In Vitro Antibacterial and Wound Healing Activities Evoked by Silver Nanoparticles Synthesized through Probiotic Bacteria
Abstract
1. Introduction
2. Results and Discussion
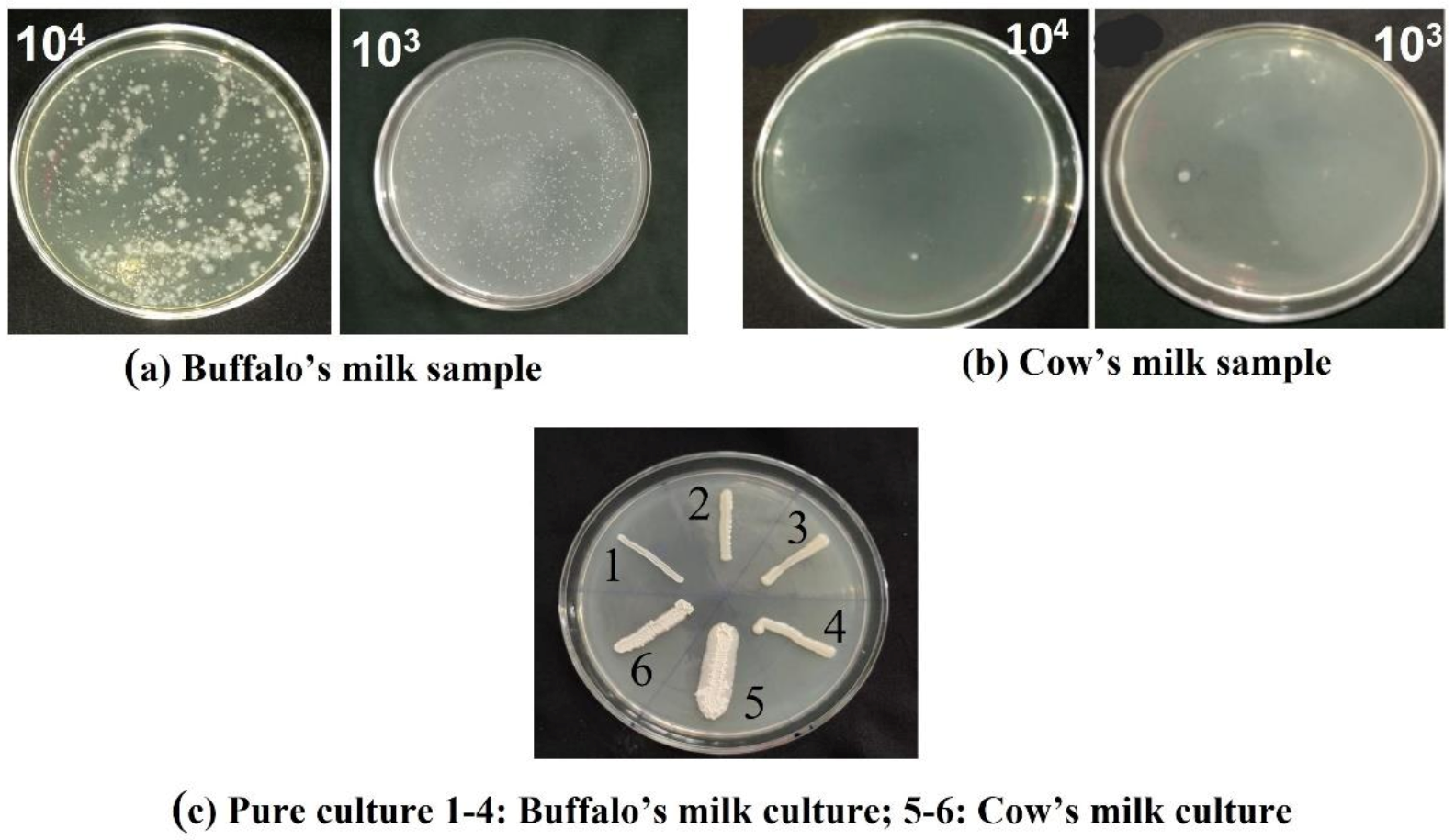
2.1. Isolated Probiotic Bacteria
2.2. Screening of Isolated Colonies
2.3. Identification of Isolated Strain
2.4. Nanoparticles Synthesis
2.5. Stability Analysis of AgNPs
2.6. FTIR Analysis of AgNPs
2.7. TEM and EDX Analysis of AgNPs
2.8. Optimisation of Synthesised Nanoparticle
2.8.1. Effect of Temperature
2.8.2. Effect of Concentration
2.8.3. Effect of pH
2.8.4. Antibacterial Activity of AgNPs
2.8.5. Antioxidant Activity
2.9. Cytotoxicity of AgNPs
2.10. In Vitro Scratch Wound-Healing Activity of AgNPs
3. Materials and Methods:
3.1. Isolation of Probiotic from Milk
- Once swabbed over LB media, they were screened against Gram-positive Bacillus subtilis and Staphylococcus aureus, as well as Gram-negative Pseudomonas aeruginosa and Escherichia coli.
