Antimicrobial and Biocide Resistance among Canine and Feline Enterococcus faecalis, Enterococcus faecium, Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii Isolates from Diagnostic Submissions
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Susceptibility of Canine and Feline E. faecalis Isolates
2.2. Antimicrobial Susceptibility of Canine and Feline E. faecium Isolates
2.3. Antimicrobial Susceptibility of Canine and Feline E. coli Isolates
2.4. Antimicrobial Susceptibility of Canine and Feline P. aeruginosa Isolates
2.5. Antimicrobial Susceptibility of Canine and Feline A. baumannii Isolates
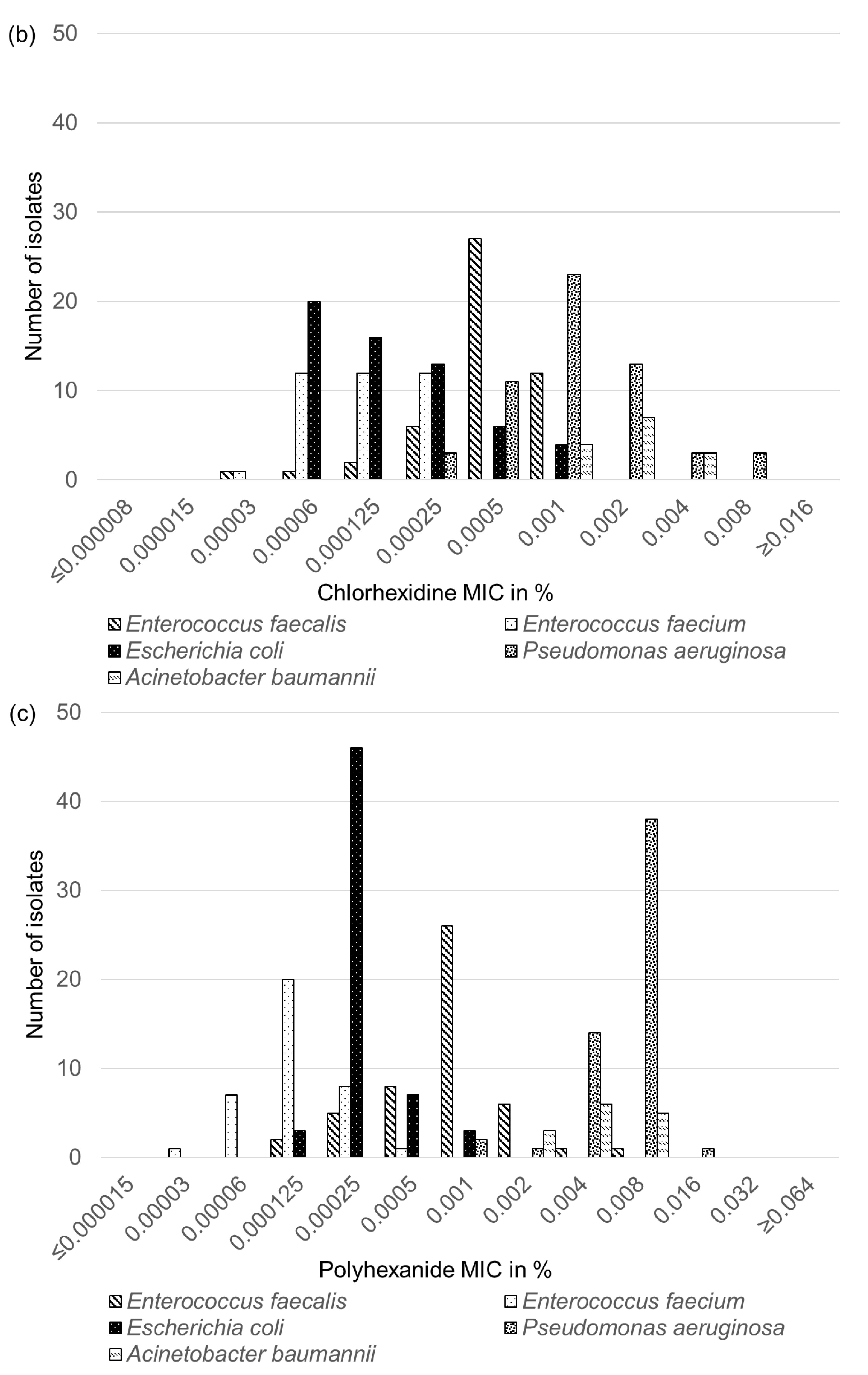
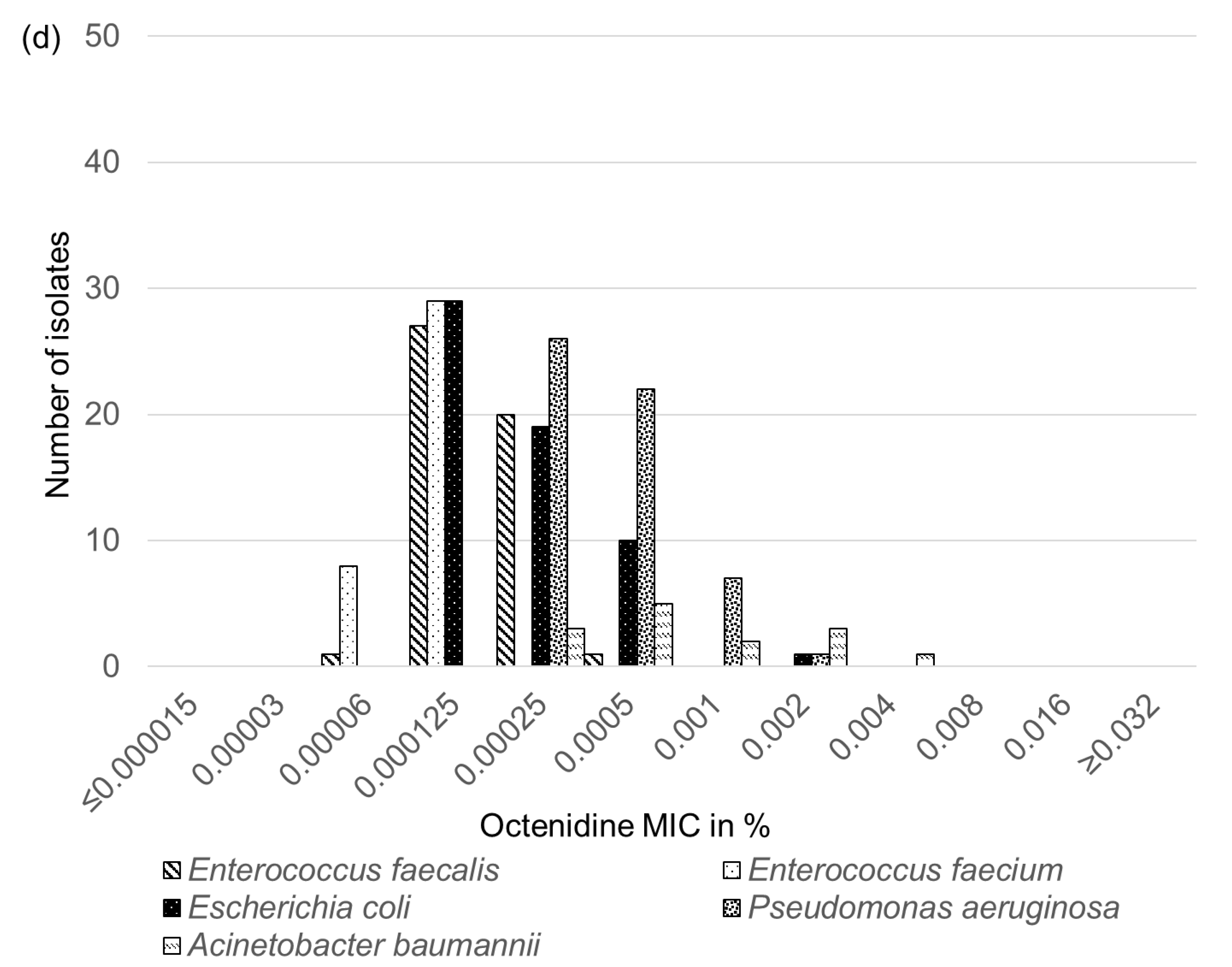
2.6. Biocide Susceptibility of Feline and Canine E. faecalis, E. faecium, E. coli, P. aeruginosa and A. baumannii Isolates
3. Discussion
4. Materials and Methods
4.1. Origin and Identification of the Isolates Investigated
4.2. Antimicrobial Susceptibility Testing
4.3. Calculation of the MAR Index
4.4. Whole Genome Sequencing and Analysis
4.5. Biocide Susceptibility Testing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Organization Antimicrobial Resistance: Global Report on Surveillance 2014; WHO: Geneva, Switzerland, 2014.
- Klous, G.; Huss, A.; Heederik, D.J.J.; Coutinho, R.A. Human-livestock contacts and their relationship to transmission of zoonotic pathogens, a systematic review of literature. One Health 2016, 2, 65–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argudín, M.A.; Deplano, A.; Meghraoui, A.; Dodémont, M.; Heinrichs, A.; Denis, O.; Nonhoff, C.; Roisin, S. Bacteria from Animals as a Pool of Antimicrobial Resistance Genes. Antibiotics 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Loeffler, A.; Kadlec, K. Bacterial resistance to antimicrobial agents and its impact on veterinary and human medicine. Vet. Dermatol. 2017, 28, 82.e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, W.K.; Shin, S.; Park, Y.K.; Noh, S.M.; Shin, S.R.; Yoo, H.S.; Park, S.C.; Park, Y.H.; Park, K.T. Distribution and antimicrobial resistance profiles of bacterial species in stray dogs, hospital-admitted dogs, and veterinary staff in South Korea. Prev. Vet. Med. 2020, 184, 105151. [Google Scholar] [CrossRef]
- van Hoek, A.H.A.M.; Dierikx, C.; Bosch, T.; Schouls, L.; van Duijkeren, E.; Visser, M. Transmission of ESBL-producing Escherichia coli between broilers and humans on broiler farms. J. Antimicrob. Chemother. 2020, 75, 543–549. [Google Scholar] [CrossRef]
- van den Bogaard, A.E.; Willems, R.; London, N.; Top, J.; Stobberingh, E.E. Antibiotic resistance of faecal enterococci in poultry, poultry farmers and poultry slaughterers. J. Antimicrob. Chemother. 2002, 49, 497–505. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Wang, Y.; He, J.; Cai, C.; Liu, Q.; Yang, D.; Zou, Z.; Shi, L.; Jia, J.; Wang, Y.; et al. Prevalence and risk analysis of mobile colistin resistance and extended-spectrum beta-lactamase genes carriage in pet dogs and their owners: A population based cross-sectional study. Emerg. Microbes Infect. 2021, 10, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sanz, E.; Ceballos, S.; Ruiz-Ripa, L.; Zarazaga, M.; Torres, C. Clonally Diverse Methicillin and Multidrug Resistant Coagulase Negative Staphylococci Are Ubiquitous and Pose Transfer Ability Between Pets and Their Owners. Front. Microbiol. 2019, 10, 485. [Google Scholar] [CrossRef] [Green Version]
- Ljungquist, O.; Ljungquist, D.; Myrenås, M.; Rydén, C.; Finn, M.; Bengtsson, B. Evidence of household transfer of ESBL-/pAmpC-producing Enterobacteriaceae between humans and dogs—A pilot study. Infect. Ecol. Epidemiol. 2016, 6, 31514. [Google Scholar] [CrossRef]
