The In Vitro Efficacy of Activated Charcoal in Fecal Ceftriaxone Adsorption among Patients Who Received Intravenous Ceftriaxone
Abstract
1. Introduction
2. Materials and Methods
2.1. Enrollment
2.2. Study Design and Sample Size Calculation
2.3. Fecal Sample Collection and Standardization
- One gram of fecal sample was mixed and homogenized with 9 mL normal saline (NSS), resulting in 1:10 fecal solutions that were comparable to fluid concentration in the human cecum [23].
- Fecal solutions were incubated at 70 °C for 30 min to inhibit any residual beta-lactamase enzyme activity that might have been present in some patients, even if they were not exposed to beta-lactam antibiotics in the previous three months [24].
- The incubated fecal solutions were centrifuged for 5 min at 3000 RCF.
- Each “fecal medium” supernatant was transferred to a 1.5 mL aliquot tube and frozen at −80 °C for stabilization of ceftriaxone concentrations throughout the study period, in compliance with standard laboratory guidelines.
2.4. Fecal Sample Mixing and ‘X’ Fecal Sample Preparation Protocol
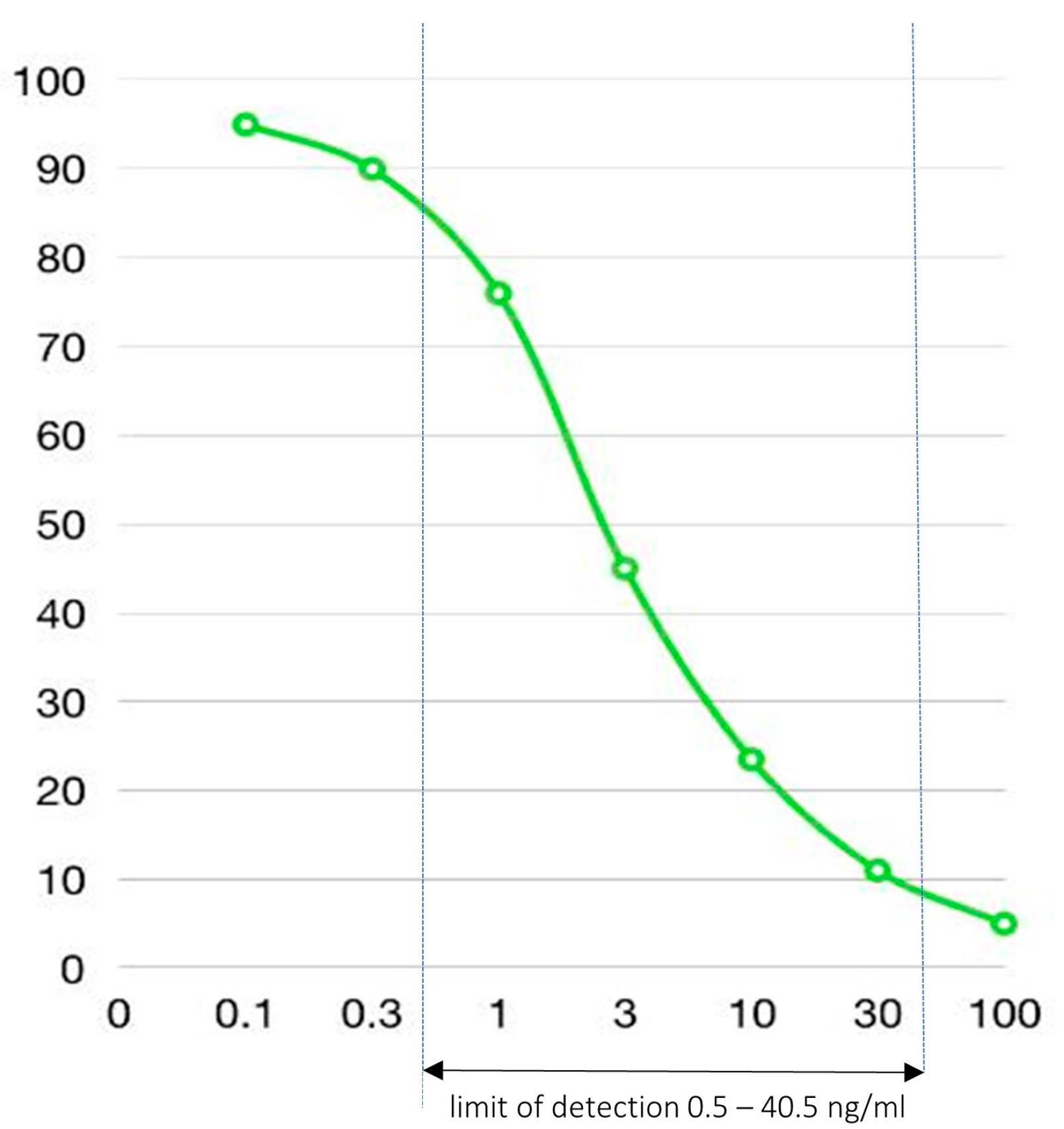
2.5. Fecal Ceftriaxone Level Measurement
2.6. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonten, M.J.; Weinstein, R. The role of colonization in the pathogenesis of nosocomial infections. Infect. Control Hosp. Epidemiol. 1996, 17, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cobas, A.E.; Gosalbes, M.J.; Friedrichs, A.; Knecht, H.; Artacho, A.; Eismann, K.; Otto, W.; Rojo, D.; Bargiela, R.; von Bergen, M.; et al. Gut microbiota disturbance during antibiotic therapy: A multi-omic approach. Gut 2013, 62, 1591–1601. [Google Scholar] [CrossRef]
- Bräutigam, H.H.; Knothe, H.; Rangoonwala, R. Impact of cefotaxime and ceftriaxone on the bowel and vaginal flora after single-dose prophylaxis in vaginal hysterectomy. Drugs 1988, 35 (Suppl. 2), 163–168. [Google Scholar] [CrossRef] [PubMed]
