Biodentine Inhibits the Initial Microbial Adhesion of Oral Microbiota In Vivo
Abstract
:1. Introduction
2. Results
2.1. Microbial Adhesion on Biodentine, MTA, AH Plus, and Bovine Dentin
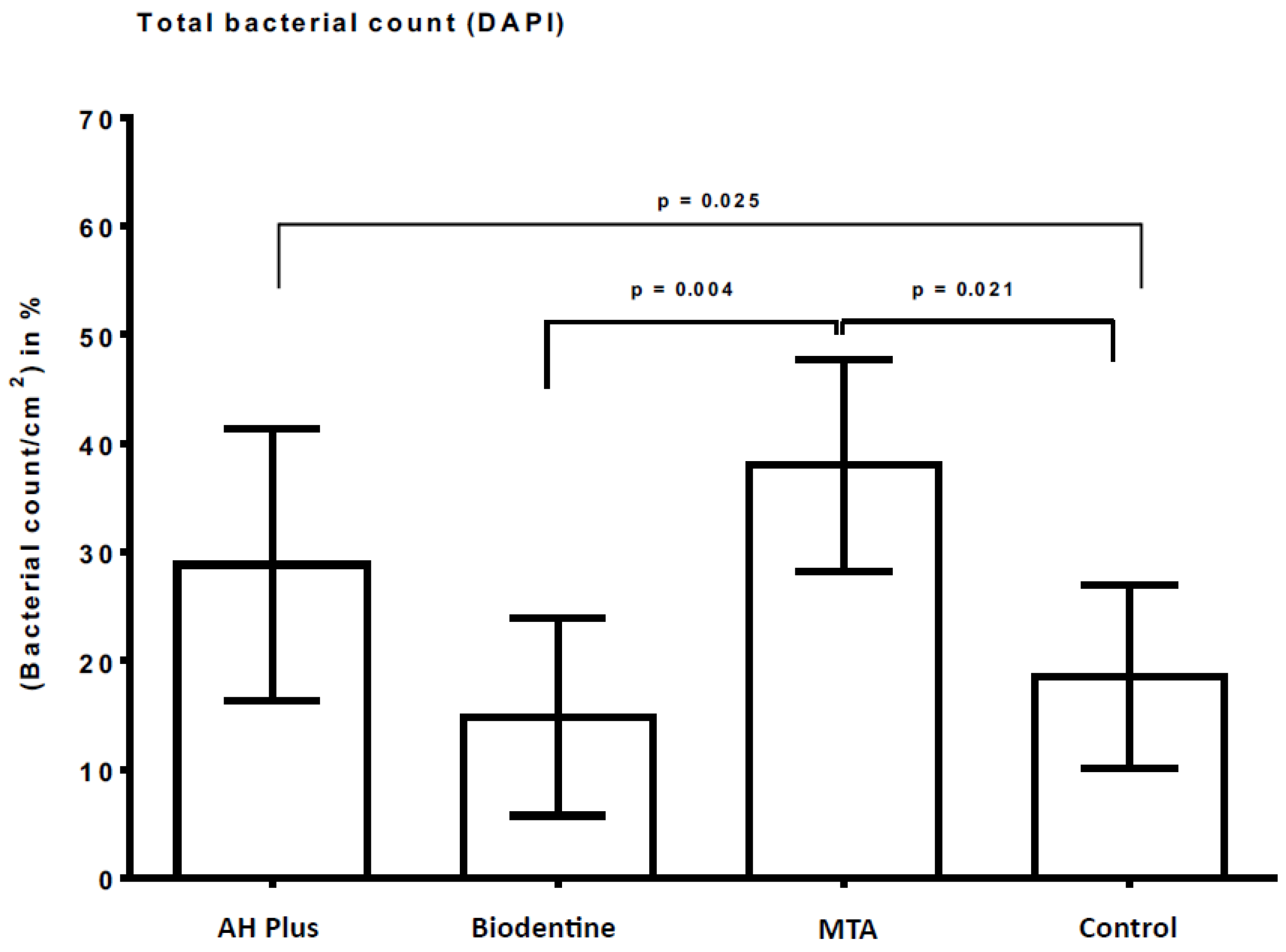
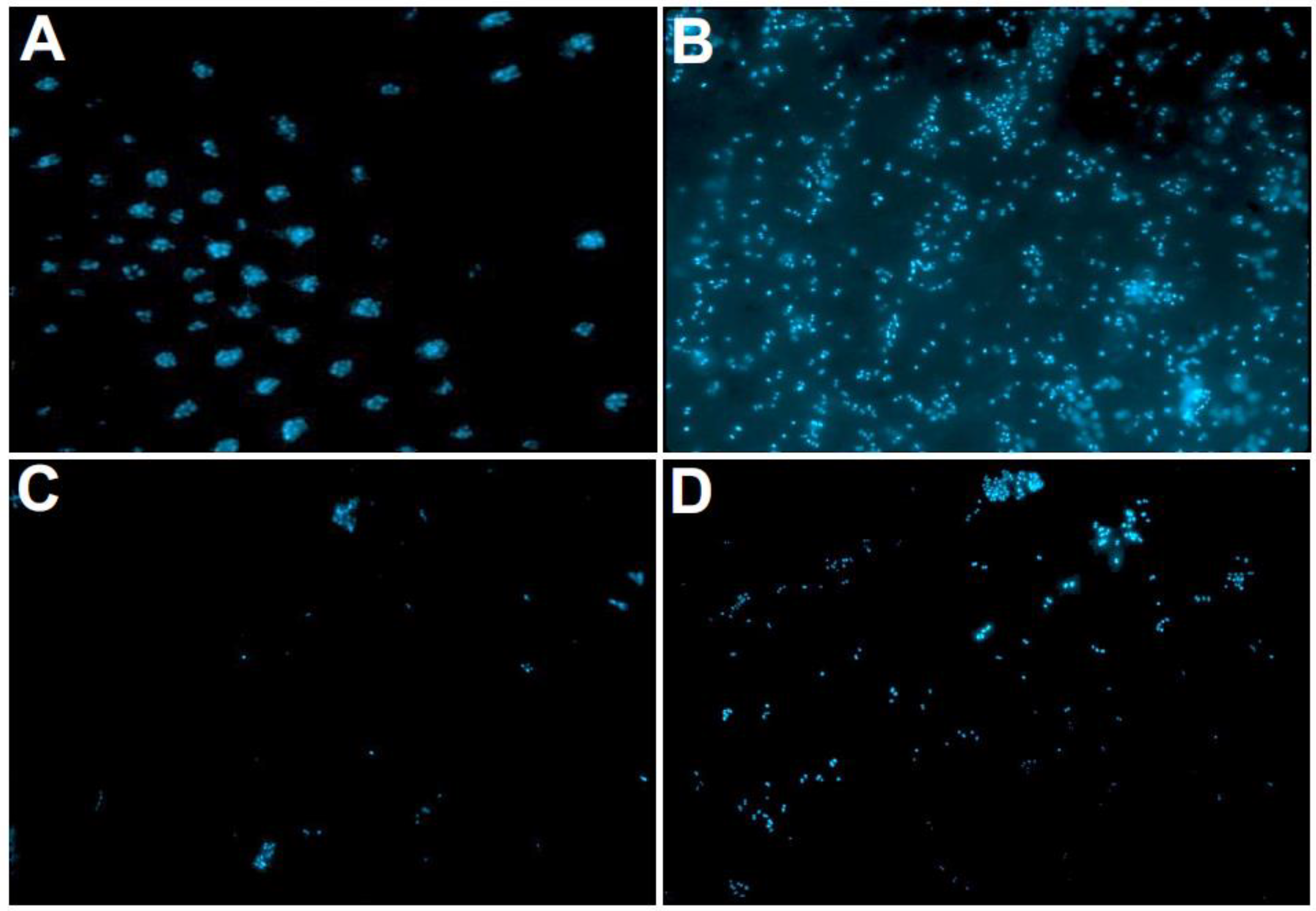
2.2. Fluorescence Microscopic Evaluation with DAPI
2.3. Live/Dead Staining
3. Discussion
4. Materials and Methods
4.1. Subject Recruitment
4.1.1. Inclusion Criteria
- -
- individuals aged ≥ 18 years
- -
- caries-free individuals
4.1.2. Exclusion Criteria
- -
- individuals with known allergy to the materials or their components and/or suffering from infectious or life-threatening diseases
- -
- breastfeeding or pregnant individuals
- -
- individuals with temporary use of antibiotics in the last six months or anti-inflammatory medication within the last 30 days
- -
- individuals with serious general illnesses, such as diabetes, HIV, hepatitis B and C, acute tumor diseases, or epilepsy
4.2. Material Samples
4.3. Intraoral Splints
4.4. Determination of the Colony Forming Units (CFUs)
4.5. DAPI (4′,6-Diamidine-2′-phenylindole Dihydrochloride) Staining
4.6. Live/Dead Staining and Fluorescence Microscopy
4.7. Image Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.J.; Monsef, M.; Torabinejad, M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J. Endod. 1993, 19, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Jung, C.; Shin, D.H.; Cho, Y.B.; Song, M. Calcium silicate-based root canal sealers: A literature review. Restor. Dent. Endod. 2020, 45, e35. [Google Scholar] [CrossRef] [PubMed]
- Aminoshariae, A.; Primus, C.; Kulild, J.C. Tricalcium silicate cement sealers: Do the potential benefits of bioactivity justify the drawbacks? J. Am. Dent. Assoc. 2022, 153, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.A.H.; Marciano, M.A.; Vivan, R.R.; Tanomaru Filho, M.; Tanomaru, J.M.G.; Camilleri, J. Tricalcium silicate-based cements: Properties and modifications. Braz. Oral. Res. 2018, 32, e70. [Google Scholar] [CrossRef] [PubMed]
- Bhavana, V.; Chaitanya, K.P.; Gandi, P.; Patil, J.; Dola, B.; Reddy, R.B. Evaluation of antibacterial and antifungal activity of new calcium-based cement (Biodentine) compared to MTA and glass ionomer cement. J. Conserv. Dent. 2015, 18, 44–46. [Google Scholar] [CrossRef]
- Koruyucu, M.; Topcuoglu, N.; Tuna, E.B.; Ozel, S.; Gencay, K.; Kulekci, G.; Seymen, F. An assessment of antibacterial activity of three pulp capping materials on Enterococcus faecalis by a direct contact test: An in vitro study. Eur. J. Dent. 2015, 9, 240–245. [Google Scholar] [CrossRef]
