Scabicidal Potential of Coconut Seed Extract in Rabbits via Downregulating Inflammatory/Immune Cross Talk: A Comprehensive Phytochemical/GC-MS and In Silico Proof
Abstract
:1. Introduction
2. Results
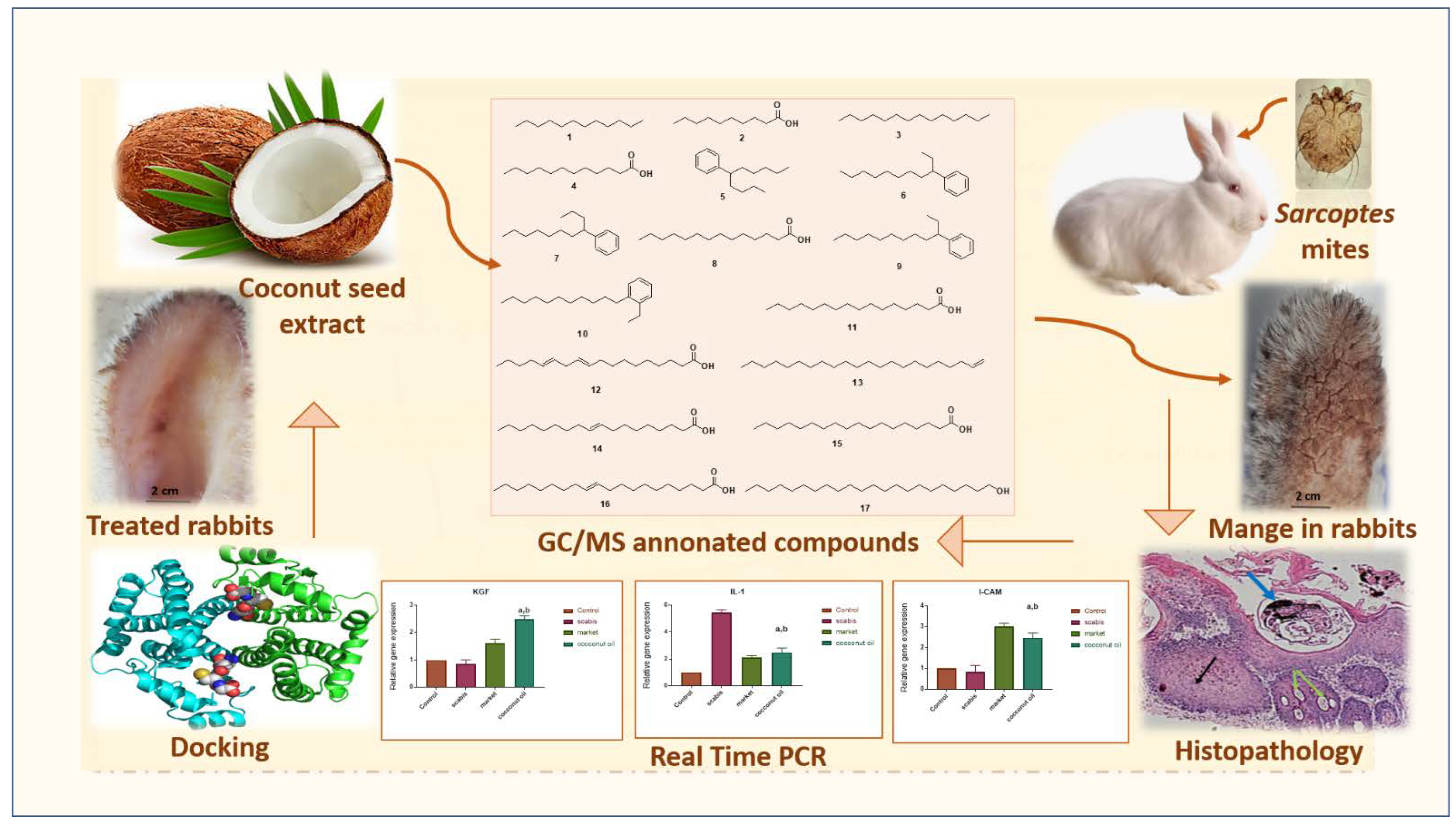
2.1. GC-MS Profiling of Coconut Seed Extract
2.2. Phytochemical Investigation of Coconut Seed Extract
2.3. The Antioxidant Potential of Coconut Seed Extract
2.4. Evaluation of the In Vitro Scabicidal Potential of Coconut Seed Extract
2.5. Evaluation of the In Vivo Efficacy of Coconut Seed Extract on Infected Rabbits
2.6. Histopathological Investigation
2.7. Gene Expression Results
2.8. Molecular Docking Study
3. Discussion
4. Material and Methods
4.1. Collection of Plant Material
4.2. GC/MS Analysis, Extraction, Fractionation, Isolation of Phytoconstituents, and In Vitro Antioxidant Potential of Coconut Seed Extract
4.3. Biological Investigation
4.3.1. Collection of Sarcoptes scabiei Mites
4.3.2. In Vitro Application of Coconut Extract on Sarcoptic Mange
4.3.3. In Vivo Application of Coconut Seed Extract
4.3.4. Histopathological Examination
4.3.5. RNA Isolation and qRT-PCR Assay
4.4. Molecular Docking Study
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elmaidomy, A.H.; Shady, N.H.; Abdeljawad, K.M.; Elzamkan, M.B.; Helmy, H.H.; Tarshan, E.A.; Adly, A.N.; Hussien, Y.H.; Sayed, N.G.; Zayed, A. Antimicrobial potentials of natural products against multidrug resistance pathogens: A comprehensive review. RSC Adv. 2022, 12, 29078–29102. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Nazir, M.; Ali, M.S.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: An update on future antibiotic drug candidates. NPR 2010, 27, 238–254. [Google Scholar] [PubMed]
- Chew, Y.-L. The beneficial properties of virgin coconut oil in management of atopic dermatitis. Pharmacogn. Rev. 2019, 13, 24. [Google Scholar] [CrossRef]
- Tabassam, S.M.; Iqbal, Z.; Jabbar, A.; Chattha, A.I. Efficacy of crude neem seed kernel extracts against natural infestation of Sarcoptes scabiei var. ovis. J. Ethnopharmacol. 2008, 115, 284–287. [Google Scholar] [CrossRef]
- Johnston, G.; Sladden, M. Scabies: Diagnosis and treatment. BMJ 2005, 331, 619–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, J.; Shikari, R.; Das, A.K.; Roy, B.; Mitra, M. Therapeutic management of sarcoptic mange in rabbit with ivermectin. Explor. Anim. Med. Res 2014, 4, 116–120. [Google Scholar]
- Hu, Z.; Chen, Z.; Yin, Z.; Jia, R.; Song, X.; Li, L.; Zou, Y.; Liang, X.; Li, L.; He, C.; et al. In vitro acaricidal activity of 1,8-cineole against Sarcoptes scabiei var. cuniculi and regulating effects on enzyme activity. Parasitol. Res. 2015, 114, 2959–2967. [Google Scholar] [CrossRef]