- To the freshly prepared MRS broth a loop full of Lactobacillus culture was added and incubated in shaker for 48 h.
- Mueller-Hinton agar plates were prepared with 100 μL of infection-causing pathogens (Gram-positive Bacillus subtilis and Staphylococcus aureus, as well as Gram-negative Pseudomonas aeruginosa and Escherichia coli) spread evenly onto the surfaces of the agar plates separately.
- The MRS broth containing Lactobacillus culture was centrifuged and 50 µL of supernatant was added to the wells. Gentamycin (MEDOX Biotech, Chennai, India) was used as the control and the plates were incubated for 24 h.
3.2. Identification of Probiotic Bacteria
3.3. Synthesis of Nanoparticles
3.4. Optimization of Nanoparticles Synthesis
3.4.1. Effect of Temperature
3.4.2. Effect of pH
3.4.3. Effect of Precursor Concentration
3.5. Biosynthesis and Stability Analysis of Synthesized Nanoparticles
3.6. Characterization of Nano Particles
3.7. Application Studies
3.7.1. Antibacterial Assay of Bio-Synthesized Nanoparticles
3.7.2. Antioxidant Activity of Bio Synthesized Nanoparticles
3.7.3. In Vitro Wound-healing Activity
Cell Culture Maintenance
Cytotoxic Effect
3.7.4. In Vitro Scratch Wound-Healing Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and nanofibers for topical drug delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Wang, L.; Kafshgari, M.H.; Meunier, M. Optical Properties and Applications of Plasmonic-Metal Nanoparticles. Adv. Funct. Mater. 2020, 30, 2005400. [Google Scholar] [CrossRef]
- Coronado, E.A.; Encina, E.R.; Stefani, F. Optical properties of metallic nanoparticles: Manipulating light, heat and forces at the nanoscale. Nanoscale 2011, 3, 4042–4059. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Neville, A.; Dowson, D.; Williams, S.; Fisher, J. Effect of metallic nanoparticles on the biotribocorrosion behaviour of Metal-on-Metal hip prostheses. Wear 2009, 267, 683–688. [Google Scholar] [CrossRef]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as Recyclable Catalysts: The Frontier between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef] [PubMed]
- Myers, P.D., Jr.; Alam, T.E.; Kamal, R.; Goswami, D.Y.; Stefanakos, E. Nitrate salts doped with CuO nanoparticles for thermal energy storage with improved heat transfer. Appl. Energy 2016, 165, 225–233. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef] [PubMed]
- Balashanmugam, P.; Kim, H.J.; Singh, V.; Kumaran, R.S. Green synthesis of silver nanoparticles using Ginkgo biloba and their bactericidal and larvicidal effects. Nanosci. Nanotechnol. Lett. 2018, 10, 433–438. [Google Scholar] [CrossRef]
- Balashanmugam, P.; Jothinathan, M.K.D.; Murugan, R.; Palani, P.; Kalaichelvan, P.T.; Kim, H.J.; Singh, V.; Kumaran, R.S. An in vitro study on the burn wound healing activity of cotton fabrics incorporated with phytosynthesized silver nanoparticles in male Wistar albino rats. Eur. J. Pharm. Sci. 2017, 100, 187–196. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 2009, 84, 151–157. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomedicine 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Ashraf, N.; Ashraf, T.; Zhou, R.B.; Yin, D.C. Biological synthesis of metallic nanoparticles (MNPs) by plants and microbes: Their cellular uptake, biocompatibility, and biomedical applications. Appl. Microbiol. Biotechnol. 2019, 103, 2913–2935. [Google Scholar] [CrossRef]