- Tischendorf, J.; de Avila, R.A.; Safdar, N. Risk of infection following colonization with carbapenem-resistant Enterobactericeae: A systematic review. Am. J. Infect. Control 2016, 44, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.G. Clinical practice. Antibiotic-associated diarrhea. N. Engl. J. Med. 2002, 346, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.L.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- Millan, B.; Park, H.; Hotte, N.; Mathieu, O.; Burguiere, P.; Tompkins, T.A.; Kao, D.; Madsen, K.L. Fecal Microbial Transplants Reduce Antibiotic-resistant Genes in Patients With Recurrent Clostridium difficile Infection. Clin. Infect. Dis. 2016, 62, 1479–1486. [Google Scholar] [CrossRef]
- Khanna, S.; Pardi, D.S.; Kelly, C.R.; Kraft, C.S.; Dhere, T.; Henn, M.R.; Lombardo, M.J.; Vulic, M.; Ohsumi, T.; Winkler, J.; et al. A Novel Microbiome Therapeutic Increases Gut Microbial Diversity and Prevents Recurrent Clostridium difficile Infection. J. Infect. Dis. 2016, 214, 173–181. [Google Scholar] [CrossRef]
- Kokai-Kun, J.F.; Sarver, J.; Carman, R. Characterization of Clostridium difficile isolates collected during a phase 2b clinical study with SYN-004 (ribaxamase) for the prevention of C. difficile infection. Anaerobe 2018, 53, 30–33. [Google Scholar] [CrossRef]
- Kaleko, M.; Bristol, J.A.; Hubert, S.; Parsley, T.; Widmer, G.; Tzipori, S.; Subramanian, P.; Hasan, N.; Koski, P.; Kokai-Kun, J.; et al. Development of SYN-004, an oral beta-lactamase treatment to protect the gut microbiome from antibiotic-mediated damage and prevent Clostridium difficile infection. Anaerobe 2016, 41, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Yuzuriha, K.; Yakabe, K.; Nagai, H.; Li, S.; Zendo, T.; Zai, K.; Kishimura, A.; Hase, K.; Kim, Y.