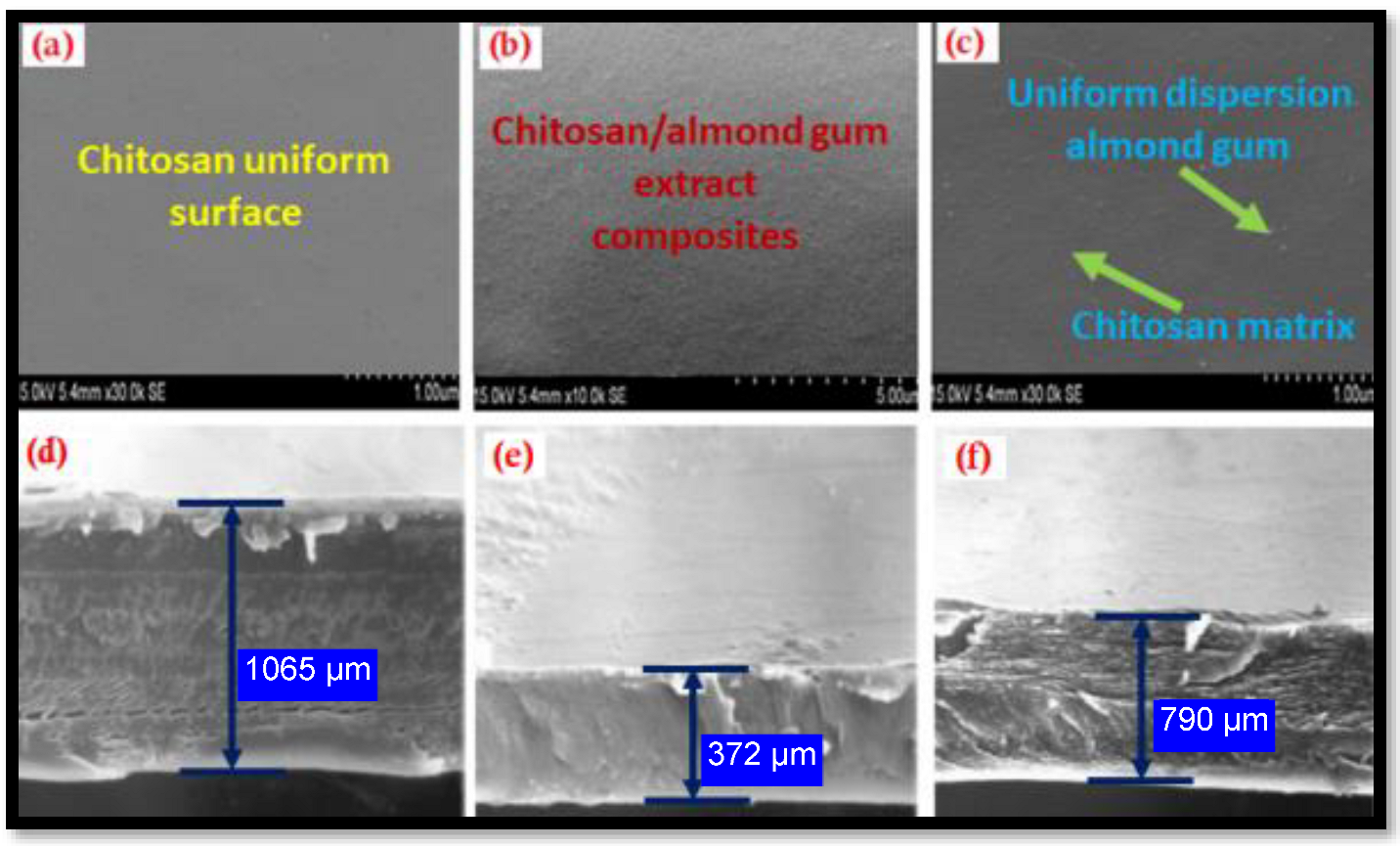
2.2. SEM Analysis of Chitosan and Its Composites
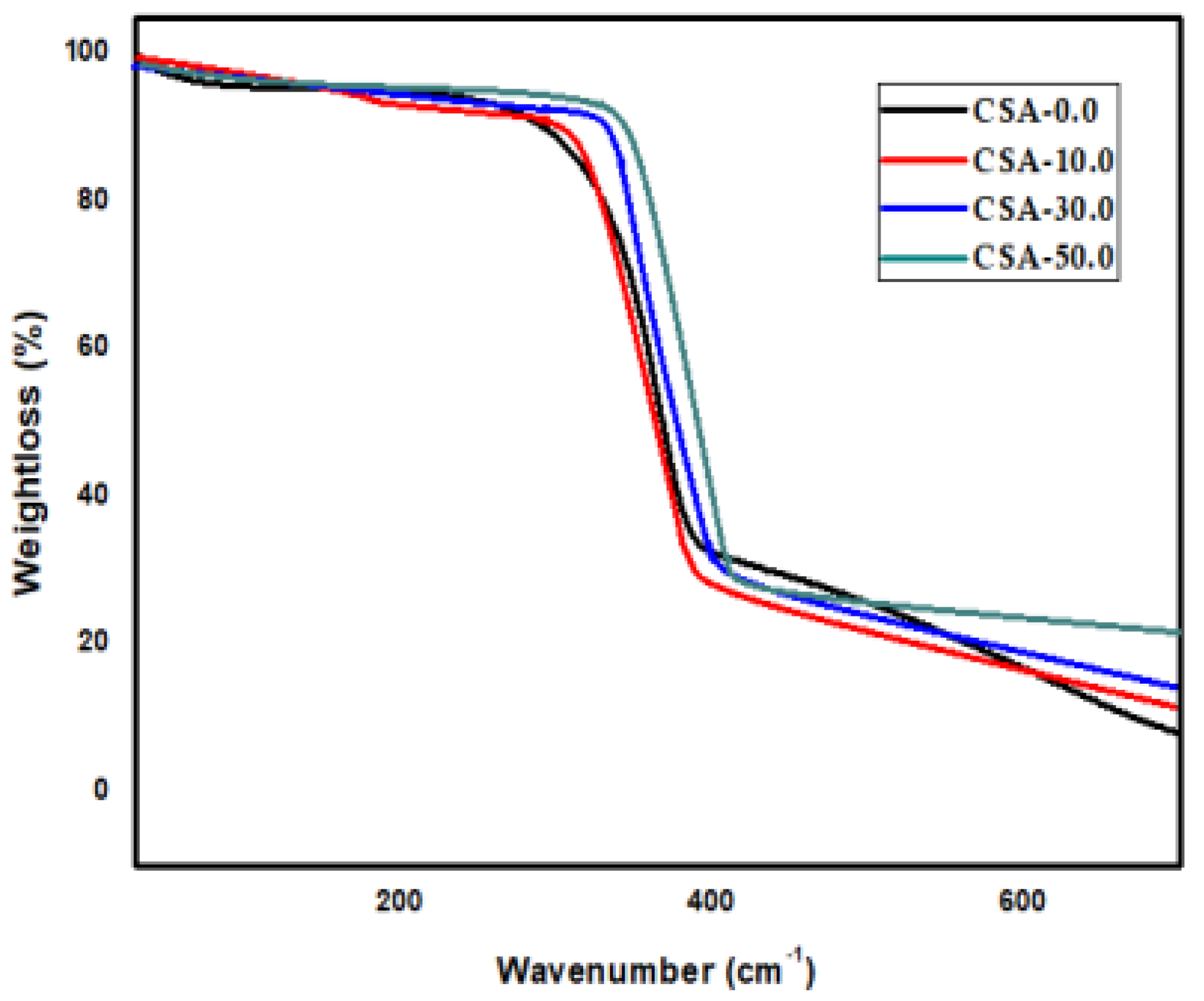
2.3. TGA Analysis
2.4. Mechanical Test of Chitosan and Chitosan/Almond Gum Composites
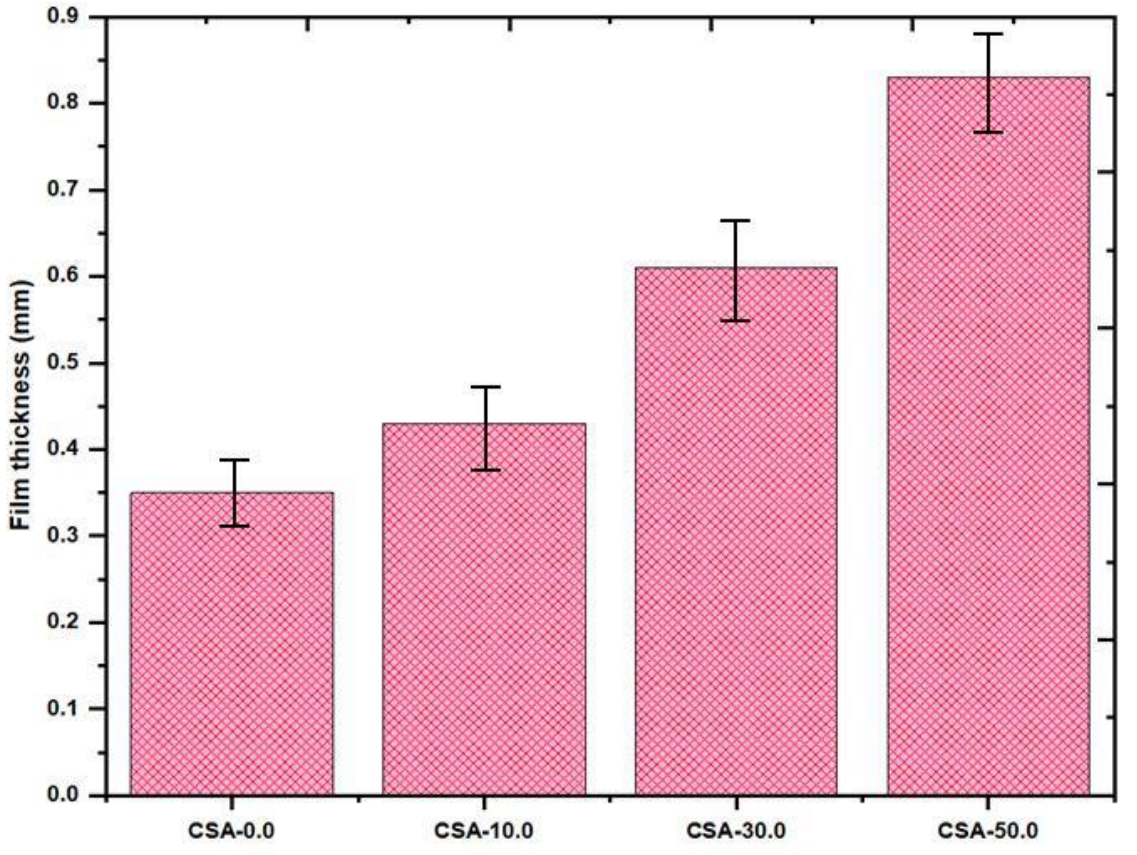
2.5. Film Thickness of Composite Film
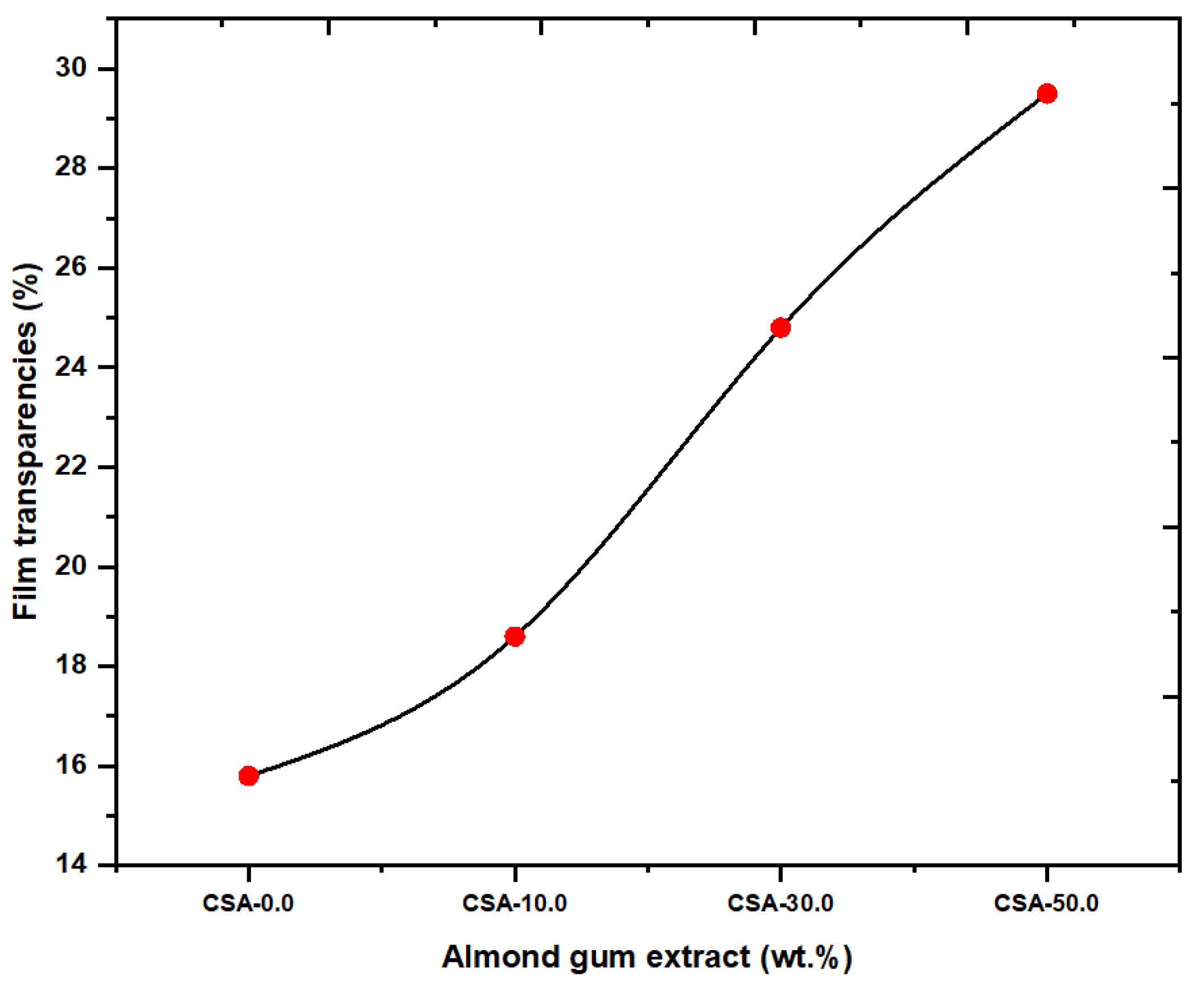
2.6. Film Transparency
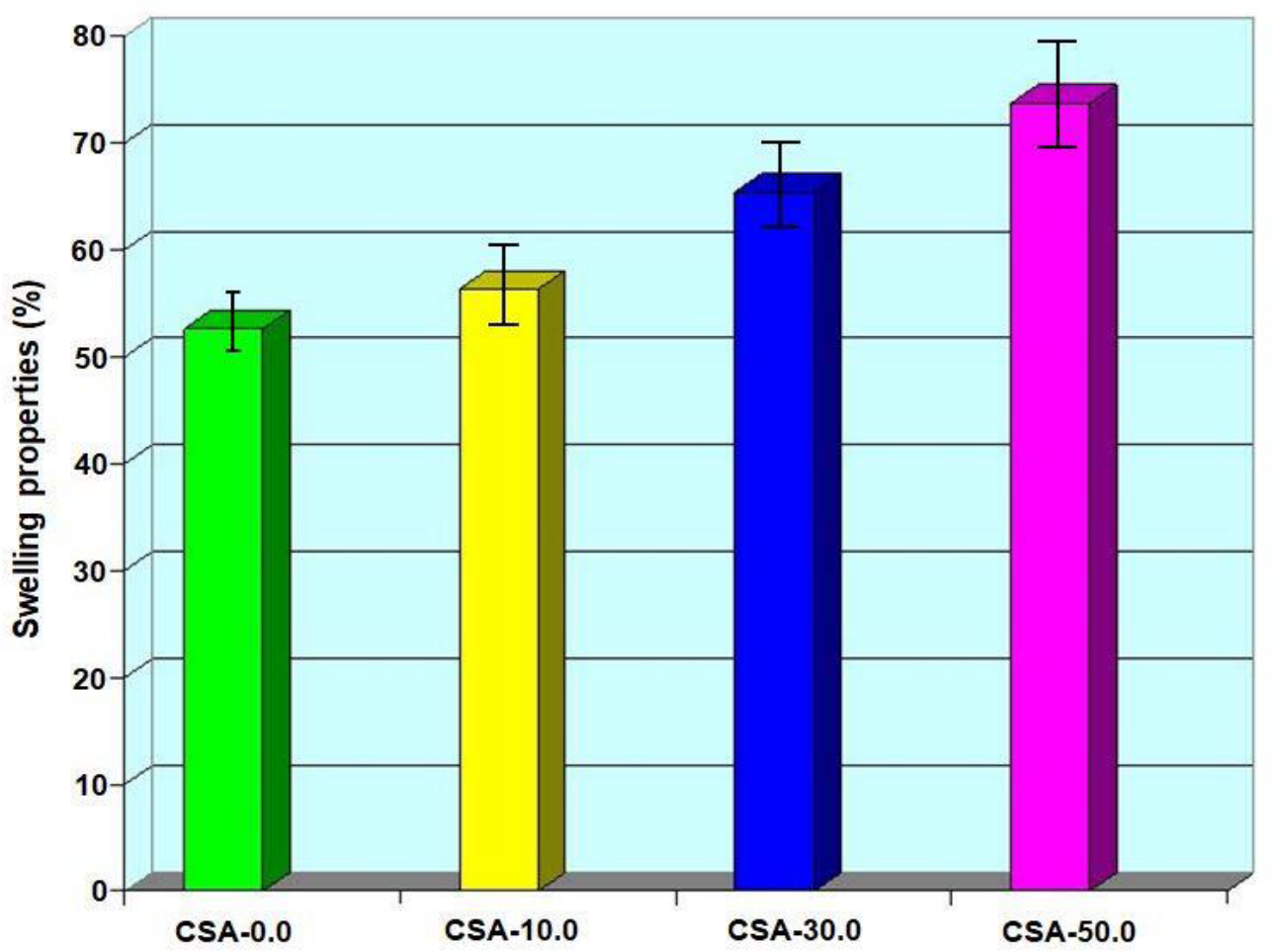
2.7. Swelling Property of Composites
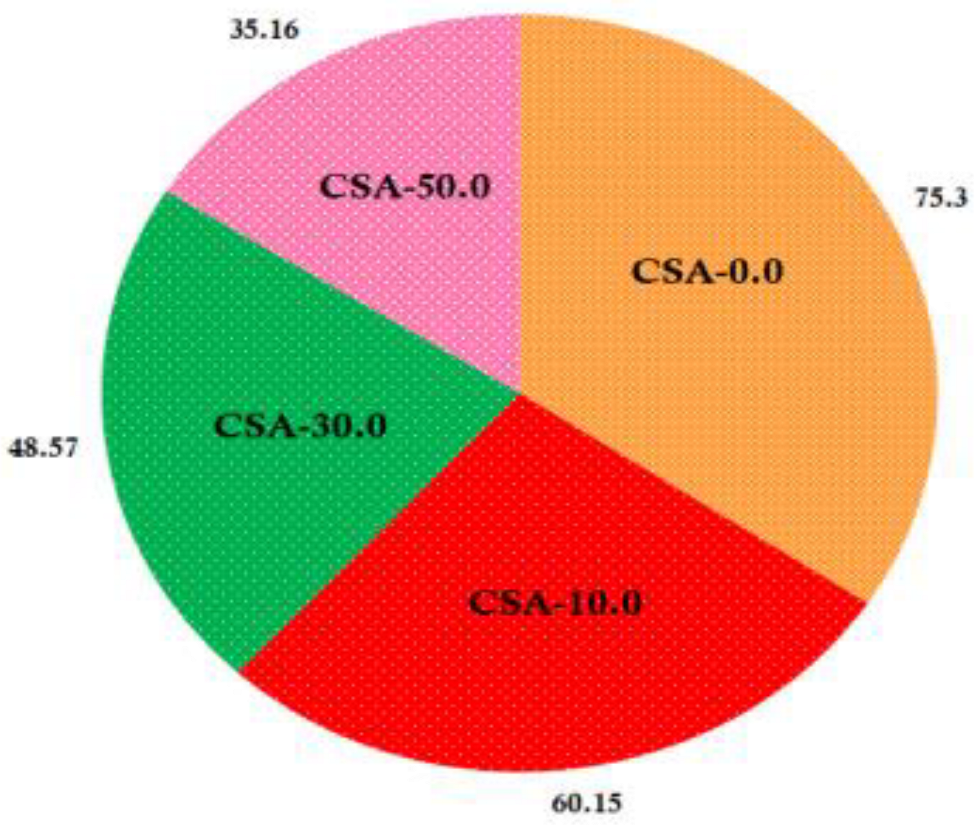
2.8. Water Solubility Test
2.9. Barrier Property
- Loading of filler decreases the area available for diffusion of gases.
- Increase in the distance that gaseous molecules must travel to cross the films.
2.10. Antimicrobial Activity
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Collection and Purification of Almond Gum
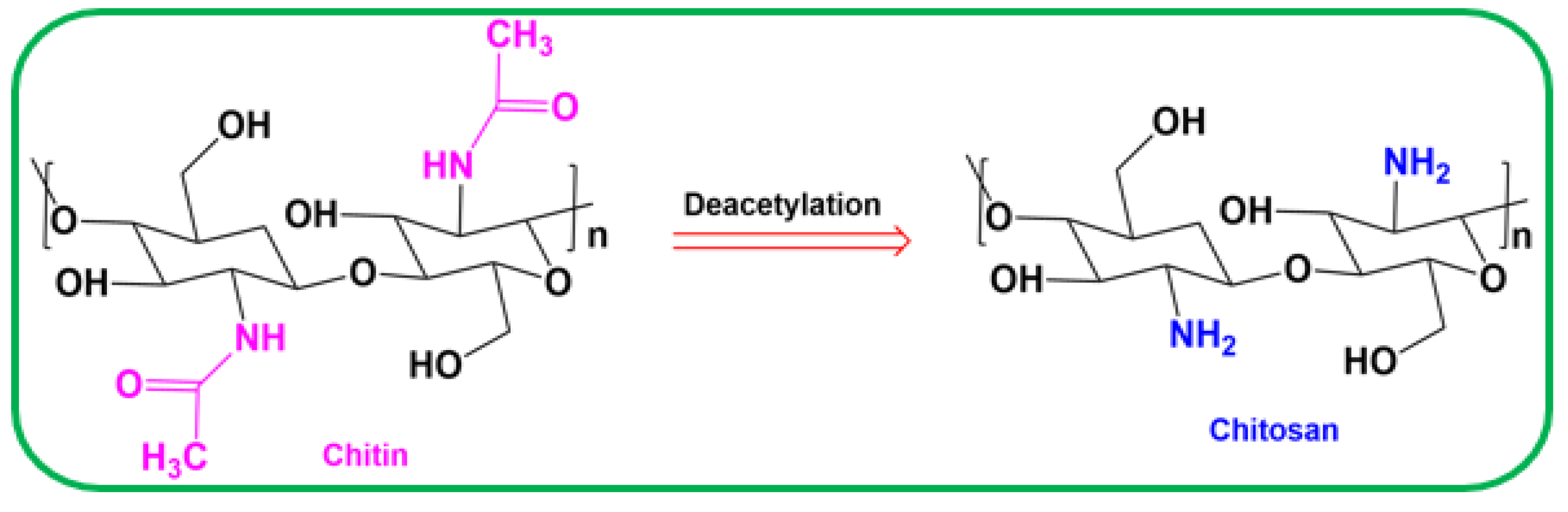
3.3. Synthesis of Chitosan Polymer
3.4. Preparation of Chitosan/Almond Gum Extract Composites
3.5. Characterization
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sunthar, T.P.M.; Boschetto, F.; Doan, N.H.; Honma, T.; Kinashi, K.; Adachi, T.; Marin, E.; Zhu, W.; Pezzotti, G. Antibacterial Property of Cellulose Acetate Composite Materials Reinforced with Aluminum Nitride. Antibiotics 2021, 10, 1292. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Rosato, A.; Salvagno, L.; Ceramella, J.; Longo, F.; Sinicropi, M.S.; Franchini, C. Searching for Small Molecules as Antibacterials: Non-Cytotoxic Diarylureas Analogues of Triclocarban. Antibiotics 2021, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Mallya, R.; Suvarna, V.; Khan, T.A.; Momin, M.; Omri, A. Nanoparticles—Attractive Carriers of Antimicrobial Essential Oils. Antibiotics 2022, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, V.; Nair, A.; Mallya, R.; Khan, T.; Omri, A. Antimicrobial Nanomaterials for Food Packaging. Antibiotics 2022, 11, 729. [Google Scholar] [CrossRef]
- Rhim, J.W.; Ng, P.K. Natural biopolymer-based nanocomposite films for packaging applications. Crit. Rev. Food Sci. Nutr. 2007, 47, 411–433. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Physicochemical and bioactivity of crosslinked chitosan-PVA film for food packaging applications. Int. J. Biol. Macromol. 2009, 45, 372–376. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N.; Tamilselvi, A. Antimicrobial, mechanical, barrier and thermal properties of bio-based poly (butylene adipate-co-terephthalate) (PBAT)/Ag2O nanocomposite films for packaging application. Polym. Adv. Technol. 2018, 29, 61–68. [Google Scholar] [CrossRef]
- Tighzert, V.L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar]
- Ciannamea, E.M.; Castillo, L.A.; Barbosa, S.E.; De, M.G. Angelis, Barrier properties and mechanical strength of bio-renewable, heat-sealable films based on gelatine, glycerol and soya bean oil for sustainable food packaging. React. Funct. Polym. 2018, 125, 29–36. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N. ZnO/PBAT nanocomposite films: Investigation on the mechanical and biological activity for antimicrobial food packaging. Polym. Adv. Technol. 2017, 28, 20–27. [Google Scholar] [CrossRef]