- Rajasekharan, S.; Martens, L.C.; Cauwels, R.; Anthonappa, R.P.; Verbeeck, R.M.H. Biodentine material characteristics and clinical applications: A 3 year literature review and update. Eur. Arch. Paediatr. Dent. 2018, 19, 1–22. [Google Scholar] [CrossRef]
- Safi, C.; Kohli, M.R.; Kratchman, S.I.; Setzer, F.C.; Karabucak, B. Outcome of Endodontic Microsurgery Using Mineral Trioxide Aggregate or Root Repair Material as Root-end Filling Material: A Randomized Controlled Trial with Cone-beam Computed Tomographic Evaluation. J. Endod. 2019, 45, 831–839. [Google Scholar] [CrossRef]
- Torabinejad, M.; Parirokh, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part II: Other clinical applications and complications. Int. Endod. J. 2018, 51, 284–317. [Google Scholar] [CrossRef]
- Malkondu, O.; Karapinar Kazandag, M.; Kazazoglu, E. A review on biodentine, a contemporary dentine replacement and repair material. Biomed. Res. Int. 2014, 2014, 160951. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Wunder, A.; Auschill, T.M.; Follo, M.; Braun, G.; Hellwig, E.; Arweiler, N.B. The in vivo dynamics of Streptococcus spp., Actinomyces naeslundii, Fusobacterium nucleatum and Veillonella spp. in dental plaque biofilm as analysed by five-colour multiplex fluorescence in situ hybridization. J. Med. Microbiol. 2007, 56, 681–687. [Google Scholar] [CrossRef]
- Dawood, A.E.; Manton, D.J.; Parashos, P.; Wong, R.; Palamara, J.; Stanton, D.P.; Reynolds, E.C. The physical properties and ion release of CPP-ACP-modified calcium silicate-based cements. Aust. Dent. J. 2015, 60, 434–444. [Google Scholar] [CrossRef]
- Kaup, M.; Schafer, E.; Dammaschke, T. An in vitro study of different material properties of Biodentine compared to ProRoot MTA. Head. Face Med. 2015, 11, 16. [Google Scholar] [CrossRef]
- Marconyak, L.J., Jr.; Kirkpatrick, T.C.; Roberts, H.W.; Roberts, M.D.; Aparicio, A.; Himel, V.T.; Sabey, K.A. A Comparison of Coronal Tooth Discoloration Elicited by Various Endodontic Reparative Materials. J. Endod. 2016, 42, 470–473. [Google Scholar] [CrossRef]
- Escobar-Garcia, D.M.; Aguirre-Lopez, E.; Mendez-Gonzalez, V.; Pozos-Guillen, A. Cytotoxicity and Initial Biocompatibility of Endodontic Biomaterials (MTA and Biodentine) Used as Root-End Filling Materials. Biomed. Res. Int. 2016, 2016, 7926961. [Google Scholar] [CrossRef]
- Kucukkaya, S.; Gorduysus, M.O.; Zeybek, N.D.; Muftuoglu, S.F. In Vitro Cytotoxicity of Calcium Silicate-Based Endodontic Cement as Root-End Filling Materials. Scientifica (Cairo) 2016, 2016, 9203932. [Google Scholar] [CrossRef]
- Saberi, E.A.; Karkehabadi, H.; Mollashahi, N.F. Cytotoxicity of Various Endodontic Materials on Stem Cells of Human Apical Papilla. Iran. Endod. J. 2016, 11, 17–22. [Google Scholar] [CrossRef]
