Abstract
Background: The clinical significance of utilizing a vancomycin loading dose in critically ill patients remains unclear. Objective: The main aim of this systematic review is to evaluate the clinical safety and efficacy of the vancomycin loading dose in critically ill patients. Methods: We performed a systematic review using PRISMA guidelines. PubMed, the Web of Science, MEDLINE, Scopus, Google Scholar, the Saudi Digital Library and other databases were searched. Studies that reported clinical outcomes among patients receiving the vancomycin LD were considered eligible. Data for this study were collected using PubMed, the Web of Science, MEDLINE, Scopus, Google Scholar and the Saudi Digital Library using the following terms: “vancomycin”, “safety”, “efficacy” and “loading dose” combined with the Boolean operator “AND” or “OR”. Results: A total of 17 articles, including 2 RCTs, 11 retrospective cohorts and 4 other studies, met the inclusion/exclusion criteria out of a total 1189 studies. Patients had different clinical characteristics representing a heterogenous group, including patients in critical condition, with renal impairment, sepsis, MRSA infection and hospitalized patients for hemodialysis or in the emergency department. Conclusions: The study shows that the target therapeutic level is achieved more easily among patients receiving a weight-based LD as compared to patients received the usual dose without an increased risk of new-onset adverse drug reactions.
1. Introduction
Vancomycin, a glycopeptide antibiotic, is a type of time-dependent antimicrobial prescribed for severe infections or healthcare-associated infections caused by methicillin-resistant Staphylococcous aureus (MRSA) [1]. It is one of the most commonly studied antimicrobials regarding therapeutic drug monitoring in order to confirm successful clinical outcomes and to reduce the risk of nephrotoxicity [2,3,4,5]. An appropriate dosing regimen is the basis of rational vancomycin therapy in order to improve clinical outcome and reduce the development of antimicrobial resistance (AMR) and dose-dependent toxicity [6,7]. Moreover, the antimicrobial efficacy of vancomycin can be determined by the time period during which the vancomycin concentration in plasma is greater than the minimal inhibitory concentration (MIC). The antimicrobial efficacy of vancomycin is highest when the vancomycin concentration reaches 4–5 times that of the MIC [8,9]. Due to an increase in MRSA and vancomycin-resistant Enterococci infection rates, there has been an excessive use of vancomycin, which has resulted in increased MICs of more than 1.5 mg/L [10,11]. Therefore, guidelines suggest a more aggressive dose regimen of vancomycin in order achieve a target trough concentration (15–20 mg/L) for life threatening infections such as MRSA or pneumonia [12]. Recently published guidelines and literature on vancomycin therapeutic drug monitoring recommend an AUC/MIC ratio of ≥400 at MIC values of 0.5 and 1 μg/mL, comparing trough concentration targets of 10 and 20 μg/mL to optimize vancomycin exposure with minimal toxicity. Therefore, in the recent era, most clinicians prefer AUC/MIC targets over trough concentration targets to optimize vancomycin therapy [9,13].
However, most vancomycin usage in critical care settings is empiric, and the concept of AUC/MIC is pointless in these settings. An appropriately weight-based vancomycin dosing likely attains the AUC target without therapeutic drug monitoring [14]. Additionally, Gram-positive microbes cannot be efficiently eliminated if the vancomycin concentration is less than 10 mg/L. Eventually, chances of vancomycin-resistant infections increase, which may cause a prolonged hospital stay and higher mortality rate [2,15,16,17].
Dose optimization antimicrobial stewardship programs using pharmacokinetics/pharmacodynamics (Pk/Pd) principles are effective strategies to ensure clinical efficacy of most narrow therapeutic index antimicrobials [7,18]. In order to quickly achieve an effective AUC/MIC target of vancomycin and optimize its use, a loading dose (LD) of vancomycin 25–30 mg/kg (actual body weight) in adults and 20–25 mg/kg in children is recommended [19]. This practice is also supported by the revised clinical guidelines on vancomycin therapeutic drug monitoring by the IDSA [9]. Irrational dosing eventually leads to the emergence of AMR [20,21,22]. Therefore, the main reason to conduct this systematic review is to evaluate the available published data regarding the clinical safety and efficacy of the vancomycin LD in the treatment of Gram-positive infections and to provide reference for clinical practice.