- Yeng, C.M.; Husseinsyah, S.; Ting, S.S. Modified corn cob filled chitosan biocomposite films. Polym. Plast. Technol. Eng. 2013, 52, 1496–1502. [Google Scholar] [CrossRef]
- Curcio, M.; Puoci, F.; Iemma, F.; Parisi, O.I.; Cirillo, G.; Spizzirri, U.G.; Picci, N. Covalent insertion of antioxidant molecules on chitosan by a free radical grafting procedure. J. Agric. Food Chem. 2009, 57, 5933–5938. [Google Scholar] [CrossRef] [PubMed]
- Darder, M.; Colilla, M.; Ruiz-Hitzky, E. Biopolymer-clay nanocomposites based on chitosan intercalated in montmorillonite. Chem. Mater. 2003, 15, 3774–3780. [Google Scholar] [CrossRef]
- Du, W.X.; Olsen, C.W.; Avena-Bustillos, R.J.; McHugh, T.H.; Levin, C.E.; Friedman, M. Storage stability and antibacterial activity against Escherichia coli O157:H7 of carvacrol in edible apple films made by two different casting methods. J. Agric. Food Chem. 2008, 56, 3082–3088. [Google Scholar] [CrossRef]
- Burrows, F.; Clifford, L.; Michael, A.; Oghenekome, O. Extraction and evaluation of chitosan from crab exoskeleton as a seed fungicide and plant growth enhancer. Am. Eur. J. Agric. Environ. Sci. 2007, 2, 103–111. [Google Scholar]