- Samyuktha, V.; Ravikumar, P.; Nagesh, B.; Ranganathan, K.; Jayaprakash, T.; Sayesh, V. Cytotoxicity evaluation of root repair materials in human-cultured periodontal ligament fibroblasts. J. Conserv. Dent. 2014, 17, 467–470. [Google Scholar] [CrossRef]
- Thompson, V.; Craig, R.G.; Curro, F.A.; Green, W.S.; Ship, J.A. Treatment of deep carious lesions by complete excavation or partial removal: A critical review. J. Am. Dent. Assoc. 2008, 139, 705–712. [Google Scholar] [CrossRef]
- Ali, A.; Bhosale, A.; Pawar, S.; Kakti, A.; Bichpuriya, A.; Agwan, M.A. Current Trends in Root Canal Irrigation. Cureus 2022, 14, e24833. [Google Scholar] [CrossRef]
- Bjorndal, L.; Kidd, E.A. The treatment of deep dentine caries lesions. Dent. Update 2005, 32, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Bjorndal, L.; Reit, C.; Bruun, G.; Markvart, M.; Kjaeldgaard, M.; Nasman, P.; Thordrup, M.; Dige, I.; Nyvad, B.; Fransson, H.; et al. Treatment of deep caries lesions in adults: Randomized clinical trials comparing stepwise vs. direct complete excavation, and direct pulp capping vs. partial pulpotomy. Eur. J. Oral. Sci. 2010, 118, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Ceci, M.; Beltrami, R.; Chiesa, M.; Colombo, M.; Poggio, C. Biological and chemical-physical properties of root-end filling materials: A comparative study. J. Conserv. Dent. 2015, 18, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Poggio, C.; Beltrami, R.; Colombo, M.; Ceci, M.; Dagna, A.; Chiesa, M. In vitro antibacterial activity of different pulp capping materials. J. Clin. Exp. Dent. 2015, 7, e584–e588. [Google Scholar] [CrossRef] [PubMed]
- Ghatole, K.; Patil, A.; Giriyappa, R.H.; Singh, T.V.; Jyotsna, S.V.; Rairam, S. Evaluation of Antibacterial Efficacy of MTA with and without Additives Like Silver Zeolite and Chlorhexidine. J. Clin. Diagn. Res. 2016, 10, ZC11–ZC14. [Google Scholar] [CrossRef]
- Poggio, C.; Arciola, C.R.; Beltrami, R.; Monaco, A.; Dagna, A.; Lombardini, M.; Visai, L. Cytocompatibility and antibacterial properties of capping materials. ScientificWorldJournal 2014, 2014, 181945. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, S.; Koh, D.; Hsu, C.Y. Salivary biomarkers for dental caries. Periodontol 2000 2016, 70, 128–141. [Google Scholar] [CrossRef]
- Hiremath, G.S.; Kulkarni, R.D.; Naik, B.D. Evaluation of minimal inhibitory concentration of two new materials using tube dilution method: An in vitro study. J. Conserv. Dent. 2015, 18, 159–162. [Google Scholar] [CrossRef]