- Noruzi, M. Biosynthesis of gold nanoparticles using plant extracts. Bioprocess Biosyst. Eng. 2015, 38, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.A.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.G.; El-Kased, R.F.; Elmazar, M.M. Thermoresponsive gels containing gold nanoparticles as smart antibacterial and wound healing agents. Sci. Rep. 2018, 8, 13674. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharma. J. 2019, 27, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Raucci, M.G.; De Gaetano, F.D.; Marotta, A. Antibacterial and bioactive silver-containing Na2O × CaO × 2SiO2 glass prepared by solgel method. J. Mater. Sci. Mater. Med. 2004, 15, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.H.; Burchette, R.J.; Siddiqi, R.A.; Huen, I.T.; Handott, L.L.; Fishman, A. The efcacy of silver-ion implanted catheters in reducing peritoneal dialysis-related infections. Perit. Dial. Int. 2003, 23, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Rani, C.; Ragunathan, C.R.; Prasannakumar, K. Biosynthesis of Silver Nano Particles Using L. Acidophilus (Probiotic Bacteria) and its Application. Int. J. Nanotechnol. Appl. 2010, 4, 217–222. [Google Scholar]
- Wilhelm, H.H.; Ulrich, S. Introduction to pre- and probiotics. Food Res. Int. 2002, 35, 109–116. [Google Scholar]
- Carlos, R.S.; Luciana, P.S.V.; Michele, R.S.; Adriane, B.P.M.; Caroline, T.Y.; Juliano, D.D.L.; Ashok, P.; Vanete, T.S. The Potential of Probiotics: A Review. Food Technol. Biotechnol. 2010, 48, 413–434. [Google Scholar]
- Cosme-Silva, L.; Dal-Fabbro, R.; Cintra, L.T.A.; Ervolino, E.; Prado, A.S.A.d.; Oliveira, D.P.d.; Marcelos, P.G.C.L.d.; Gomes-Filho, J.E. Dietary supplementation with multi-strain formula of probiotics modulates inflammatory and immunological markers in apical periodontitis. J. Appl. Oral Sci. 2021, 29, e20210483. [Google Scholar] [CrossRef]
- Cosme-Silva, L.; Dal-Fabbro, R.; Cintra, L.T.A.; Ervolino, E.; Plazza, F.; Mogami Bomfim, S.; Duarte, P.C.T.; Junior, V.; Gomes-Filho, J.E. Reduced bone resorption and inflammation in apical periodontitis evoked by dietary supplementation with probiotics in rats. Int. Endod. J. 2020, 53, 1084–1092. [Google Scholar] [CrossRef]
- Lehtoranta, L.; Pitkäranta, A.; Korpela, R. Probiotics in respiratory virus infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1289–1302. [Google Scholar] [CrossRef] [PubMed]
- Soghra, K.; Hamideh, M.H.; Mohammad, T.; Mohammad, R.N.; Abbas, A.I.F. Probiotics as an alternative strategy for prevention and treatment of human diseases: A review. Inflamm. Allergy-Drug Targets 2012, 11, 79–89. [Google Scholar]
- Sahar, K.; Mohammad, R.; Hosna, H.; Mahmoud, B.; Leila; Mahmoud, K. Isolation and identification of probiotic Lactobacillus from local dairy and evaluating their antagonistic effect on pathogens. Int. J. Pharm. Investig. 2017, 15, 241–246. [Google Scholar]
- Losada, M.A.; Olleros, T. Towards a healthier diet for the colon: The influence of fructo oligosaccharides and lactobacilli on intestinal health. Nutr. Res. 2006, 22, 71–84. [Google Scholar] [CrossRef]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides produced by lactic acid bacteria: From health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biotechnol. 2016, 7, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Anjana; Santosh Kumar, T. Bacteriocin-Producing Probiotic Lactic Acid Bacteria in Controlling Dysbiosis of the Gut Microbiota. Front. Cell. Infect. Microbiol. 2022, 12, 851140. [Google Scholar] [CrossRef] [PubMed]
- Rebecca, K.; Thomas, K.; James, G. The role of topical probiotics on wound healing: A review of animal and human studies. Int. Wound J. 2020, 17, 1687–1694. [Google Scholar]