2. Results
2.1. Literature Search
A total of 1189 articles was identified after literature search from five databases. After applying inclusion and exclusion criteria, 93 full-text relevant articles were separated. In these research articles, nine review papers were excluded because they were not original studies. In the remaining 82 articles, 13 articles were preclinical studies and 10 epidemiology studies, 29 research papers without an LD group (LDG) and 13 research papers on unrelated topics. Finally, 17 articles, including 2 RCTs [23,24], 11 retrospective cohorts, and 4 other studies, were included [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. The PRISMA flow diagram reporting the procedure of selection of studies is shown in Figure 1.
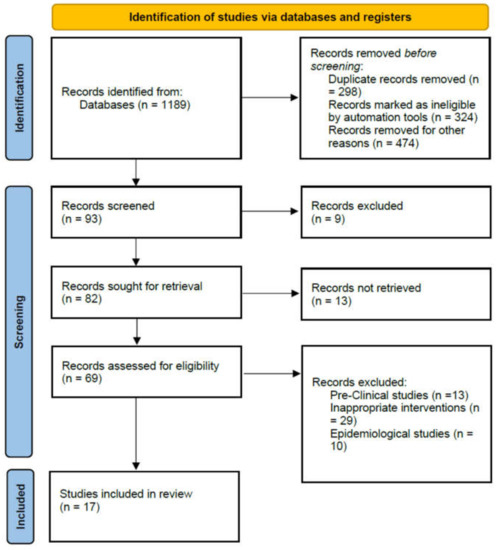
Figure 1.
PRISMA flow diagram reporting the procedure of selection of studies.
2.2. Study Characteristics
The important features of the research articles included in this review are shown in Table 1. One RCT was a double-blind study that was conducted among children aged 2–18 years old [23]. The second RCT evaluated the use of LD among patients admitted to the emergency department (ED) [24]. In the remaining 14 studies, 10 were retrospective cohort studies, 1 prospective, 1 concurrent and 2 studies conducted both retrospectively and prospectively in order to compare the results with each other. Six studies were conducted in patients with MRSA infections [25,26,27,28,29,30], while the remaining were conducted in patients with different infectious diseases [23,24,31,32,33,34,35,36,37,38,39]. The studies included intensive care unit (ICU) patients, patients with severe renal impairment, sepsis patients, hospitalized hemodialysis patients, emergency department patients and MRSA-infected patients. The quality of the included research articles was evaluated, and the results are presented in Table 2 and Table 3.

Table 1.
Loading dose of vancomycin in included studies and implications.

Table 2.
Quality assessment of cohort studies.

Table 3.
Risk of bias assessment for randomized controlled trials.
2.3. Attainment of Target Therapeutic Concentration and Clinical Response
Both in RCT and non-RCT subgroups, the overall attainment of target therapeutic TC (15 to 20 mg/L) was notably higher in the LDG than in the control group (CG). However, the difference was not significantly higher in the non-RCT group. The studies showed a better clinical response along with negative blood cultures in the LDG than in the CG. One observational study highlighted that the nomogram led to a reliable attainment of concentrations of vancomycin (≥15 mg/L) without increasing nephrotoxicity among patients admitted to the ICU [36]. Ortwine et al. reported that patients receiving an LD of ≥ 20 mg/kg of vancomycin on the basis of weight might not be the optimal dosing strategy [28]. Dolan and his colleagues reported that the majority of children who received an LD of 20 to 25 mg/kg of vancomycin had a subtherapeutic concentration [32].
2.4. Nephrotoxicity and Other Adverse Events
The total number of patients who were inflicted with renal toxicity was significantly less in the LDG than in the CG. Results of RCTs also revealed a significantly higher incidence of nephrotoxicity in the LDG. Marvin and his colleagues reported that a high LD of vancomycin does not increase nephrotoxicity when compared to a lower dose in renally impaired patients [31]. However, Demirjian et al. reported that children who received an LD of 30 mg/kg infused over 2 h reported the occurrence of nephrotoxicity and red man syndrome [23]. Besides nephrotoxicity, other common adverse drug reactions of vancomycin included flushing, pruritus and a rash. Only RCTs were compared to other adverse drug reactions between the LDG and CG, and there was no clinically significant difference between the two groups. The research articles stated the mortality rate after receiving the vancomycin LD, but there was no significant difference between the LDG and CG.