- Song, F.; Koo, H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J. Dent. Res. 2015, 8, 1027–1034. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Solanki, N.P.; Venkappa, K.K.; Shah, N.C. Biocompatibility and sealing ability of mineral trioxide aggregate and biodentine as root-end filling material: A systematic review. J. Conserv. Dent. 2018, 1, 10–15. [Google Scholar] [CrossRef]
- Ionescu, A.; Wutscher, E.; Brambilla, E.; Schneider-Feyrer, S.; Giessibl, F.J.; Hahnel, S. Influence of surface properties of resin-based composites on in vitro Streptococcus mutans biofilm development. Eur. J. Oral Sci. 2012, 5, 458–645. [Google Scholar] [CrossRef]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Aksel, H.; Kucukkaya Eren, S.; Askerbeyli Ors, S.; Karaismailoglu, E. Surface and vertical dimensional changes of mineral trioxide aggregate and biodentine in different environmental conditions. J. Appl. Oral. Sci. 2018, 27, e20180093. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Iacono, F.; Agee, K.; Siboni, F.; Tay, F.; Pashley, D.H.; Prati, C. Setting time and expansion in different soaking media of experimental accelerated calcium-silicate cements and ProRoot MTA. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2009, 108, e39–e45. [Google Scholar] [CrossRef]
- Camilleri, J.; Grech, L.; Galea, K.; Keir, D.; Fenech, M.; Formosa, L.; Damidot, D.; Mallia, B. Porosity and root dentine to material interface assessment of calcium silicate-based root-end filling materials. Clin. Oral. Investig. 2014, 18, 1437–1446. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Siboni, F.; Botero, T.; Bossu, M.; Riccitiello, F.; Prati, C. Calcium silicate and calcium hydroxide materials for pulp capping: Biointeractivity, porosity, solubility and bioactivity of current formulations. J. Appl. Biomater. Funct. Mater. 2015, 13, 43–60. [Google Scholar] [CrossRef]
- Camilleri, J.; Laurent, P.; About, I. Hydration of Biodentine, Theracal LC, and a prototype tricalcium silicate-based dentin replacement material after pulp capping in entire tooth cultures. J. Endod. 2014, 40, 1846–1854. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M. The oral cavity—A key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clin. Oral. Investig. 2009, 13, 123–139. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Shalavi, S.; Yazdizadeh, M. Antimicrobial activity of calcium hydroxide in endodontics: A review. Chonnam Med. J. 2012, 48, 133–140. [Google Scholar] [CrossRef]
- Schmidt, J.; Buenger, L.; Krohn, S.; Kallies, R.; Zeller, K.; Schneider, H.; Ziebolz, D.; Berg, T.; Haak, R. Effect of a bioactive cement on the microbial community in carious dentin after selective caries removal—An in-vivo study. J. Dent. 2020, 92, 103264. [Google Scholar] [CrossRef]