- Ahmadova, A.; Todorov, S.D.; Hadji-Sfaxi, I.; Choiset, Y.; Rabesona, H.; Messaoudi, S. Antimicrobial and antifungal activities of Lactobacillus curvatus strain isolated from homemade Azerbaijani cheese. Anaerobe 2013, 20, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Babak, H.; Yousef, N.; Ali, A.; Norhafizah, A.; Dayang, R.; Rozita, R.; Abolfazl, B.; Ahmad, Y.K. Isolation and characterization of probiotics from dairies. Iran. J. Microbiol. 2017, 9, 234–243. [Google Scholar]
- Ben, A.K.; Vaughan, E.E.; de Vos, W.M. Advanced molecular tools for the identification of lactic acid bacteria. J. Nutr. 2007, 137, 741S–747S. [Google Scholar]
- Haghshenas, B.; Nami, Y.; Abdullah, N.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Anti-proliferative effects of Enterococcus strains isolated from fermented dairy products on different cancer cell lines. J. Funct. Foods 2014, 11, 363–374. [Google Scholar] [CrossRef]
- Sikder, M.; Lead, J.R.; Chandler, G.T.; Baalousha, M. A rapid approach for measuring silver nanoparticle concentration and dissolution in seawater by UV-Vis. Sci. Total Environ. 2018, 618, 597–607. [Google Scholar] [CrossRef]
- Naseer, Q.A.; Xuec, X.; Wang, X.; Dang, S.; Din, S.U.; Kalsoom; Jamil, J. Synthesis of silver nanoparticles using Lactobacillus bulgaricus and assessment of their antibacterial potential. Braz. J. Biol. 2022, 82, e232434. [Google Scholar] [CrossRef]
- Poonam, S.; Rajani, R.; Rajendra, M. Dairy Products Mediated Green Synthesis of Silver Nanoparticles: A Comparative Study. JETIR 2021, 8, 415–424. [Google Scholar]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd, Chichester, UK, 2000; pp. 10815–10837.
- Nourbakhsh, H.; Madadlou, A.; Emam-Djomeh, Z.; Wang, Y.; Gunasekaran, S. One-pot nanoparticulation of potentially bioactive peptides and gallic acid encapsulation. Food Chem. 2016, 210, 317–324. [Google Scholar] [CrossRef]
- Vass, E.; Hollosi, M.; Besson, F.; Buchet, R. Vibrational Spectroscopic Detection of Beta- and Gamma-Turns in Synthetic and Natural Peptides and Proteins. Chem. Rev. 2003, 103, 1917–1954. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Heuer, C.; Luinge, H.J.; Lutz, E.T.; Schukken, Y.H.; van der Maas, J.H.; Wilmink, H.; Noordhuizen, J.P. Determination of Acetone in Cow Milk by Fourier Transform Infrared Spectroscopy for the Detection of Subclinical Ketosis, J. Dairy Sci. 2001, 84, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Khan, K.Y.; Majeed, H.; Abid, M.; Xu, L.; Wu, F.; Xu, X. Soymilk-Cow’s milk ACE-inhibiting enzyme modified cheese. Food Chem. 2017, 237, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Abbasi, A.Z.; Pfeiffer, C.; Hussain, S.Z.; Khalid, Z.M.; Gil, P.R.; Parak, W.J.; Hussain, I. Protein-mediated synthesis, pH-induced reversible agglomeration, toxicity and cellular interaction of silver nanoparticles. Colloids Surf. B Biointerfaces 2013, 102, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Kasthuri, J.; Veerapandian, S.; Rajendiran, N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf. B 2009, 68, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, J.; Pan, D.; Wu, Z.; Guo, Y.; Zeng, X.; Lian, L. Metabolomics analysis of Lactobacillus plantarum ATCC 14917 adhesion activity under initial acid and alkali stress. PLoS ONE 2018, 13, e0196231. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, M.; Abdul, R.; Rosfarizan, M.; Uswatun, H. Microbial Mediated Synthesis of Silver nanoparticles by Lactobacillus plantarum TA4 and Its Antibacterial and, Antioxidant Activity. Appl. Sci. 2020, 7, 6973. [Google Scholar]
- Hamed, B.; Soheila, H.; Pouneh, E.; Milad, A. Microbial mediated preparation, characterization and optimization of gold nanoparticles. Braz. J. Microbiol. 2014, 45, 1493–1500. [Google Scholar]