- Ghanem, A.; Skonberg, D. Effect of preparation method on the capture and release of biologically active molecules in chitosan gel beads. J. Appl. Polym. Sci. 2002, 84, 405–413. [Google Scholar] [CrossRef]
- Wang, J.; Feng, L.; Feng, Y.; Yan, A.; Ma, X. Preparation and properties of organic rectorite/epoxy resin nano-composites. Polym. Plast. Technol. Eng. 2012, 51, 1583–1588. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Hamdi, S. Use of almond gum and gum arabic as novel edible coating to delay postharvest ripening and to maintain sweet cherry (Prunus avium) quality during storage. J. Food Process. Preserv. 2015, 39, 1499–1508. [Google Scholar] [CrossRef]
- Rezaei, A.; Nasirpour, A.; Tavanai, H. Fractionation and some physicochemical properties of almond gum (Amygdalus communis L.) exudates. Food Hydroco. 2016, 60, 461–469. [Google Scholar] [CrossRef]
- Srivastava, M.; Kapoor, V.P. Seed galactomannans: An overview. Chem. Biodiv. 2005, 2, 295–317. [Google Scholar] [CrossRef]
- Farooq, U.; Sharma, P.K.; Malviya, R. Extraction and characterization of almond (Prunus sulcis) gum as pharmaceutical excipient. Am. Eur. J. Agric. Environ. Sci. 2014, 14, 269–274. [Google Scholar]
- Russo, G.M.; Simon, G.P.; Incarnato, L.C. Correlation between rheological, mechanical, and barrier properties in new copolyamide-based nanocomposite films. Macromolecules 2006, 39, 3855–3864. [Google Scholar] [CrossRef]