3. Discussion
While existing clinical practice guidelines recommend LDs of vancomycin for life threatening infections, limited published articles are available endorsing or disproving this recommendation [4,8]. Research articles evaluated in this systematic review had different methodologies, but the main data were derived from retrospective cohort studies. The recommended daily dose of vancomycin is 2 g intravenously either divided as 500 mg four times daily or 1 g twice daily for patients with a normal renal function as per directions provided by the Food and Drug Administration (FDA) of the United States. However, an FDA-approved label does not state the use of the vancomycin LD. Conversely, in various published clinical guidelines, LD is strongly recommended for patients in critically condition (including those with meningitis, sepsis, pneumonia or infective endocarditis) due to suspected MRSA infection [2,4].
Numerous research articles confirmed an improved clinical response among patients administered with an LD of vancomycin [25,30]. A meta-analysis reported that an LD group can achieve an optimal therapeutic concentration significantly better that a nonloading dose group [40]. It may take a long time for vancomycin to achieve target plasma concentrations. Therefore, in severely ill patients, an LD allows for the quick attainment of a target TC of 15–20 mg/mL. The results of RCT indicated that the vancomycin LD is a better treatment option for the management of serious infections of MRSA, compared to non-LD therapy [26]. This systematic review confirmed the results of studies stating that a LDG can attain an optimal TC significantly better than a non-LDG, whereas another study indicated that a standard dose of vancomycin (500 mg four times a day) is subtherapeutic in critically ill patients [41]. Therefore, studies suggest that a 15 mg/kg LD should be given to patients in critical condition due to suspected infections of Gram-positive microbes [42]. In most adult patients, when the LD is administered, the optimal TC (15 to 20 mg/L) can be attained within 24 h before the second dose. Likewise, the compliance rate of an optimal TC in children is higher in the LDG than in the CG [23].
Nephrotoxicity is one of the major side effects of vancomycin in patients, especially those who are critically ill [43]. Considering that the LD of vancomycin may result in a high risk of nephrotoxicity, it remains unclear whether the LD of vancomycin is safe for the treatment of serious infections. A meta-analysis reported that the risk of nephrotoxicity was lower in the LD group when compared with the control group, revealing that the LD was not associated with increased nephrotoxicity [40]. An LD of vancomycin reduces the risk of nephrotoxicity. The effective control of life-threatening infections such as sepsis through better antibacterial activity might slow down the progression of renal damage, shorten the hospitalization and reduce mortality [44]. The incidence of other adverse drug reactions (e.g., red man syndrome) was also not significantly higher in the LDG [23]. For patient safety, vancomycin should be infused slowly in the LD to avoid infusion-related adverse reactions [23,34]. Moreover, patients who receive prolonged therapy of vancomycin for more than 1 week should have their serum creatinine level checked 2–3 times weekly along with the routine monitoring of the urine output. Vancomycin antibacterial activity is time-dependent with a prolonged post antibiotic effect. Therefore, keeping the TC above an effective concentration can improve the clinical efficacy. The literature showed that a continuous vancomycin infusion along with an LD increases the clinical efficacy of vancomycin as compared to a normal infusion time [45].
Owing to the lack of a huge sample size and controlled study design, these retrospective cohorts were not enough to endorse the safety and efficacy of an LD. Controlled studies with adequate statistical analyses are required in order to endorse the clinical safety and efficacy of the LD of vancomycin. Future research should focus closely on patients with life-threatening infections, for instance, those with bacteremia, hospital-acquired pneumonia, infective endocarditis, osteomyelitis, meningitis or confirmed MRSA infections. Moreover, the development of validated vancomycin dosing nomograms should integrate well-defined guidelines related to the administration of standardized LDs in life-threatening infections to achieve a rapid TC. The use of LDs should be justified through high-quality RCTs. This systematic review had a few limitations and should be seen pragmatically. Firstly, there is a need to add more RCTs in order to validate the results, as only a few RCTs were included. Secondly, some clinical outcomes of studies included in this systematic review were not presented, as only a few studies stated the clinical outcomes, morbidity and mortality. Furthermore, heterogeneity existed in the included studies.