- Saravanan, M.; Priya, A.; Raja, S.; Konathala, R. Biomimetic synthesis of silver nanoparticles from Streptomyces atrovirens and their potential anticancer activity against human breast cancer cells. IET Nanobiotechnol. 2017, 11, 965–972. [Google Scholar]
- Aparajita, V.; Mohan, S.M. Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. J. Radiat. Res. Appl. Sci. 2015, 45, 209–316. [Google Scholar]
- Khalil, M.M.H.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2013, 7, 1131e1139. [Google Scholar] [CrossRef]
- Yacaman, M.J.; Ascencio, J.A.; Liu, H.B.; Gardea-Torresdey, J. Structure shape and stability of nanometric sized particles. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2001, 19, 1091–1103. [Google Scholar] [CrossRef]
- Li, J.; Rong, K.; Zhao, H.; Li, F.; Lu, Z.; Chen, R. Highly selective antibacterial activities of silver nanoparticles against Bacillus subtilis. J. Nanosci. Nanotechnol. 2013, 13, 6806–6813. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Tyagi, A.K.; Kumar, P.; Malik, A. Antibacterial potential of Jatropha curcas synthesized silver nanoparticles against food borne pathogens. Front. Microbiol. 2016, 7, 1748. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 27, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Netala, V.R.; Kotakadi, V.S.; Nagam, V.; Bobbu, P.; Ghosh, S.B.; Tartte, V. First report of biomimetic synthesis of silver nanoparticles using aqueous callus extract of Centella asiatica and their antimicrobial activity. Appl. Nanosci. 2014, 5, 123–129. [Google Scholar] [CrossRef]
- Rashid, M.M.O.; Akhter, K.N.; Chowdhury, J.A.; Hossen, F.; Hussain, M.S.; Hossain, M.T. Characterization of phytoconstituents and evaluation of antimicrobial activity of silver extract nanoparticles synthesized from Momordica charantia fruit extract. BMC Complement. Altern. Med. 2017, 17, 336–341. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Raza, A.; Khan, M.A.; Ahmad, I.; Islam, N.U.; Saravanan, M.; Ubaid, M.F.; Ali, M.; Shinwari, Z.K. Green synthesis of silver nanoparticles via plant extracts: Beginning a new era in cancer theranostics. Nanomedicine 2016, 12, 3157–3177. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, G.; Kaur, P.; Bajaj, R.; Rawat, M. A review on green synthesis and characterization of silver nanoparticles and their application: A green nanoworld. World J. Pharm. Pharm. Sci. 2016, 6, 7. [Google Scholar]
- Kim, S.H.; Lee, H.S.; Ryu, D.S.; Choi, S.J.; Lee, D.S. Antibacterial Activity of Silver-nanoparticles Against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Hussein, R.R.S.; Farghali, A.A.; Hassanein, A.H.A.; Ibraheem, I.B.M. Biosynthesis of silver nanoparticles by using of the marine alga Gracilariaparvispora and its antagonistic efficacy against some common skin infecting pathogens. Aust. J. Basic Appl. Sci. 2017, 11, 219–227. [Google Scholar]
- Reddy, N.J.; Nagoor Vali, D.; Rani, M. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Pradhan, S.; Ashe, S.; Nayak, B. Biologically synthesized silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J. Colloid Interface Sci. 2015, 457, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Amro, N.A.; Kotra, L.P.; Wadu-Mesthrige, K.; Bulychev, A.; Mobashery, S.; Liu, G. High-resolution atomic force microscopy studies of the Escherichia coli outer membrane: Structural basis for permeability. Langmuir 2000, 16, 2789–2796. [Google Scholar] [CrossRef]