- Ghosh, A.; Dowd, S.E.; Zurek, L. Dogs leaving the ICU carry a very large multi-drug resistant enterococcal population with capacity for biofilm formation and horizontal gene transfer. PLoS ONE 2011, 6, e22451. [Google Scholar] [CrossRef] [Green Version]
- Nittayasut, N.; Yindee, J.; Boonkham, P.; Yata, T.; Suanpairintr, N.; Chanchaithong, P. Multiple and High-Risk Clones of Extended-Spectrum Cephalosporin-Resistant and blaNDM-5-Harbouring Uropathogenic Escherichia coli from Cats and Dogs in Thailand. Antibiotics 2021, 10, 1374. [Google Scholar] [CrossRef] [PubMed]
- Mišić, D.; Asanin, J.; Spergser, J.; Szostak, M.; Loncaric, I. OXA-72-Mediated Carbapenem Resistance in Sequence Type 1 Multidrug (Colistin)-Resistant Acinetobacter baumannii Associated with Urinary Tract Infection in a Dog from Serbia. Antimicrob. Agents Chemother. 2018, 62, e00219-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanchaithong, P.; Prapasarakul, N.; Sirisopit Mehl, N.; Suanpairintr, N.; Teankum, K.; Collaud, A.; Endimiani, A.; Perreten, V. Extensively drug-resistant community-acquired Acinetobacter baumannii sequence type 2 in a dog with urinary tract infection in Thailand. J. Glob. Antimicrob. Resist. 2018, 13, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Dégi, J.; Moțco, O.A.; Dégi, D.M.; Suici, T.; Mareș, M.; Imre, K.; Cristina, R.T. Antibiotic Susceptibility Profile of Pseudomonas aeruginosa Canine Isolates from a Multicentric Study in Romania. Antibiotics 2021, 10, 846. [Google Scholar] [CrossRef]
- Nocera, F.P.; Ambrosio, M.; Fiorito, F.; Cortese, L.; De Martino, L. On Gram-Positive- and Gram-Negative-Bacteria-Associated Canine and Feline Skin Infections: A 4-Year Retrospective Study of the University Veterinary Microbiology Diagnostic Laboratory of Naples, Italy. Animals 2021, 11, 1603. [Google Scholar] [CrossRef] [PubMed]
- Stępień-Pyśniak, D.; Bertelloni, F.; Dec, M.; Cagnoli, G.; Pietras-Ożga, D.; Urban-Chmiel, R.; Ebani, V.V. Characterization and Comparison of Enterococcus spp. Isolates from Feces of Healthy Dogs and Urine of Dogs with UTIs. Animals 2021, 11, 2845. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti. Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Santaniello, A.; Sansone, M.; Fioretti, A.; Menna, L.F. Systematic Review and Meta-Analysis of the Occurrence of ESKAPE Bacteria Group in Dogs, and the Related Zoonotic Risk in Animal-Assisted Therapy, and in Animal-Assisted Activity in the Health Context. Int. J. Environ. Res. Public Health 2020, 17, 3278. [Google Scholar] [CrossRef]
- Scientific Committee on Emerging and Newly Identified Health Risks. Assessment of the Antibiotic Resistance Effects of Biocides; European Commission Health & Consumer Protection DG Directorate C: Brussels, Belgium, 2009. [Google Scholar]
- Borio, S.; Colombo, S.; La Rosa, G.; De Lucia, M.; Damborg, P.; Guardabassi, L. Effectiveness of a combined (4% chlorhexidine digluconate shampoo and solution) protocol in MRS and non-MRS canine superficial pyoderma: A randomized, blinded, antibiotic-controlled study. Vet. Dermatol. 2015, 26, 339–344.e72. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, A.; Cobb, M.A.; Bond, R. Comparison of a chlorhexidine and a benzoyl peroxide shampoo as sole treatment in canine superficial pyoderma. Vet. Rec. 2011, 169, 249. [Google Scholar] [CrossRef] [PubMed]