- Indumathi, M.P.; Sarojini, K.S.; Rajarajeswari, G.R. Antimicrobial and biodegradable chitosan/cellulose acetate phthalate/ZnO nano composite films with optimal oxygen permeability and hydrophobicity for extending the shelf life of black grape fruits. Int. J. Biol. Macromol. 2019, 132, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Sanuja, S.; Agalya, A.; Umapathy, M.J. Synthesis and characterisation of zinc oxide neem oil-chitosan bionanocomposite for food packaging applications. Int. J. Biol. Macromol. 2015, 74, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Pristijon, T.R.; Golding, J.B.; Stathopoulos, C.E. Lagerstroemia speciosa fruit mediated synthesis of silver nanoparticles and its application as filler in agar-based nanocomposite films for antimicrobial food packaging. Food Packag. Shelf Life 2017, 14, 99–106. [Google Scholar]
- Wu, Q.; Zhang, L. Structure and properties of casting films blended with starch and waterborne polyurethane. J. Appl. Polym. Sci. 2001, 79, 2006–2013. [Google Scholar] [CrossRef]
- Van Der Watt, E.; Pretorius, J.C. Purification and identification of active antibacterial components in Carpobrotus edulis L. J. Ethnopharmacol. 2001, 76, 87–91. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Krkic, N.; Lazic, V.; Gvozdenovic, J. Chitosan biofilm properties as affected by the addition of oregano essential oil. J. Process. Energy Agric. 2011, 15, 165–168. [Google Scholar]
- Bhushette, P.R.; Annapure, U.S. Comparative study of Acacia nilotica exudate gum and acacia gum. Int. J. Biol. Macromol. 2017, 102, 266–271. [Google Scholar] [CrossRef]