- Mirzajani, F.; Ghassempour, A.; Aliahmadi, A.; Esmaeili, M.A. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Res. Microbiol. 2011, 162, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Hara, K.; Kudo, J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl. Environ. Microbiol. 2005, 71, 7589–7593. [Google Scholar] [CrossRef]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome. Res. 2006, 5, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of solver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, C.; Wang, H. Impact of silver nanoparticles on human cells: Effect of particle size, shape and physical properties. Nanotoxicology 2010, 4, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zeng, J.; Moran, C.H.; Xia, Y. Recent developments in shape-controlled synthesis of silver nanocrystals. J. Phys. Chem. C 2012, 116, 21647–21656. [Google Scholar] [CrossRef]
- Hosseinidoust, Z.; Basenet, M.; van de Ven, T.G. Tufenkji, N. One-pot green synthesis of anisotropic silver nanoparticles. Environ. Sci. Nano 2016, 3, 1259–1264. [Google Scholar] [CrossRef]
- Helmlinger, J.; Sengstock, C.; Groß-Heitfeld, C.; Mayer, C.; Schildhaue, T.A.; Koller, M.; Epple, M. Silver nanoparticles with different size and shape: Equal cytotoxicity, but different antibacterial effects. RSC Adv. 2016, 6, 18490–18501. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.; Ullah, A.K.; Hossain, K.F.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Mishra, P. Antimicrobial and antibiofilm activity of curcumin-silver nanoparticles with improved stability and selective toxicity to bacteria over mammalian cells. Med. Microbiol. Immunol. 2018, 207, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Baygar, T. Characterization of silk sutures coated with propolis and biogenic silver nanoparticles (AgNPs); an eco-friendly solution with wound healing potential against surgical site infections (SSIs). Turk. J. Med. Sci. 2019, 50, 258–266. [Google Scholar]
- Daniela, M.S.; Claudia, Y.H.; Adnan, Y.T.; Maricê, N.d.O. Evaluation of different selective media for enumeration of probiotic micro-organisms in combination with yogurt starter cultures in fermented milk. Afr. J. Microbiol. Res. 2011, 5, 3901–3906. [Google Scholar] [CrossRef]
- Gayathri, V.; Nivedha, S.; Pujita, V.; Ivo, R.S. Green synthesis of copper nanoparticles using bracts of Musa paradisiaca (Monthan) and study of its antimicrobial and antioxidant activity. Res. J. Pharm. Technol. 2020, 13, 781–786. [Google Scholar] [CrossRef]
- Arumugam, T.; Senthil Kumar, P.; Hemavathy, R.V.; Swetha, V.; Karishma Sri, R. Isolation, structure elucidation and anticancer activity from Brevibacillus brevis EGS 9 that combats Multi Drug Resistant actinobacteria. Microb. Pathogen. 2018, 115, 146–153. [Google Scholar] [CrossRef]
- Balashanmugam, P.; Prabhu, D.; Devasena, T.; Hak, J.S.; Kim, K.W.; Jung, Y.S.; Song, H.J.; Kim, H.J.; Kumaran, R.S. Facile synthesis of silver nanoparticles using Asian spider flower and its in vitro cytotoxic activity against human breast carcinoma cells. Processes 2020, 8, 430. [Google Scholar]
- Vijayakumar, G.; Kesavan, H.; Kannan, A.; Arulanandam, D.; Kim, J.H.; Kim, K.J.; Song, H.J.; Kim, H.J.; Rangarajulu, S.K. Phytosynthesis of Copper Nanoparticles Using Extracts of Spices and Their Antibacterial Properties. Processes, 2021; 9, 1341. [Google Scholar]
- Kouhkan, M.; Ahangar, P.; Babaganjeh, L.; Ashrafi, M. Biosynthesis of Copper Oxide nanoparticles Using Lactobacillus casei Subsp. Casei and its Anticancer and Antibacterial Activities. Curr. Nanosci. 2020, 102, 216–223. [Google Scholar]