- Jeffers, J.G. Topical therapy for drug-resistant pyoderma in small animals. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Prince, N.H.; Nonemaker, W.S.; Norgard, R.C.; Prince, D.L. Drug resistance with topical antiseptics. J. Pharm. Sci. 1978, 67, 1629–1631. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Davies, A.; Russell, A.D. Sensitivity and resistance of Escherichia coli and Staphylococcus aureus to chlorhexidine. Lett. Appl. Microbiol. 1992, 14, 33–36. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Schmitt, R.; Wöhrmann, A.; Stefanik, D.; Seifert, H. Resistance to disinfectants in epidemiologically defined clinical isolates of Acinetobacter baumannii. J. Hosp. Infect. 2007, 66, 174–181. [Google Scholar] [CrossRef]
- Bock, L.J.; Wand, M.E.; Sutton, J.M. Varying activity of chlorhexidine-based disinfectants against Klebsiella pneumoniae clinical isolates and adapted strains. J. Hosp. Infect. 2016, 93, 42–48. [Google Scholar] [CrossRef]
- Schug, A.R.; Bartel, A.; Scholtzek, A.D.; Meurer, M.; Brombach, J.; Hensel, V.; Fanning, S.; Schwarz, S.; Feßler, A.T. Biocide susceptibility testing of bacteria: Development of a broth microdilution method. Vet. Microbiol. 2020, 248, 108791. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals, 5th ed.; CLSI Supplement VET01S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Liu, H.; Wang, Y.; Wu, C.; Schwarz, S.; Shen, Z.; Jeon, B.; Ding, S.; Zhang, Q.; Shen, J. A novel phenicol exporter gene, fexB, found in enterococci of animal origin. J. Antimicrob. Chemother. 2012, 67, 322–325. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lv, Y.; Cai, J.; Schwarz, S.; Cui, L.; Hu, Z.; Zhang, R.; Li, J.; Zhao, Q.; He, T.; et al. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J. Antimicrob. Chemother. 2015, 70, 2182–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, S.; Silley, P.; Simjee, S.; Woodford, N.; van Duijkeren, E.; Johnson, A.P.; Gaastra, W. Assessing the antimicrobial susceptibility of bacteria obtained from animals. Vet. Microbiol. 2010, 141, 13. [Google Scholar] [CrossRef] [PubMed]
- Pietta, E.; Montealegre, M.C.; Roh, J.H.; Cocconcelli, P.S.; Murray, B.E. Enterococcus faecium PBP5-S/R, the missing link between PBP5-S and PBP5-R. Antimicrob. Agents Chemother. 2014, 58, 6978–6981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- el Amin, N.A.; Jalal, S.; Wretlind, B. Alterations in GyrA and ParC associated with fluoroquinolone resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 1999, 43, 947–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CLSI. Understanding Susceptibility Test. Data as a Component of Antimicrobial Stewardship in Veterinary Settings Performance, 1st ed.; CLSI Report VET09; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- Richter, A.; Feßler, A.T.; Böttner, A.; Köper, L.M.; Wallmann, J.; Schwarz, S. Reasons for antimicrobial treatment failures and predictive value of in-vitro susceptibility testing in veterinary practice: An overview. Vet. Microbiol. 2020, 245, 108694. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.T.; Lubbers, B.V.; Schwarz, S.; Watts, J.L. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J. Antimicrob. Chemother. 2018, 73, 1460–1463. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Belas, A.; Franco, A.; Aboim, C.; Gama, L.T.; Pomba, C. Increase in antimicrobial resistance and emergence of major international high-risk clonal lineages in dogs and cats with urinary tract infection: 16 year retrospective study. J. Antimicrob. Chemother. 2018, 73, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Ben Said, L.; Dziri, R.; Sassi, N.; Lozano, C.; Ben Slama, K.; Ouzari, I.; Torres, C.; Klibi, N. Species distribution, antibiotic resistance and virulence traits in canine and feline enterococci in Tunisia. Acta Vet. Hung. 2017, 65, 173–184. [Google Scholar] [CrossRef]