- Malsawmtluangi, C.; Thanzami, K.; Lalhlenmawia, H.; Selvan, V.; Palanisamy, S.; Kandasamy, R.; Pachuau, L. Physicochemical characteristics and antioxidant activity of Prunus cerasoides D. Don gum exudates. Int. J. Biol. Macromol. 2014, 69, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Gokkurt, T.; Findık, F.; Unal, H.; Mimaroglu, A. Extension in shelf life of fresh food using nanomaterials food packages. Polym. Plast. Technol. 2012, 51, 701–706. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N. TiO2 nanoparticles/poly (butylene adipate-coterephthalate) bionanocomposite films for packaging applications. Polym. Adv. Technol. 2017, 28, 1699–1706. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N.; Thiyagu, T.T. Preparation, characterization and mechanical properties of k-Carrageenan/SiO2 nanocomposite films for antimicrobial food packaging. Bull. Mater. Sci. 2017, 40, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. J. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Suneetha, M.; Rao, K.M.; Han, S.S. Mussel-inspired cell/tissue-adhesive, hemostatic hydrogels for tissue engineering applications. ACS Omega 2019, 4, 12647–12656. [Google Scholar] [CrossRef] [Green Version]
- Tongdeesoontorn, W.; Mauer, L.J.; Wongruong, S.; Sriburi, P.; Rachtanapun, P. Mechanical and physical properties of cassava starch-gelatin composite films. Int. J. Polym. Mater. 2012, 61, 778–792. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N. Nano-silica reinforced poly (butylene adipate-co- terephthalate) nanocomposites: Preparation, characterization and properties. Polym. Bull. 2019, 76, 4785–4801. [Google Scholar] [CrossRef]