-G.; Mori, T.; et al. Protection of gut microbiome from antibiotics: Development of a vancomycin-specific adsorbent with high adsorption capacity. Biosci. Microbiota Food Health 2020, 39, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yakabe, K.; Zai, K.; Liu, Y.; Kishimura, A.; Hase, K.; Kim, Y.-G.; Mori, T.; Katayama, Y. Specific adsorption of a β-lactam antibiotic in vivo by an anion-exchange resin for protection of the intestinal microbiota. Biomater. Sci. 2021, 9, 7219–7227. [Google Scholar] [CrossRef] [PubMed]
- Albertson, T.E.; Owen, K.P.; Sutter, M.E.; Chan, A.L. Gastrointestinal decontamination in the acutely poisoned patient. Int. J. Emerg. Med. 2011, 4, 65. [Google Scholar] [CrossRef]
- Derlet, R.W.; Albertson, T.E. Activated charcoal--past, present and future. West J. Med. 1986, 145, 493–496. [Google Scholar]
- Murray, P.R.; Masur, H. Current approaches to the diagnosis of bacterial and fungal bloodstream infections in the intensive care unit. Crit. Care Med. 2012, 40, 3277–3282. [Google Scholar] [CrossRef]
- Ahmed, M.J. Adsorption of quinolone, tetracycline, and penicillin antibiotics from aqueous solution using activated carbons: Review. Environ. Toxicol. Pharmacol. 2017, 50, 1–10. [Google Scholar] [CrossRef]
- Grall, N.; Massias, L.; Nguyen, T.T.; Sayah-Jeanne, S.; Ducrot, N.; Chachaty, E.; de Gunzburg, J.; Andremont, A. Oral DAV131, a charcoal-based adsorbent, inhibits intestinal colonization by β-lactam-resistant Klebsiella pneumoniae in cefotaxime-treated mice. Antimicrob. Agents Chemother. 2013, 57, 5423–5425. [Google Scholar] [CrossRef]
- De Gunzburg, J.; Ghozlane, A.; Ducher, A.; Le Chatelier, E.; Duval, X.; Ruppé, E.; Armand-Lefevre, L.; Sablier-Gallis, F.; Burdet, C.; Alavoine, L.; et al. Protection of the Human Gut Microbiome From Antibiotics. J. Infect. Dis. 2018, 217, 628–636. [Google Scholar] [CrossRef]