- Torabinejad, M.; Hong, C.U.; McDonald, F.; Pitt Ford, T.R. Physical and chemical properties of a new root-end filling material. J. Endod. 1995, 21, 349–353. [Google Scholar] [CrossRef]
- Clauder, T. Present status and future directions—Managing perforations. Int. Endod. J. 2022, 55 (Suppl. 4), 872–891. [Google Scholar] [CrossRef]
- Zizka, R.; Sedy, J.; Gregor, L.; Voborna, I. Discoloration after Regenerative Endodontic Procedures: A Critical Review. Iran. Endod. J. 2018, 13, 278–284. [Google Scholar] [CrossRef]
- Mozynska, J.; Metlerski, M.; Lipski, M.; Nowicka, A. Tooth Discoloration Induced by Different Calcium Silicate-based Cements: A Systematic Review of In Vitro Studies. J. Endod. 2017, 43, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, K.; Mistakidis, I.; Beltes, P.; Karagiannis, V. Spectrophotometric analysis of coronal discolouration induced by grey and white MTA. Int. Endod. J. 2013, 46, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Auschill, T.M.; Hellwig, E.; Sculean, A.; Hein, N.; Arweiler, N.B. Impact of the intraoral location on the rate of biofilm growth. Clin. Oral. Investig. 2004, 8, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.J.; Al-Ahmad, A.; Follo, M.; Spitzmuller, B.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Visualization of initial bacterial colonization on dentine and enamel in situ. J. Microbiol. Methods 2010, 81, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Prada-Lopez, I.; Quintas, V.; Vilaboa, C.; Suarez-Quintanilla, D.; Tomas, I. Devices for In situ Development of Non-disturbed Oral Biofilm. A Systematic Review. Front. Microbiol. 2016, 7, 1055. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M.; Attin, T. Enzymes in the acquired enamel pellicle. Eur. J. Oral. Sci. 2005, 113, 2–13. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Follo, M.; Selzer, A.C.; Hellwig, E.; Hannig, M.; Hannig, C. Bacterial colonization of enamel in situ investigated using fluorescence in situ hybridization. J. Med. Microbiol. 2009, 58, 1359–1366. [Google Scholar] [CrossRef]
- Hazan, R.; Que, Y.A.; Maura, D.; Rahme, L.G. A method for high throughput determination of viable bacteria cell counts in 96-well plates. BMC Microbiol. 2012, 12, 259. [Google Scholar] [CrossRef]
- Senges, C.; Wrbas, K.T.; Altenburger, M.; Follo, M.; Spitzmuller, B.; Wittmer, A.; Hellwig, E.; Al-Ahmad, A. Bacterial and Candida albicans adhesion on different root canal filling materials and sealers. J. Endod. 2011, 37, 1247–1252. [Google Scholar] [CrossRef]