4. Materials and Methods
4.1. Literature Search
PubMed, the Web of Science, MEDLINE, Scopus, Google Scholar and the Saudi Digital Library were explored from their beginning up to December 2021. The search terms used for this systematic review were “vancomycin”, “safety”, “efficacy” and “loading dose” combined with the Boolean operator “AND,” or “OR”. Both text words and mesh terms were used. Additionally, references of the initially identified original research articles and related review papers were also checked for relevant research papers. Only articles written in English language were included in this systematic review.
4.2. Study Selection
The 2020 PRISMA guidelines (preferred reporting items for systematic reviews and meta-analyses) were used to conduct this systematic review. Randomized controlled trials (RCTs) and observational and cohort studies that presented serum TC of vancomycin after the use of vancomycin LD intravenously as either an empiric or targeted antimicrobial therapy were included. Studies that focused on laboratory research, oral vancomycin use, preclinical research, sample size of less than 10 and epidemiology were excluded. A study protocol was developed to assess eligibility for inclusion. Literature search and study selection were carried out by two independent researchers (AH and ZS). Prospective and retrospective open-label and observational studies and randomized clinical trials (RCTs) were included.
4.3. Data Extraction
Data extraction was carried out using the predesigned data extraction form for this systematic review. The following data were extracted from each study: (1) author(s) and year of publication; (2) reference; (3) study design; (4) sample size; (5) characteristics of the patient; (6) dosing practice; (7) outcomes that included PK data, therapeutic outcomes and toxicity; (8) inference.
4.4. Article Quality Assessment
The quality of each article was evaluated using a New Castle-Ottawa Scale (NOS) for retrospective studies and Cochrane bias tool for RCTs [46,47,48]. Two of the reviewers assessed the quality of each included study independently. They compared their results and disagreements were resolved by detailed discussion.
5. Conclusions
Multisite studies reported that the use of a vancomycin loading dose achieved optimal therapeutic and AUC/MIC targets. Moreover, the loading dose lowered the risk of nephrotoxicity and other adverse reactions. Based on the existing literature, the LD is reported to be an effective and safe treatment option for critically ill patients. However, there is a need to conduct high-quality large-scale RCTs in order to further validate the efficacy and safety of the vancomycin LD.
Author Contributions
Conceptualization, A.H., H.S.F., S.S.A., M.A., M.K.A., A.S.A., A.A. and A.S.; methodology, A.H., S.S.A., M.E.E., S.S.A.A., A.F.A., M.B. and Z.S.; review, A.H., S.S.A. and Z.S.; analysis, A.H., M.B., S.S.A.A., J.M.A. and A.S.; resources, A.H., M.E.E., S.S.A.A., A.A.K., M.S.I. and N.A.O.; writing—original draft preparation, A.H., N.A.O., S.S.A.A., A.J.M., S.S.A., A.A.K., J.M.A. and N.A.O.; writing—review and editing, A.S., Z.S., M.K.A., M.S.I., H.S.F., A.A.K., M.B. and A.S.; supervision, H.S.F., A.A.K., M.A., A.A. and A.S.; funding acquisition, A.H., A.F.A., N.A.O., M.E.E. and H.S.F. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Deanship of Scientific research at the Umm Al-Qura University for supporting this work by grant code: 22UQU4290073DSR02).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rybak, M.J. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 2006, 42, S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Takesue, Y.; Ohmagari, N.; Mochizuki, T.; Mikamo, H.; Seki, M.; Takakura, S.; Tokimatsu, I.; Takahashi, Y.; Kasahara, K. Practice guidelines for therapeutic drug monitoring of vancomycin: A consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J. Infect. Chemother. 2013, 19, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.-K.; Chen, Y.-L.; Chen, K.; Zhang, X.-L.; Du, G.-H.; He, B.; Li, D.-K.; Liu, Y.-N.; Yang, K.-H.; Zhang, Y.-Y. Therapeutic drug monitoring of vancomycin: A guideline of the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. J. Antimicrob. Chemother. 2016, 71, 3020–3025. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R., Jr.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef]