- Alam, H.; Nafeesa, K.; Mohammad, A.; Syed, A.; Saravanan, M. Synthesis of Selenium nanoparticles Using Probiotic Bacteria Lactobacillus acidophilus and Their Enhanced Antimicrobial Activity Against Resistant Bacteria. J. Clust. Sci. 2019, 63, 501–503. [Google Scholar] [CrossRef]
- Amr, T.M.S.; Ahmad, S.A.; Hessa, A.B.; Khalid, A.A.R. Production of silver nanoparticles with strong and stable antimicrobial activity against highly pathogenic and multidrug resistant bacteria. Sci. World J. 2014, 2014, 704708. [Google Scholar]
- Suriati, G.; Nureen, N. Morphology and optical properties of AgNPs: Effect of reducing agent to surfactant ratio. ARPN J. Eng. Appl. Sci. 2016, 11, 10. [Google Scholar]
- Pannerselvam, B.; Thiyagarajan, D.; Pazhani, A.; Thangavelu, K.P.; Kim, H.J.; Rangarajulu, S.K. Copperpod Plant Synthesized AgNPs Enhance Cytotoxic and Apoptotic Effect in Cancer Cell Lines. Processes 2021, 9, 888. [Google Scholar] [CrossRef]
- Mouritzen, M.V.; Andrea, A.; Qvist, K.; Poulsen, S.S.; Jenssen, H. Immunomodulatory potential of Nisin A with application in wound healing. Wound Repair Regen. 2019, 27, 650–660. [Google Scholar] [CrossRef]
- Ong, J.S.; Taylor, T.D.; Yong, C.C.; Khoo, B.Y.; Liong, M.T. Lactobacillus plantarum USM8613 Aids in Wound Healing and Suppresses Staphylococcus aureus Infection at Wound Sites. Probiotics Antimicrob. Proteins 2019, 12, 125–137. [Google Scholar] [CrossRef]
- Nicole, C.A.; Magda, B.S.; Abdu, F.A. Growth and Maintenance of Vero Cell Lines. Curr. Protoc. Microbiol. 2008, 11, A-4E. [Google Scholar]
- Mossman, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Malarvizhi, G.; Karpagam, S.C. Evaluating the natural fibre reinforced polymer biocomposite for the development of novel wound dressing materials. Int. J. Res. Ayurveda. Pharm. 2017, 8, 124–129. [Google Scholar]
- Felice, F.; Zambito, Y.; Belardinelli, E. Effect of different chitosan derivatives on in vitro scratch wound assay: A comparative study. Int. J. Biol. Macromol. 2015, 76, 236–241. [Google Scholar] [CrossRef]















| Microorganism | Zone of Inhibition (mm) | |||
|---|---|---|---|---|
| Positive Control&&&&(Gentamycin) | Pure Culture 3 | Pure Culture 6 | ||
| Gram −ve | Escherichia coli | 21 | 09 | 08 |
| Pseudomonas aeruginosa | 20 | 12 | 11 | |
| Gram +ve | Staphylococcus aureus | 21 | 10 | 12 |
| Bacillus subtilis | 19 | 11 | 09 | |
| Spectral Band (cm−1) | Functional Group |
|---|---|
| 3450–3320 | Hydroxyl group and H-bonded |
| 1635 | Amide I (C=O stretching vibration) |
| 1350 | N-H bending vibration |
| 1232 | C-N stretching vibration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijayakumar, G.; Kim, H.J.; Rangarajulu, S.K. In Vitro Antibacterial and Wound Healing Activities Evoked by Silver Nanoparticles Synthesized through Probiotic Bacteria. Antibiotics 2023, 12, 141. https://doi.org/10.3390/antibiotics12010141
Vijayakumar G, Kim HJ, Rangarajulu SK. In Vitro Antibacterial and Wound Healing Activities Evoked by Silver Nanoparticles Synthesized through Probiotic Bacteria. Antibiotics. 2023; 12(1):141. https://doi.org/10.3390/antibiotics12010141
Chicago/Turabian StyleVijayakumar, Gayathri, Hyung Joo Kim, and Senthil Kumaran Rangarajulu. 2023. "In Vitro Antibacterial and Wound Healing Activities Evoked by Silver Nanoparticles Synthesized through Probiotic Bacteria" Antibiotics 12, no. 1: 141. https://doi.org/10.3390/antibiotics12010141
APA StyleVijayakumar, G., Kim, H. J., & Rangarajulu, S. K. (2023). In Vitro Antibacterial and Wound Healing Activities Evoked by Silver Nanoparticles Synthesized through Probiotic Bacteria. Antibiotics, 12(1), 141. https://doi.org/10.3390/antibiotics12010141