- Coles, T.B.; Lynn, R.C. Antiparasitic drugs. In Georgis’ Parasitology for Veterinarians, 10th ed.; WB Saunders Co.: Saint Louis, MO, USA, 2014; Volume 2014, pp. 264–325. [Google Scholar]
- Dunstand-Guzmán, E.; Hallal-Calleros, C.; Morales-Montor, J.; Hernández-Velázquez, V.M.; Zárate-Ramos, J.J.; Hoffman, K.L.; Peña-Chora, G.; Flores-Pérez, F.I. Therapeutic use of Bacillus thuringiensis in the treatment of psoroptic mange in naturally infested New Zealand rabbits. Vet. Parasitol. 2017, 238, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Mounsey, K.E.; Liu, X.; Walton, S.F. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasites Vectors 2017, 10, 385. [Google Scholar] [CrossRef] [Green Version]
- Morgan, M.S.; Arlian, L.G.; Markey, M.P. Sarcoptes scabiei mites modulate gene expression in human skin equivalents. PLoS ONE 2013, 8, e71143. [Google Scholar] [CrossRef]
- Bernigaud, C.; Samarawickrama, G.R.; Jones, M.K.; Gasser, R.B.; Fischer, K. The challenge of developing a single-dose treatment for scabies. Trends Parasitol. 2019, 35, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Obaid, M.K.; Islam, N.; Alouffi, A.; Khan, A.Z.; da Silva Vaz Jr, I.; Tanaka, T.; Ali, A. Acaricides Resistance in Ticks: Selection, Diagnosis, Mechanisms, and Mitigation. Front. Cell. Infect. Microbiol. 2022, 12, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Ghany, W.A. Mange in Rabbits: An Ectoparasitic Disease with a Zoonotic Potential. Vet. Med. Int. 2022, 2022, 5506272. [Google Scholar] [CrossRef] [PubMed]
- Maher Zahran, E.; Mohamad, S.A.; Yahia, R.; Badawi, A.M.; Sayed, A.M.; Ramadan Abdelmohsen, U. Anti-otomycotic potential of nanoparticles of Moringa oleifera leaf extract: An integrated in vitro, in silico and phase 0 clinical study. Food Funct. 2022, 13, 11083–11096. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, S.A.; Zahran, E.M.; Fadeel, M.R.A.; Albohy, A.; Safwat, M.A. New acaciin-loaded self-assembled nanofibers as mpro inhibitors against bcv as a surrogate model for SARS-CoV-2. Int. J. Nanomed. 2021, 16, 1789. [Google Scholar] [CrossRef]
- Senhoras, E.M. Oportunidades da cadeia agroindustrial do coco verde: Do coco verde nada se perde, tudo se desfruta. Rev. Urutágua Mar. 2004, 5, 8–11. [Google Scholar]
- Mat, K.; Abdul Kari, Z.; Rusli, N.D.; Che Harun, H.; Wei, L.S.; Rahman, M.M.; Mohd Khalid, H.N.; Mohd Ali Hanafiah, M.H.; Mohamad Sukri, S.A.; Raja Khalif, R.I.A. Coconut Palm: Food, Feed, and Nutraceutical Properties. Animals 2022, 12, 2107. [Google Scholar] [CrossRef]