- Haseeb, A.; Faidah, H.S.; Algethamy, M.; Alghamdi, S.; Alhazmi, G.A.; Alshomrani, A.O.; Alqethami, B.R.; Alotibi, H.S.; Almutiri, M.Z.; Almuqati, K.S. Antimicrobial Usage and Resistance in Makkah Region Hospitals: A Regional Point Prevalence Survey of Public Hospitals. Int. J. Environ. Res. Public Health 2022, 19, 254. [Google Scholar] [CrossRef]
- Saleem, Z.; Saeed, H.; Hassali, M.A.; Godman, B.; Asif, U.; Yousaf, M.; Ahmed, Z.; Riaz, H.; Raza, S.A. Pattern of inappropriate antibiotic use among hospitalized patients in Pakistan: A longitudinal surveillance and implications. Antimicrob. Resist. Infect. Control. 2019, 8, 188. [Google Scholar] [CrossRef]
- Haseeb, A.; Faidah, H.S.; Alghamdi, S.; Alotaibi, A.F.; Elrggal, M.E.; Mahrous, A.J.; Almarzoky Abuhussain, S.S.; Obaid, N.A.; Algethamy, M.; AlQarni, A. Dose Optimization of Colistin: A Systematic Review. Antibiotics 2021, 10, 1454. [Google Scholar] [CrossRef]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2020, 71, 1361–1364. [Google Scholar]
- Rybak, M.J.; Lomaestro, B.M.; Rotschafer, J.C.; Moellering, R.C., Jr.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic Monitoring of Vancomycin in Adults: Summary of Consensus Recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2009, 29, 1275–1279. [Google Scholar] [CrossRef]
- Simor, A.E.; Gilbert, N.L.; Gravel, D.; Mulvey, M.R.; Bryce, E.; Loeb, M.; Matlow, A.; McGeer, A.; Louie, L.; Campbell, J. Methicillin-resistant Staphylococcus aureus colonization or infection in Canada: National surveillance and changing epidemiology, 1995–2007. Infect. Control. Hosp. Epidemiol. 2010, 31, 348–356. [Google Scholar] [CrossRef]
- Moise-Broder, P.A.; Forrest, A.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 2004, 43, 925–942. [Google Scholar] [CrossRef]
- Tsutsuura, M.; Moriyama, H.; Kojima, N.; Mizukami, Y.; Tashiro, S.; Osa, S.; Enoki, Y.; Taguchi, K.; Oda, K.; Fujii, S. The monitoring of vancomycin: A systematic review and meta-analyses of area under the concentration-time curve-guided dosing and trough-guided dosing. BMC Infect. Dis. 2021, 21, 153. [Google Scholar] [CrossRef]
- Dilworth, T.J.; Schulz, L.T.; Rose, W.E. Vancomycin advanced therapeutic drug monitoring: Exercise in futility or virtuous endeavor to improve drug efficacy and safety? Clin. Infect. Dis. 2021, 72, e675–e681. [Google Scholar] [CrossRef]
- Kullar, R.; Davis, S.L.; Levine, D.P.; Rybak, M.J. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: Support for consensus guidelines suggested targets. Clin. Infect. Dis. 2011, 52, 975–981. [Google Scholar] [CrossRef]
- Holmes, N.E.; Turnidge, J.D.; Munckhof, W.J.; Robinson, J.O.; Korman, T.M.; O’Sullivan, M.V.; Anderson, T.L.; Roberts, S.A.; Warren, S.J.; Gao, W. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 2013, 57, 1654–1663. [Google Scholar] [CrossRef]
- Gawronski, K.M.; Goff, D.A.; Brown, J.; Khadem, T.M.; Bauer, K.A. A stewardship program’s retrospective evaluation of vancomycin AUC24/MIC and time to microbiological clearance in patients with methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. Clin. Ther. 2013, 35, 772–779. [Google Scholar] [CrossRef]