- Patel, I.H.; Chen, S.; Parsonnet, M.; Hackman, M.R.; Brooks, M.A.; Konikoff, J.; Kaplan, S.A. Pharmacokinetics of ceftriaxone in humans. Antimicrob. Agents Chemother. 1981, 20, 634–641. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Roche Laboratories Inc., ROCEPHIN®(ceftriaxone sodium) FOR INJECTION Package Insert. Available online: www.accessdata.fda.gov/drugsatfda_docs/label/2004/50585s057,50624s027lbl.pdf (accessed on 5 January 2023).
- Peris, J.-E.; Pascual-Arce, M.; Granero, L.; Chesa-Jiménez, J.; Almela, M. Renal and nonrenal clearances of ceftriaxone at the steady-state and its relation to plasma protein binding. Eur. J. Pharm. Sci. 1995, 3, 133–138. [Google Scholar] [CrossRef]
- Debongnie, J.C.; Phillips, S. Capacity of the human colon to absorb fluid. Gastroenterology 1978, 74, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, H.; Ishibashi, M.; Arakawa, T.; Tokunaga, M. Highly efficient renaturation of beta-lactamase isolated from moderately halophilic bacteria. FEBS Lett. 2004, 558, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.; Ganio, M.S.; Seifert, T.; Overgaard, M.; Secher, N.H.; Crandall, C.G. Pulmonary artery and intestinal temperatures during heat stress and cooling. Med. Sci. Sports Exerc. 2012, 44, 857–862. [Google Scholar] [CrossRef] [PubMed]


| X patient Fecal Sample | Demographic Data (Age—Years, Sex) | Primary Diagnosis | Date of IV Ceftriaxone Initiation (mm/dd/yy) | Post 1st IV Ceftriaxone Period Prior to Fecal Sample Collection (hours) | Number of Ceftriaxone Doses Received before Fecal Sample Collection |
|---|---|---|---|---|---|
| X1 | 35, female | CBD stone (Elective ERCP) | 1/13/2020 | 17 | 1 |