- Leener, E.D.; Decostere, A.; De Graef, E.M.; Moyaert, H.; Haesebrouck, F. Presence and mechanism of antimicrobial resistance among enterococci from cats and dogs. Microb. Drug Resist. 2005, 11, 395–403. [Google Scholar] [CrossRef]
- van den Bunt, G.; Top, J.; Hordijk, J.; de Greeff, S.C.; Mughini-Gras, L.; Corander, J.; van Pelt, W.; Bonten, M.J.M.; Fluit, A.C.; Willems, R.J.L. Intestinal carriage of ampicillin- and vancomycin-resistant Enterococcus faecium in humans, dogs and cats in the Netherlands. J. Antimicrob. Chemother. 2018, 73, 607–614. [Google Scholar] [CrossRef]
- Abbott, Y.; Kirby, B.M.; Karczmarczyk, M.; Markey, B.K.; Leonard, F.C.; Fitzgerald, S. High-level gentamicin-resistant and vancomycin-resistant Enterococcus faecium isolated from a wound in a dog. J. Small Anim. Pract. 2009, 50, 194–197. [Google Scholar] [CrossRef]
- Simjee, S.; White, D.G.; McDermott, P.F.; Wagner, D.D.; Zervos, M.J.; Donabedian, S.M.; English, L.L.; Hayes, J.R.; Walker, R.D. Characterization of Tn1546 in vancomycin-resistant Enterococcus faecium isolated from canine urinary tract infections: Evidence of gene exchange between human and animal enterococci. J. Clin. Microbiol. 2002, 40, 4659–4665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.J.; Xu, X.R.; Schwarz, S.; Wang, X.M.; Dai, L.; Zheng, H.J.; Liu, S. Characterization of the IncA/C plasmid pSCEC2 from Escherichia coli of swine origin that harbours the multiresistance gene cfr. J. Antimicrob. Chemother. 2014, 69, 385–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirel, L.; Madec, J.Y.; Lupo, A.; Schink, A.K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolle, I.; Prenger-Berninghoff, E.; Stamm, I.; Scheufen, S.; Hassdenteufel, E.; Guenther, S.; Bethe, A.; Pfeifer, Y.; Ewers, C. Emergence of OXA-48 carbapenemase-producing Escherichia coli and Klebsiella pneumoniae in dogs. J. Antimicrob. Chemother. 2013, 68, 2802–2808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, S.; Wong, H.S.; Turnidge, J.; Johnson, J.R.; Trott, D.J. Carbapenemase-producing bacteria in companion animals: A public health concern on the horizon. J. Antimicrob. Chemother. 2014, 69, 1155–1157. [Google Scholar] [CrossRef] [Green Version]
- Guerra, B.; Fischer, J.; Helmuth, R. An emerging public health problem: Acquired carbapenemase-producing microorganisms are present in food-producing animals, their environment, companion animals and wild birds. Vet. Microbiol. 2014, 171, 290–297. [Google Scholar] [CrossRef]
- Poirel, L.; Stephan, R.; Perreten, V.; Nordmann, P. The carbapenemase threat in the animal world: The wrong culprit. J. Antimicrob. Chemother. 2014, 69, 2007–2008. [Google Scholar] [CrossRef] [Green Version]
- Grobbel, M.; Lübke-Becker, A.; Alesík, E.; Schwarz, S.; Wallmann, J.; Werckenthin, C.; Wieler, L.H. Antimicrobial susceptibility of Escherichia coli from swine, horses, dogs and cats as determined in the BfT-GermVet monitoring program 2004-2006. Berl. Münch. Tierärztl. Wochenschr. 2007, 120, 391–401. [Google Scholar]
- Werckenthin, C.; Alesík, E.; Grobbel, M.; Lübke-Becker, A.; Schwarz, S.; Wieler, L.H.; Wallmann, J. Antimicrobial susceptibility of Pseudomonas aeruginosa from dogs and cats as well as Arcanobacterium pyogenes from cattle and swine as determined in the BfT-GermVet monitoring program 2004–2006. Berl. Münch. Tierärztl. Wochenschr. 2007, 120, 412–422. [Google Scholar]
- Köper, L.M.; Bode, C.; Bender, A.; Reimer, I.; Heberer, T.; Wallmann, J. Eight years of sales surveillance of antimicrobials for veterinary use in Germany-What are the perceptions? PLoS ONE 2020, 15, e0237459. [Google Scholar] [CrossRef]