- Haseeb, A.; Abourehab, M.A.; Almalki, W.A.; Almontashri, A.M.; Bajawi, S.A.; Aljoaid, A.M.; Alsahabi, B.M.; Algethamy, M.; AlQarni, A.; Iqbal, M.S. Trimethoprim-Sulfamethoxazole (Bactrim) Dose Optimization in Pneumocystis jirovecii Pneumonia (PCP) Management: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 2833. [Google Scholar] [CrossRef]
- Rybak, M.J.; Lomaestro, B.M.; Rotscahfer, J.C.; Moellering, R.C., Jr.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Vancomycin therapeutic guidelines: A summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2009, 49, 325–327. [Google Scholar] [CrossRef]
- Alhifany, A.A.; Alqurashi, A.F.; Al-Agamy, M.H.; Alkhushaym, N.; Alhomoud, F.; Alhomoud, F.K.; Almangour, T.A. Employment of mapping technology in antimicrobial resistance reporting in Saudi Arabia. Geospat. Health 2020, 15, 868. [Google Scholar] [CrossRef]
- Almeleebia, T.M.; Alhifany, A.A.; Almutairi, F.; Alshibani, M.; Alhossan, A.M. Regulating antimicrobial sales in Saudi Arabia: Achievements and challenges. Int. J. Clin. Pract. 2021, 75, e13833. [Google Scholar] [CrossRef]
- Saleem, Z.; Godman, B.; Azhar, F.; Kalungia, A.C.; Fadare, J.; Opanga, S.; Markovic-Pekovic, V.; Hoxha, I.; Saeed, A.; Al-Gethamy, M. Progress on the national action plan of Pakistan on antimicrobial resistance (AMR): A narrative review and the implications. Expert Rev. Anti Infect. Ther. 2022, 20, 71–93. [Google Scholar] [CrossRef] [PubMed]
- Demirjian, A.; Finkelstein, Y.; Nava-Ocampo, A.; Arnold, A.; Jones, S.; Monuteaux, M.; Sandora, T.J.; Patterson, A.; Harper, M.B. A randomized controlled trial of a vancomycin loading dose in children. Pediatr. Infect. Dis. J. 2013, 32, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Rosini, J.M.; Laughner, J.; Levine, B.J.; Papas, M.A.; Reinhardt, J.F.; Jasani, N.B. A randomized trial of loading vancomycin in the emergency department. Ann. Pharmacother. 2015, 49, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Wesolek, J.L.; McNorton, K.; Delgado, G., Jr.; Giuliano, C.A. Effect of vancomycin initial dosing on time to systemic inflammatory response syndrome resolution in patients with methicillin-resistant Staphylococcus aureus bacteremia. J. Chemother. 2018, 30, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Takesue, Y.; Nakajima, K.; Ichiki, K.; Ishikawa, K.; Takai, Y.; Yamada, K.; Wada, Y.; Tsuchida, T.; Otani, N. Vancomycin loading dose is associated with increased early clinical response without attainment of initial target trough concentration at a steady state in patients with methicillin-resistant Staphylococcus aureus infections. J. Clin. Pharm. Ther. 2020, 45, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.G.; Huh, K.; Sohn, Y.M.; Park, H.J.; Na, S.J.; Jeon, K. Effect of vancomycin loading dose on clinical outcome in critically ill patients with methicillin-resistant Staphylococcus aureus pneumonia. J. Thorac. Dis. 2021, 13, 768. [Google Scholar] [CrossRef] [PubMed]
- Ortwine, J.K.; Zasowski, E.J.; Pogue, J.M.; Hanni, C.; Giuliano, C.; Casapao, A.M.; Mynatt, R.; Rybak, M.J. Relationship status between vancomycin loading dose and treatment failure in patients with MRSA bacteremia: It’s complicated. Infect. Dis. Ther. 2019, 8, 627–640. [Google Scholar] [CrossRef]
- Flannery, A.H.; Wallace, K.L.; Rhudy, C.N.; Olmsted, A.S.; Minrath, R.C.; Pope, S.M.; Cook, A.M.; Burgess, D.S.; Morris, P.E. Efficacy and safety of vancomycin loading doses in critically ill patients with methicillin-resistant Staphylococcus aureus infection. Ther. Adv. Infect. Dis. 2021, 8, 20499361211005965. [Google Scholar] [CrossRef]
- Cheong, J.; Makmor-Bakry, M.; Lau, C.; Rahman, R.A. The relationship between trough concentration of vancomycin and effect on methicillin-resistant Staphylococcus aureus in critically ill patients. S. Afr. Med. J. 2012, 102, 616–619. [Google Scholar] [CrossRef]