| X2 | 69, male | Acute cellulitis | 1/13/2020 | 14 | 1 |
| X3 | 69, female | Infected wound | 1/13/2020 | 41 | 2 |
| X4 | 52, male | Acute pyelonephritis | 1/15/2020 | 38 | 2 |
| X5 | 69, male | Bacterial pneumonia | 1/19/2020 | 42 | 2 |
| X6 | 79, female | Acute cholangitis | 3/2/2020 | 58 | 3 |
| X7 | 45, male | Acute pyelonephritis | 3/10/2020 | 49 | 3 |
| X8 | 89, female | Acute pyelonephritis | 3/13/2020 | 73 | 4 |
| XNull | 25, female | Healthy volunteer | NA | NA | 0 |
| Xpatient Fecal Sample (Optimal Dilution) | Baseline (No AC) | Mixing with Low-Dose AC 30 mg/g Feces | Mixing with Intermediate-Dose AC 150 mg/g Feces | Mixing with High-Dose AC 500 mg/g Feces |
|---|---|---|---|---|
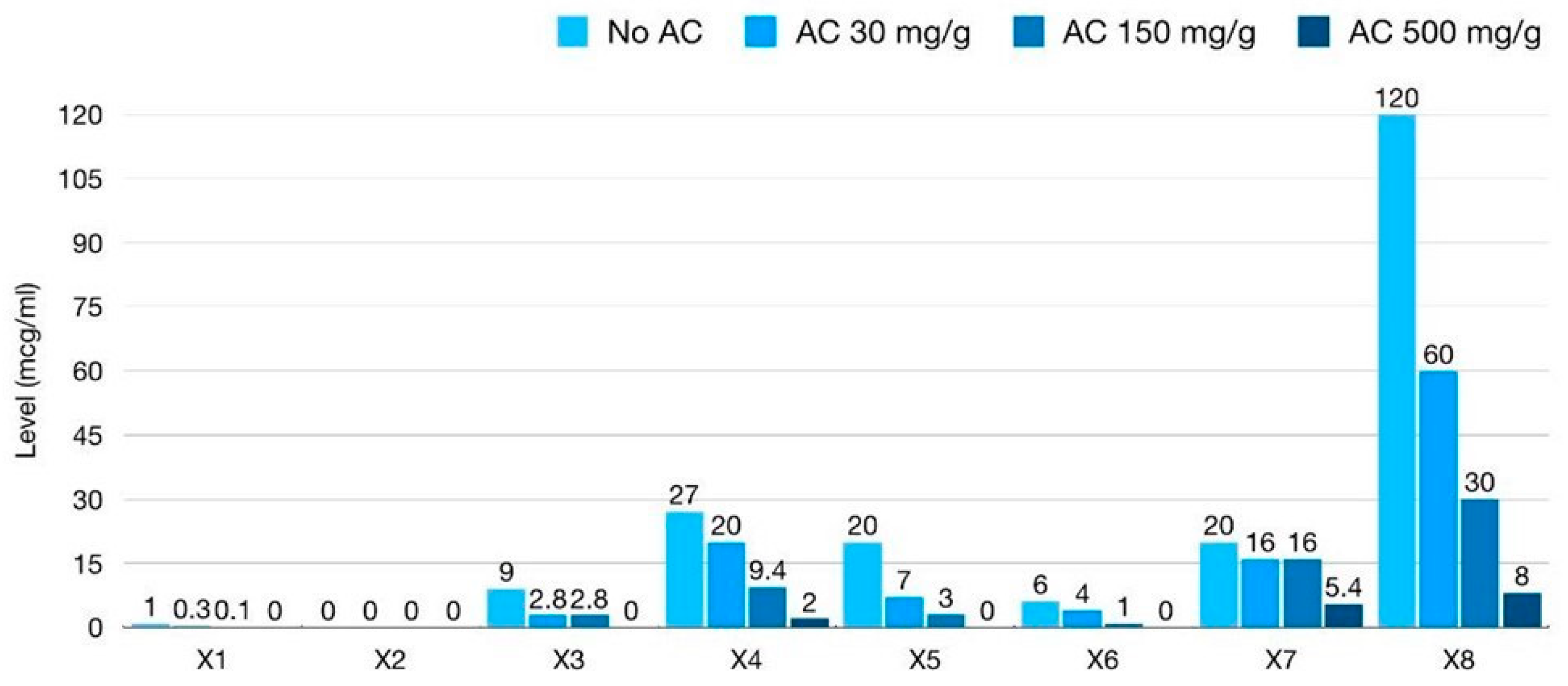
| X1 (100× dilution) | 10 ng/mL | 3 ng/mL | 1 ng/mL | <0.5 ng/mL |
| X2 (100× dilution) | 0.5 # ng/mL | <0.5 ng/mL | <0.5 ng/mL | <0.5 ng/mL |
| X3 (2000× dilution) | 4.5 ng/mL | 1.4 ng/mL | 1.4 ng/mL | <0.5 ng/mL |
| X4 (2000× dilution) | 13.5 ng/mL | 10 ng/mL | 4.7 ng/mL | 1 ng/mL |
| X5 (2000× dilution) | 10 ng/mL | 3.5 ng/mL | 1.5 ng/mL | <0.5 ng/mL |
| X6 (2000× dilution) | 3 ng/mL | 2 ng/mL | 0.5 ng/mL | <0.5 ng/mL |
| X7 (2000× dilution) | 10 ng/mL | 8 ng/mL | 8 ng/mL | 2.7 ng/mL |
| X8 (2000× dilution) | >40.5 (60) * ng/mL | 30 ng/mL | 15 ng/mL | 4 ng/mL |
| XNull (100× dilution) | <0.5 ng/mL | <0.5 ng/mL | <0.5 ng/mL | <0.5 ng/mL |
| XNull (2000× dilution) | <0.5 ng/mL | <0.5 ng/mL | <0.5 ng/mL | <0.5 ng/mL |
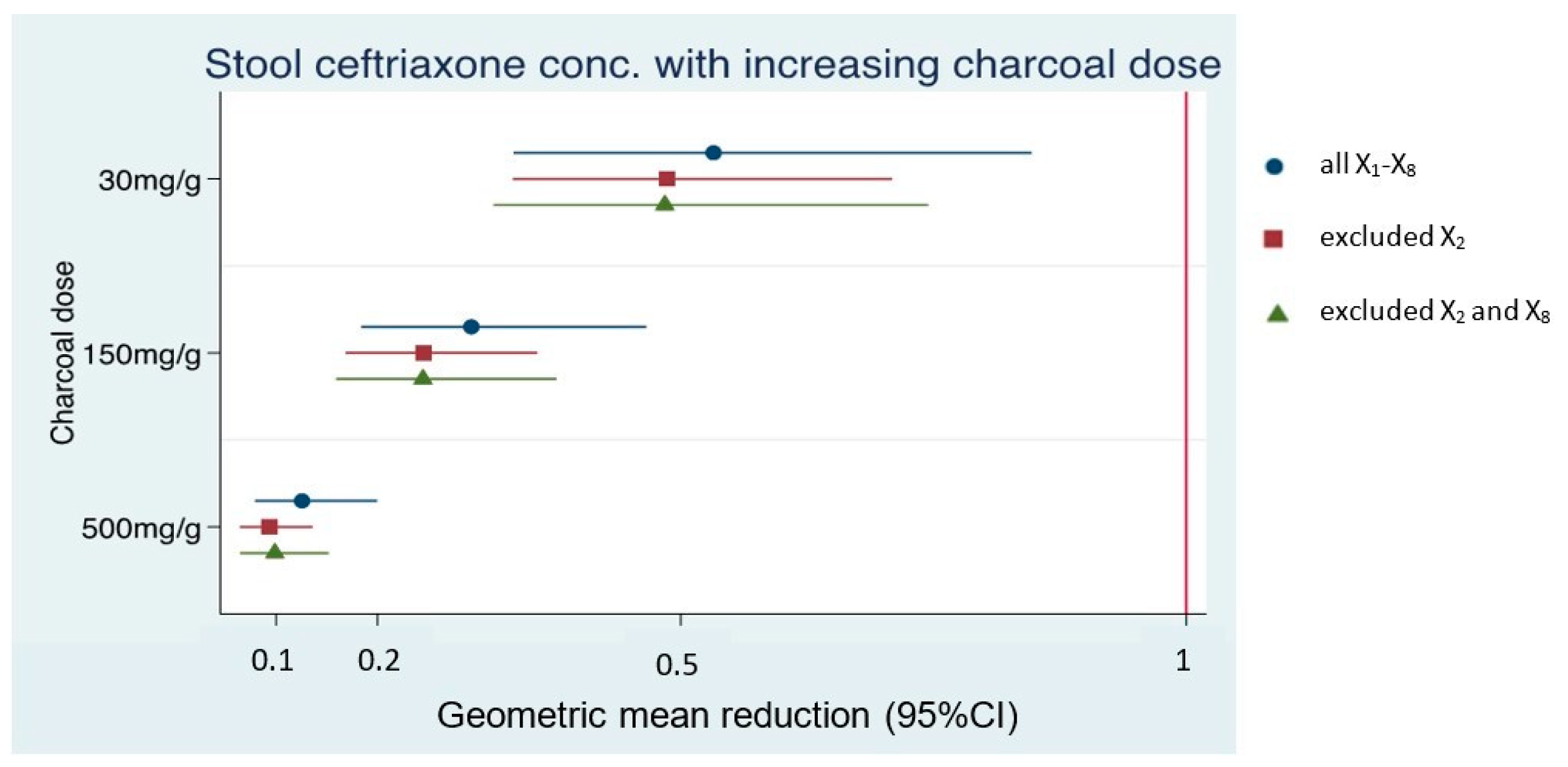
| Dataset 1; N = 8 (All X1–8) | ||
| Low dose AC | GMR 0.53 (0.33–0.85), p-value = 0.008 | 47% adsorption |
| Intermediate dose AC | GMR 0.29 (0.18–0.47), p-value < 0.001 | 71% adsorption |
| High dose AC | GMR 0.13 (0.08–0.2), p-value < 0.001 | 87% adsorption |
| Dataset 2; N = 7 (exclude X2) | ||