- EMEA. Commitee for Veterinary Medicinal Products Benzalkonium Chloride Summary Report. The European Agency for the Evaluation of Medicinal Products. EMEA/MRL/306/97. 1997. Available online: https://www.ema.europa.eu/en/documents/mrl-report/benzalkonium-chloride-summary-report-committee-veterinary-medicinal-products_en.pdf (accessed on 1 December 2021).
- Mišić, D.; Kiskaroly, F.; Szostak, M.P.; Cabal, A.; Ruppitsch, W.; Bernreiter-Hofer, T.; Milovanovic, V.; Feßler, A.T.; Allerberger, F.; Spergser, J.; et al. The First Report of mcr-1-Carrying Escherichia coli Originating from Animals in Serbia. Antibiotics 2021, 10, 1063. [Google Scholar] [CrossRef] [PubMed]
- Bernreiter-Hofer, T.; Schwarz, L.; Müller, E.; Cabal-Rosel, A.; Korus, M.; Mišić, D.; Frankenfeld, K.; Abraham, K.; Grünzweil, O.; Weiss, A.; et al. The Pheno- and Genotypic Characterization of Porcine Escherichia coli Isolates. Microorganisms 2021, 9, 1676. [Google Scholar] [CrossRef] [PubMed]
- Grünzweil, O.M.; Palmer, L.; Cabal, A.; Szostak, M.P.; Ruppitsch, W.; Kornschober, C.; Korus, M.; Mišić, D.; Bernreiter-Hofer, T.; Korath, A.D.J.; et al. Presence of β-Lactamase-producing Enterobacterales and Salmonella Isolates in Marine Mammals. Int. J. Mol. Sci. 2021, 22, 5905. [Google Scholar] [CrossRef]
- Werner, G.; Fleige, C.; Feßler, A.T.; Timke, M.; Kostrzewa, M.; Zischka, M.; Peters, T.; Kaspar, H.; Schwarz, S. Improved identification including MALDI-TOF mass spectrometry analysis of group D streptococci from bovine mastitis and subsequent molecular characterization of corresponding Enterococcus faecalis and Enterococcus faecium isolates. Vet. Microbiol. 2012, 160, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Kauter, A.; Epping, L.; Ghazisaeedi, F.; Lübke-Becker, A.; Wolf, S.A.; Kannapin, D.; Stoeckle, S.D.; Semmler, T.; Günther, S.; Gehlen, H.; et al. Frequency, Local Dynamics, and Genomic Characteristics of ESBL-Producing Escherichia coli Isolated From Specimens of Hospitalized Horses. Front. Microbiol. 2021, 12, 671676. [Google Scholar] [CrossRef] [PubMed]
- Ewers, C.; Klotz, P.; Leidner, U.; Stamm, I.; Prenger-Berninghoff, E.; Göttig, S.; Semmler, T.; Scheufen, S. OXA-23 and ISAba1-OXA-66 class D beta-lactamases in Acinetobacter baumannii isolates from companion animals. Int. J. Antimicrob. Agents 2017, 49, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Krumperman, P.H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 1983, 46, 165–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Antimicrobial Agent(s) | Number of Isolates for Which the MIC Value (mg/L) Is | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.008 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | |
| Penicillin G | - | - | - | - | - | - | 5 | 12 | 32 | - | - | - | - | |||||
| Ampicillin | - | 1 | 2 | 4 | 5 | 35 | 2 | - | - | - | - | - | - | |||||
| Amoxicillin-clavulanic acid (2:1) | - | 1 | 3 | 7 | 16 | 22 | - | - | - | - | - | - | - | |||||
| Cephalothin | - | - | - | - | - | - | - | - | 6 | 43 | - | - | - | |||||
| Cefotaxime | - | - | - | 1 | - | - | 1 | - | - | - | - | - | 47 | |||||
| Cefoperazone | - | - | - | - | - | - | - | 4 | 12 | 28 | 5 | |||||||
| Ceftiofur | - | - | 1 | - | 1 | 1 | - | - | 1 | 1 | 6 | 15 | 23 | |||||
| Florfenicol | - | - | - | 2 | 20 | 26 | 1 | - | - | - | - | - | - | |||||
| Erythromycin | - | - | - | - | 4 | 3 | 6 | 20 | 12 | - | - | - | 4 | |||||
| Tylosin | - | - | - | 3 | 20 | 15 | 6 | 1 | - | - | - | - | 4 | |||||
| Tilmicosin | - | - | - | - | - | - | 1 | 1 | 28 | 13 | 3 | - | 3 | |||||
| Clindamycin | - | - | 1 | - | - | - | 1 | - | 2 | 30 | 12 | 1 | 2 | |||||
| Streptomycin | - | - | - | - | - | - | - | - | 6 | 31 | 8 | - | 4 | |||||