- Lin, Y.; Bilotti, E.; Bastiaansen, C.W.M.; Peijs, T. Transparent semi-crystalline polymeric materials and their nanocomposites: A review. Polym. Eng. Sci. 2020, 60, 2351–2376. [Google Scholar] [CrossRef]
- Ramji, V.; Vishnuvarthanan, M. Influence of NiO supported silica nanoparticles on mechanical, barrier, optical and antibacterial properties of polylactic acid (PLA) bio nanocomposite films for food packaging applications. Silicon 2022, 14, 531–538. [Google Scholar] [CrossRef]


| Samples | Mechanical Strength | |
|---|---|---|
| Tensile Strength (MPa) | Elongation at Break (%) | |
| CSA-0.0 | 11.33 ± 1.06 b | 424.52 ± 1.85 a |
| CSA-10.0 | 13.33 ± 2.08 a | 387.41 ± 2.22 c |
| CSA-30.0 | 15.68 ± 1.87 c | 369.15 ± 2.90 c |
| CSA-50.0 | 17.51 ± 0.75 b | 334.70 ± 0.60 b |
| Samples | Barrier Properties | |
|---|---|---|
| OTR (cc/m2/24 h) | WVTR (g/m2/day) | |
| CSA-0.0 | 90.6 ± 2.05 b | 22.1 ± 0.71 a |
| CSA-10.0 | 74.0 ± 0.98 a | 19.5 ± 1.22 c |
| CSA-30.0 | 52.5 ± 0.61 c | 15.9 ± 0.99 c |
| CSA-50.0 | 32.9 ± 1.95 b | 11.6 ± 1.62 b |
| Samples | Antimicrobial Activity (Zone of Inhabitation in mm) | |
|---|---|---|
| S. aureus | E. coli | |
| CSA-0.0 | 20.0 ± 2.56 a | 20.0 ± 2.61 a |
| CSA-10.0 | 20.8 ± 3.08 a | 21.8 ± 4.65 c |
| CSA-30.0 | 22.1 ± 1.85 c | 24.5 ± 2.48 b |