| Low dose AC | GMR 0.49 (0.33–0.71), p-value < 0.001 | 51% adsorption |
| Intermediate dose AC | GMR 0.25 (0.17–0.36), p-value < 0.001 | 75% adsorption |
| High dose AC | GMR 0.09 (0.06–0.14), p-value < 0.001 | 91% adsorption |
| Dataset 3; N = 6 (exclude X2 and X8) | ||
| Low dose AC | GMR 0.48 (0.31–0.74), p-value = 0.001 | 52% adsorption |
| Intermediate dose AC | GMR 0.25 (0.16–0.38), p-value < 0.001 | 75% adsorption |
| High dose AC | GMR 0.1 (0.06–0.15), p-value < 0.001 | 90% adsorption |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torvorapanit, P.; Kawang, K.; Chariyavilaskul, P.; Kerr, S.J.; Chatsuwan, T.; Nilaratanakul, V. The In Vitro Efficacy of Activated Charcoal in Fecal Ceftriaxone Adsorption among Patients Who Received Intravenous Ceftriaxone. Antibiotics 2023, 12, 127. https://doi.org/10.3390/antibiotics12010127
Torvorapanit P, Kawang K, Chariyavilaskul P, Kerr SJ, Chatsuwan T, Nilaratanakul V. The In Vitro Efficacy of Activated Charcoal in Fecal Ceftriaxone Adsorption among Patients Who Received Intravenous Ceftriaxone. Antibiotics. 2023; 12(1):127. https://doi.org/10.3390/antibiotics12010127
Chicago/Turabian StyleTorvorapanit, Pattama, Kornthara Kawang, Pajaree Chariyavilaskul, Stephen J Kerr, Tanittha Chatsuwan, and Voraphoj Nilaratanakul. 2023. "The In Vitro Efficacy of Activated Charcoal in Fecal Ceftriaxone Adsorption among Patients Who Received Intravenous Ceftriaxone" Antibiotics 12, no. 1: 127. https://doi.org/10.3390/antibiotics12010127
APA StyleTorvorapanit, P., Kawang, K., Chariyavilaskul, P., Kerr, S. J., Chatsuwan, T., & Nilaratanakul, V. (2023). The In Vitro Efficacy of Activated Charcoal in Fecal Ceftriaxone Adsorption among Patients Who Received Intravenous Ceftriaxone. Antibiotics, 12(1), 127. https://doi.org/10.3390/antibiotics12010127