| Gentamicin | - | - | - | - | - | 1 | 10 | 31 | 7 | - | - | - | - | |||||
| Neomycin | - | - | - | - | - | - | 1 | - | 1 | 8 | 39 | |||||||
| Ciprofloxacin | - | - | - | - | - | - | - | 30 | 16 | 1 | - | 1 | 1 | |||||
| Enrofloxacin | - | - | - | - | - | - | 8 | 36 | 3 | - | - | 1 | 1 | |||||
| Marbofloxacin | - | - | - | - | - | - | - | 2 | 38 | 7 | - | - | 2 | |||||
| Nalidixic acid | - | - | - | - | - | - | - | - | - | - | - | 1 | 48 | |||||
| Trimethoprim-sulfamethoxazole (1:19) | 6 | 23 | 15 | 1 | 2 | - | 1 | - | 1 | - | - | - | - | |||||
| Tetracycline | 4 | 15 | 5 | 7 | - | - | - | - | 4 | 14 | - | - | - | |||||
| Doxycycline | 4 | 17 | 4 | 6 | - | - | 1 | 11 | 4 | 1 | - | - | 1 | |||||
| Linezolid | - | - | - | - | 1 | 15 | 30 | 2 | 1 | - | - | - | - | |||||
| Tiamulin | - | - | - | - | - | 1 | - | - | - | 1 | - | - | 47 | |||||
| Vancomycin | - | - | - | - | - | - | 31 | 15 | 3 | - | - | - | - | |||||
| Quinupristin-dalfopristin | - | - | - | - | 1 | - | 2 | - | 3 | 37 | 6 | - | - | |||||
| Antimicrobial Agent (s) | Number of Isolates for Which the MIC Value (mg/L) Is | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.008 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | |
| Penicillin G | - | - | - | - | - | 1 | - | 4 | 3 | - | 1 | - | 28 | |||||
| Ampicillin | - | - | 1 | - | 1 | 4 | 2 | 1 | - | - | 1 | 9 | 18 | |||||
| Amoxicillin-clavulanic acid (2:1) | - | - | 1 | 2 | 2 | 3 | 1 | - | - | 1 | 9 | 2 | 16 | |||||
| Cephalothin | - | - | - | - | - | - | - | 1 | 2 | 2 | 2 | 1 | 29 | |||||
| Cefotaxime | - | - | - | 1 | - | - | 1 | - | - | 1 | - | - | 34 | |||||
| Cefoperazone | - | - | - | - | - | - | - | 2 | 1 | 3 | 31 | |||||||
| Ceftiofur | - | 1 | - | - | 1 | - | - | - | - | - | 1 | 1 | 33 | |||||
| Florfenicol | - | - | 1 | 1 | - | 29 | 5 | - | 1 | - | - | - | - | |||||
| Erythromycin | - | - | - | - | - | - | 2 | 4 | 5 | 3 | 1 | - | 22 | |||||
| Tylosin | - | - | 1 | 7 | 9 | 1 | 2 | 2 | - | - | - | - | 15 | |||||
| Tilmicosin | - | - | - | - | - | - | - | 2 | 12 | 8 | - | - | 15 | |||||
| Clindamycin | - | - | 3 | 6 | 1 | 1 | 1 | 1 | 6 | 4 | 2 | 1 | 11 | |||||
| Streptomycin | - | - | - | - | - | - | - | 8 | 9 | - | - | - | 20 | |||||
| Gentamicin | - | - | - | - | - | 4 | 17 | 4 | - | 1 | - | - | 11 | - | ||||
| Neomycin | - | - | - | - | 1 | 3 | 5 | 11 | 3 | 1 | 13 | |||||||
| Ciprofloxacin | - | - | - | - | - | - | - | - | - | 6 | 3 | 3 | 25 | |||||
| Enrofloxacin | - | - | - | - | - | - | - | - | - | 7 | 3 | 2 | 25 | |||||
| Marbofloxacin | - | - | - | - | - | - | - | - | - | 4 | 6 | 2 | 25 | |||||
| Nalidixic acid | - | - | - | - | - | - | - | - | - | - | 2 | 2 | 33 | |||||
| Trimethoprim-sulfamethoxazole (1:19) | 12 | 6 | 9 | 8 | 2 | - | - | - | - | - | - | - | - | |||||
| Tetracycline | - | 1 | 7 | 1 | - | - | 1 | - | 2 | 3 | 22 | - | - | |||||
| Doxycycline | - | 6 | 3 | - | - | 1 | 2 | 6 | 17 | 2 | - | - | - | |||||
| Linezolid | - | - | - | - | 2 | 1 | 26 | 8 | - | - | - | - | - | |||||
| Tiamulin | - | - | - | - | 2 | 8 | 1 | 2 | - | - | - | 1 | 23 | |||||
| Vancomycin | - | - | - | - | - | 19 | 11 | 5 | 1 | - | - | - | 1 | |||||
| Quinupristin-dalfopristin | - | - | - | - | 1 | 9 | 4 | 5 | 16 | 2 | - | - | - | |||||
| Antimicrobial Agent (s) | Number of Isolates for Which the MIC Value (mg/L) Is | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.008 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | |
| Penicillin G | - | - | - | - | - | - | - | - | - | 1 | - | 12 | 46 | |||||
| Ampicillin | - | - | - | - | 1 | - | 4 | 6 | - | - | - | - | 31 | |||||
| Ampicillin (UTI) | - | - | - | - | - | - | 7 | 4 | - | - | - | - | 6 | |||||