| CSA-50.0 | 27.4 ± 3.94 b | 29.7 ± 3.02 b |
| Chitosan/Almond Gum Composites (wt. %) | Samples Name |
|---|---|
| 100.0–0.0 | CSA-0.0 |
| 90.0–10.0 | CSA-10.0 |
| 70.0–30.0 | CSA-30.0 |
| 50.0–50.0 | CSA-50.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesan, R.; Sekar, S.; Raorane, C.J.; Raj, V.; Kim, S.-C. Hydrophilic Composites of Chitosan with Almond Gum: Characterization and Mechanical, and Antimicrobial Activity for Compostable Food Packaging. Antibiotics 2022, 11, 1502. https://doi.org/10.3390/antibiotics11111502
Venkatesan R, Sekar S, Raorane CJ, Raj V, Kim S-C. Hydrophilic Composites of Chitosan with Almond Gum: Characterization and Mechanical, and Antimicrobial Activity for Compostable Food Packaging. Antibiotics. 2022; 11(11):1502. https://doi.org/10.3390/antibiotics11111502
Chicago/Turabian StyleVenkatesan, Raja, Surya Sekar, Chaitany Jayprakash Raorane, Vinit Raj, and Seong-Cheol Kim. 2022. "Hydrophilic Composites of Chitosan with Almond Gum: Characterization and Mechanical, and Antimicrobial Activity for Compostable Food Packaging" Antibiotics 11, no. 11: 1502. https://doi.org/10.3390/antibiotics11111502
APA StyleVenkatesan, R., Sekar, S., Raorane, C. J., Raj, V., & Kim, S.-C. (2022). Hydrophilic Composites of Chitosan with Almond Gum: Characterization and Mechanical, and Antimicrobial Activity for Compostable Food Packaging. Antibiotics, 11(11), 1502. https://doi.org/10.3390/antibiotics11111502