- Tennert, C.; Fuhrmann, M.; Wittmer, A.; Karygianni, L.; Altenburger, M.J.; Pelz, K.; Hellwig, E.; Al-Ahmad, A. New bacterial composition in primary and persistent/secondary endodontic infections with respect to clinical and radiographic findings. J. Endod. 2014, 40, 670–677. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M.; Rehmer, O.; Braun, G.; Hellwig, E.; Al-Ahmad, A. Fluorescence microscopic visualization and quantification of initial bacterial colonization on enamel in situ. Arch. Oral. Biol. 2007, 52, 1048–1056. [Google Scholar] [CrossRef]
- Deimling, D.; Breschi, L.; Hoth-Hannig, W.; Ruggeri, A.; Hannig, C.; Nekrashevych, Y.; Prati, C.; Hannig, M. Electron microscopic detection of salivary alpha-amylase in the pellicle formed in situ. Eur. J. Oral. Sci. 2004, 112, 503–509. [Google Scholar] [CrossRef]
- Karygianni, L.; Ruf, S.; Hellwig, E.; Follo, M.; Vach, K.; Al-Ahmad, A. Antimicrobial Photoinactivation of In Situ Oral Biofilms by Visible Light Plus Water-Filtered Infrared A and Tetrahydroporphyrin-tetratosylate (THPTS). Microorganisms 2021, 9, 145. [Google Scholar] [CrossRef]





| AH Plus | Biodentine | MTA | Control | |
|---|---|---|---|---|
| Anaerobic | 27.8% ± 21.2% a | 10.5% ± 15.7% b | 8.5% ± 12.4% b | 53.2% ± 32.0% a |
| Anaerobic | 20.5% ± 24.4% a | 9.3 ± 17.1% a | 12.2% ± 22.7% a | 41.3% ± 29.9% a |
| AH Plus | Biodentine | MTA | Control | |
|---|---|---|---|---|
| Mean value | 29% | 15% | 38% | 18% |
| SD | 13% | 9% | 10% | 9% |
| AH Plus | Biodentine | MTA | Control | |||||
|---|---|---|---|---|---|---|---|---|
| Active | Non-Active | Active | Non-Active | Active | Non-Active | Active | Non-Active | |
| Mean value | 34% | 11% | 15% | 17% | 14% | 68% | 37% | 4% |
| SD | 7% | 4% | 8% | 8% | 8% | 12% | 18% | 4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ahmad, A.; Haendel, M.; Altenburger, M.J.; Karygianni, L.; Hellwig, E.; Wrbas, K.T.; Vach, K.; Tennert, C. Biodentine Inhibits the Initial Microbial Adhesion of Oral Microbiota In Vivo. Antibiotics 2023, 12, 4. https://doi.org/10.3390/antibiotics12010004
Al-Ahmad A, Haendel M, Altenburger MJ, Karygianni L, Hellwig E, Wrbas KT, Vach K, Tennert C. Biodentine Inhibits the Initial Microbial Adhesion of Oral Microbiota In Vivo. Antibiotics. 2023; 12(1):4. https://doi.org/10.3390/antibiotics12010004
Chicago/Turabian StyleAl-Ahmad, Ali, Michael Haendel, Markus Joerg Altenburger, Lamprini Karygianni, Elmar Hellwig, Karl Thomas Wrbas, Kirstin Vach, and Christian Tennert. 2023. "Biodentine Inhibits the Initial Microbial Adhesion of Oral Microbiota In Vivo" Antibiotics 12, no. 1: 4. https://doi.org/10.3390/antibiotics12010004
APA StyleAl-Ahmad, A., Haendel, M., Altenburger, M. J., Karygianni, L., Hellwig, E., Wrbas, K. T., Vach, K., & Tennert, C. (2023). Biodentine Inhibits the Initial Microbial Adhesion of Oral Microbiota In Vivo. Antibiotics, 12(1), 4. https://doi.org/10.3390/antibiotics12010004