| Amoxicillin-clavulanic acid (2:1) | - | - | - | - | - | 1 | 3 | 8 | 8 | 14 | 3 | 4 | 1 | |||||
| Amoxicillin-clavulanic acid (2:1) (UTI) | - | - | - | - | - | - | 2 | 8 | 3 | 2 | 2 | - | - | |||||
| Imipenem | - | - | - | 39 | 18 | 1 | - | - | 1 | - | - | - | - | |||||
| Cephalothin | - | - | - | - | 1 | - | - | 8 | 17 | 8 | 2 | - | 23 | |||||
| Cefotaxime | - | 1 | 17 | 14 | 2 | 1 | - | - | 1 | 1 | 2 | 2 | 18 | |||||
| Cefoperazone | 1 | 4 | 12 | 6 | 1 | 2 | 4 | 2 | 1 | 3 | 23 | |||||||
| Ceftiofur | - | - | 2 | 8 | 23 | 2 | 1 | - | - | 4 | - | - | 19 | |||||
| Florfenicol | - | - | - | - | - | 10 | 35 | 10 | 1 | 1 | - | 1 | 1 | |||||
| Streptomycin | - | - | - | - | 13 | 16 | 3 | 2 | - | 6 | 7 | 3 | 9 | |||||
| Gentamicin | - | 1 | 29 | 15 | 3 | - | - | 1 | 5 | 4 | 1 | - | - | |||||
| Neomycin | - | 1 | 18 | 26 | 4 | 1 | 1 | - | 1 | 2 | 5 | |||||||
| Ciprofloxacin | 1 | 17 | 11 | 2 | 2 | 1 | 2 | - | - | - | 4 | 4 | 15 | |||||
| Enrofloxacin | - | 6 | 20 | 5 | 1 | 1 | 2 | 1 | 1 | - | - | 5 | 17 | |||||
| Marbofloxacin | - | 5 | 21 | 5 | 2 | - | 1 | 2 | - | - | 7 | 12 | 4 | |||||
| Nalidixic acid | - | - | - | - | 4 | 23 | 3 | 2 | - | 3 | - | 1 | 23 | |||||
| Trimethoprim-sulfamethoxazole (1:19) | - | 1 | 19 | 9 | 2 | 2 | 2 | - | - | - | - | 1 | 23 | |||||
| Tetracycline | - | - | - | 11 | 16 | 7 | - | - | - | 6 | 14 | 5 | - | |||||
| Doxycycline | - | - | - | 2 | 14 | 13 | 3 | 6 | 6 | 10 | 4 | 1 | - | |||||
| Colistin | 1 | - | - | 6 | 51 | - | 1 | - | - | - | - | - | - | |||||
| Antimicrobial Agent (s) | Number of Isolates for Which the MIC Value (mg/L) Is | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.008 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | |
| Penicillin G | - | - | - | - | - | - | - | - | - | - | - | - | 56 | |||||
| Ampicillin | - | - | - | - | - | - | - | - | - | - | - | - | 56 | |||||
| Amoxicillin-clavulanic acid (2:1) | - | - | - | - | - | - | - | - | - | - | - | - | 56 | |||||
| Imipenem | - | - | - | - | - | 8 | 19 | 21 | 5 | 3 | - | - | - | |||||
| Cephalothin | - | - | - | - | - | - | - | - | - | - | - | - | 56 | |||||
| Cefotaxime | - | - | - | - | - | - | - | - | - | 11 | 22 | 16 | 7 | |||||
| Cefoperazone | - | - | - | - | 1 | 3 | 24 | 20 | 5 | 3 | - | |||||||
| Ceftiofur | - | - | - | - | - | - | 1 | - | - | 14 | 27 | 11 | 3 | |||||
| Florfenicol | - | - | - | - | - | - | - | 2 | 14 | 28 | 10 | 2 | ||||||
| Streptomycin | - | - | - | - | - | 2 | 16 | 23 | 9 | 2 | 1 | 3 | ||||||
| Gentamicin | - | - | 1 | 10 | 26 | 14 | 4 | 1 | - | - | - | - | - | |||||
| Neomycin | - | - | - | - | 6 | 16 | 12 | 11 | 5 | 5 | 1 | |||||||
| Ciprofloxacin | - | - | - | 6 | 22 | 11 | 7 | 1 | 2 | 3 | 2 | - | 2 | |||||
| Enrofloxacin | - | - | - | - | - | 3 | 21 | 14 | 7 | 3 | 1 | 2 | 5 | |||||
| Marbofloxacin | - | - | - | - | 11 | 22 | 8 | 5 | 4 | 1 | 1 | 4 | ||||||
| Nalidixic acid | - | - | - | - | - | - | - | - | 23 | 18 | 3 | 12 | ||||||
| Trimethoprim-sulfamethoxazole (1:19) | - | - | - | - | - | - | 1 | 3 | 27 | 16 | 5 | 1 | 3 | |||||
| Tetracycline | - | - | - | - | - | 1 | 3 | 34 | 12 | 6 | - | - | - | |||||
| Doxycycline | - | - | - | - | - | 2 | 6 | 27 | 18 | 3 | - | - | ||||||
| Colistin | - | - | - | 3 | 40 | 13 | - | - | - | - | - | - | ||||||
| Antimicrobial Agent (s) | Number of Isolates for Which the MIC Value (mg/L) Is | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.008 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | |
| Penicillin G | - | - | - | - | - | - | - | - | - | - | - | 7 | 7 | |||||
| Ampicillin | - | - | - | - | - | - | - | - | 7 | 3 | - | - | 4 | |||||
| Amoxicillin-clavulanic acid (2:1) | - | - | - | - | - | - | - | 1 | 7 | 2 | - | - | 4 | |||||