- Marvin, J.L.; Levine, B.J.; Papas, M.; Rosini, J.M. An evaluation of the incidence of nephrotoxicity after a loading dose of vancomycin in patients with severe renal impairment. J. Emerg. Med. 2019, 56, 701–708. [Google Scholar] [CrossRef]
- Dolan, E.; Hellinga, R.; London, M.; Ryan, K.; Dehority, W. Effect of Vancomycin Loading Doses on the Attainment of Target Trough Concentrations in Hospitalized Children. J. Pediatr. Pharmacol. Ther. 2020, 25, 423–430. [Google Scholar] [CrossRef]
- Al-Mazraawy, B.O.; Girotto, J.E. Comparing Vancomycin Area Under the Curve with a Pharmacist Protocol that Incorporates Trough and Maximum Doses at a Children’s Hospital. J. Pediatr. Pharmacol. Ther. 2021, 26, 740–745. [Google Scholar] [CrossRef]
- Rosini, J.M.; Davis, J.J.; Muenzer, J.; Levine, B.J.; Papas, M.A.; Comer, D.; Arnold, R. High single-dose vancomycin loading is not associated with increased nephrotoxicity in emergency department sepsis patients. Acad. Emerg. Med. 2016, 23, 744–746. [Google Scholar] [CrossRef][Green Version]
- Truong, J.; Levkovich, B.; Padiglione, A. Simple approach to improving vancomycin dosing in intensive care: A standardised loading dose results in earlier therapeutic levels. Intern. Med. J. 2012, 42, 23–29. [Google Scholar] [CrossRef]
- Golenia, B.S.; Levine, A.R.; Moawad, I.M.; Yeh, D.D.; Arpino, P.A. Evaluation of a vancomycin dosing nomogram based on the modification of diet in renal disease equation in intensive care unit patients. J. Crit. Care 2013, 28, 710–716. [Google Scholar] [CrossRef]
- Álvarez, O.; Plaza-Plaza, J.C.; Ramirez, M.; Peralta, A.; Amador, C.A.; Amador, R. Pharmacokinetic assessment of vancomycin loading dose in critically ill patients. Antimicrob. Agents Chemother. 2017, 61, e00280-17. [Google Scholar] [CrossRef]
- Hodiamont, C.; Juffermans, N.; Berends, S.; van Vessem, D.; Hakkens, N.; Mathôt, R.; de Jong, M.; van Hest, R. Impact of a vancomycin loading dose on the achievement of target vancomycin exposure in the first 24 h and on the accompanying risk of nephrotoxicity in critically ill patients. J. Antimicrob. Chemother. 2021, 76, 2941–2949. [Google Scholar] [CrossRef]
- Denetclaw, T.H.; Dowling, T.C.; Steinke, D. Performance of a divided-load intravenous vancomycin dosing strategy for critically ill patients. Ann. Pharmacother. 2013, 47, 1611–1617. [Google Scholar] [CrossRef]
- Mei, H.; Wang, J.; Che, H.; Wang, R.; Cai, Y. The clinical efficacy and safety of vancomycin loading dose: A systematic review and meta-analysis. Medicine 2019, 98, e17639. [Google Scholar] [CrossRef]
- Soto, J.; Sacristan, J.; Alsar, M. Necessity of a loading dose when using vancomycin in critical-ill patients. J. Antimicrob. Chemother. 1991, 27, 875. [Google Scholar] [CrossRef] [PubMed]
- Mohammedi, I.; Descloux, E.; Argaud, L.; Le Scanff, J.; Robert, D. Loading dose of vancomycin in critically ill patients: 15 mg/kg is a better choice than 500 mg. Int. J. Antimicrob. Agents 2006, 27, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Filippone, E.J.; Kraft, W.K.; Farber, J.L. The Nephrotoxicity of Vancomycin. Clin. Pharmacol. Ther. 2017, 102, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Wang, W. Acute renal failure and sepsis. N. Engl. J. Med. 2004, 351, 159–169. [Google Scholar] [CrossRef]
- Vuagnat, A.; Stern, R.; Lotthe, A.; Schuhmacher, H.; Duong, M.; Hoffmeyer, P.; Bernard, L. High dose vancomycin for osteomyelitis: Continuous vs. intermittent infusion. J. Clin. Pharm. Ther. 2004, 29, 351–357. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Newcastle-Ottawa Quality Assessment Scale Cohort Studies. 2014. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 22 September 2021).
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2019; pp. 205–228. [Google Scholar]
- Ma, L.-L.; Wang, Y.-Y.; Yang, Z.-H.; Huang, D.; Weng, H.; Zeng, X.-T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).