| Imipenem | - | - | - | 2 | 7 | 1 | - | 4 | - | - | - | - | - | |||||
| Cephalothin | - | - | - | - | - | - | - | - | - | - | - | - | 14 | |||||
| Cefotaxime | - | - | - | - | - | - | - | - | - | 9 | 1 | - | 4 | |||||
| Cefoperazone | - | - | - | - | - | - | - | - | 1 | 7 | 6 | |||||||
| Ceftiofur | - | - | - | - | - | - | - | - | 4 | 4 | 1 | 2 | 3 | |||||
| Florfenicol | - | - | - | - | - | - | - | - | 1 | 1 | 11 | 1 | - | |||||
| Streptomycin | - | - | - | - | 2 | 1 | 3 | 3 | 1 | - | - | - | 4 | |||||
| Gentamicin | - | - | 4 | 5 | - | 1 | 1 | - | - | - | 3 | - | - | |||||
| Neomycin | - | - | 4 | 4 | 2 | - | - | 1 | 2 | 1 | - | |||||||
| Ciprofloxacin | - | - | - | 2 | 6 | 1 | - | - | - | - | - | - | 5 | |||||
| Enrofloxacin | - | - | 7 | 2 | - | - | - | - | - | - | 3 | 2 | - | |||||
| Marbofloxacin | - | - | 2 | 5 | 2 | - | - | - | - | - | 4 | 1 | - | |||||
| Nalidixic acid | - | - | - | - | - | 4 | 4 | 1 | - | - | - | - | 5 | |||||
| Trimethoprim-sulfamethoxazole (1:19) | - | - | - | 7 | 3 | - | - | - | - | 1 | - | - | 3 | |||||
| Tetracycline | - | - | 2 | 5 | 3 | - | 1 | - | - | - | - | 3 | - | |||||
| Doxycycline | 2 | 6 | 1 | 2 | - | - | - | - | - | 3 | - | - | - | |||||
| Colistin | - | - | - | - | 13 | 1 | - | - | - | - | - | - | - | |||||
| Infections | E. faecalis (n = 49) | E. faecium (n = 37) | E. coli (n = 59) | P. aeruginosa (n = 56) | A. baumannii (n = 14) |
|---|---|---|---|---|---|
| Wound infections | |||||
| Dog | 16 | 11 | 17 | 12 | 4 |
| Cat | 7 | 2 | 5 | - | 1 |
| Skin infections * | |||||
| Dog | 10 | 4 | 10 | 34 | 3 |
| Cat | - | 1 | - | 1 | - |
| Urinary tract infections | |||||
| Dog | 3 | 8 | 3 | 3 | - |
| Cat | 7 | 2 | 14 | - | - |
| Respiratory tract infections | |||||
| Dog | 1 | - | 1 | 3 | |
| Cat | - | - | - | 2 | - |
| Others ** | |||||
| Dog | 5 | 4 | 5 | 4 | - |
| Cat | - | 5 | 4 | - | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feßler, A.T.; Scholtzek, A.D.; Schug, A.R.; Kohn, B.; Weingart, C.; Hanke, D.; Schink, A.-K.; Bethe, A.; Lübke-Becker, A.; Schwarz, S. Antimicrobial and Biocide Resistance among Canine and Feline Enterococcus faecalis, Enterococcus faecium, Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii Isolates from Diagnostic Submissions. Antibiotics 2022, 11, 152. https://doi.org/10.3390/antibiotics11020152
Feßler AT, Scholtzek AD, Schug AR, Kohn B, Weingart C, Hanke D, Schink A-K, Bethe A, Lübke-Becker A, Schwarz S. Antimicrobial and Biocide Resistance among Canine and Feline Enterococcus faecalis, Enterococcus faecium, Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii Isolates from Diagnostic Submissions. Antibiotics. 2022; 11(2):152. https://doi.org/10.3390/antibiotics11020152
Chicago/Turabian StyleFeßler, Andrea T., Anissa D. Scholtzek, Angela R. Schug, Barbara Kohn, Christiane Weingart, Dennis Hanke, Anne-Kathrin Schink, Astrid Bethe, Antina Lübke-Becker, and Stefan Schwarz. 2022. "Antimicrobial and Biocide Resistance among Canine and Feline Enterococcus faecalis, Enterococcus faecium, Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii Isolates from Diagnostic Submissions" Antibiotics 11, no. 2: 152. https://doi.org/10.3390/antibiotics11020152
APA StyleFeßler, A. T., Scholtzek, A. D., Schug, A. R., Kohn, B., Weingart, C., Hanke, D., Schink, A.-K., Bethe, A., Lübke-Becker, A., & Schwarz, S. (2022). Antimicrobial and Biocide Resistance among Canine and Feline Enterococcus faecalis, Enterococcus faecium, Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii Isolates from Diagnostic Submissions. Antibiotics, 11(2), 152. https://doi.org/10.3390/antibiotics11020152