- Aachmann, F.L.; Sørlie, M.; Skjåk-Bræk, G.; Eijsink, V.G.; Vaaje-Kolstad, G. NMR structure of a lytic polysaccharide monooxygenase provides insight into copper binding, protein dynamics, and substrate interactions. Proc. Natl. Acad. Sci. USA 2012, 109, 18779–18784. [Google Scholar] [CrossRef] [Green Version]
- Elmaidomy, A.H.; Mohyeldin, M.M.; Ibrahim, M.M.; Hassan, H.M.; Amin, E.; Rateb, M.E.; Hetta, M.H.; El Sayed, K.A. Acylated iridoids and rhamnopyranoses from premna odorata (lamiaceae) as novel mesenchymal–epithelial transition factor receptor inhibitors for the control of breast cancer. Phytother. Res. 2017, 31, 1546–1556. [Google Scholar] [CrossRef]
- Mohamed, R.; Ibrahim Mohamed, D.; Khalil, M.; El Masry, N.; Rasheed, N. Histopathological, Clinico-Biochemical and Therapeutic Studies on Different Types of Mange in Domestic Rabbits. Assiut Vet. Med. J. 2017, 63, 90–101. [Google Scholar]
- Shou-Min, F. Insect glutathione S-transferase: A review of comparative genomic studies and response to xenobiotics. Bull. Insectol. 2012, 65, 265–271. [Google Scholar]
- Walton, S.F.; Beroukas, D.; Roberts-Thomson, P.; Currie, B. New insights into disease pathogenesis in crusted (Norwegian) scabies: The skin immune response in crusted scabies. Br. J. Dermatol. 2008, 158, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.M.; Abdelmohsen, U.R.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. Diversity, phytochemical and medicinal potential of the genus Ocimum L.(Lamiaceae). Phytochem. Rev. 2020, 19, 907–953. [Google Scholar] [CrossRef]
- Ahmed, Z.B.; Yousfi, M.; Viaene, J.; Dejaegher, B.; Demeyer, K.; Vander Heyden, Y. Four Pistacia atlantica subspecies (atlantica, cabulica, kurdica and mutica): A review of their botany, ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2021, 265, 113329. [Google Scholar] [CrossRef] [PubMed]
- Vermaak, I.; Kamatou, G.P.P.; Komane-Mofokeng, B.; Viljoen, A.; Beckett, K. African seed oils of commercial importance—Cosmetic applications. S. Afr. J. Bot. 2011, 77, 920–933. [Google Scholar] [CrossRef] [Green Version]
- Mala, A.; Tulika, T. Therapeutic efficacy of Centella asiatica (L.) and Momordica charantia: As traditional medicinal plant. J. Plant Sci. 2015, 3, 1–9. [Google Scholar]
- Vimala, G.; Gricilda Shoba, F. A review on antiulcer activity of few Indian medicinal plants. Int. J. Microbiol. 2014, 2014, 519590. [Google Scholar] [CrossRef] [Green Version]
- Lalouckova, K.; Skrivanova, E.; Rondevaldova, J.; Frankova, A.; Soukup, J.; Kokoska, L. In vitro antagonistic inhibitory effects of palm seed crude oils and their main constituent, lauric acid, with oxacillin in Staphylococcus aureus. Sci. Rep. 2021, 11, 177. [Google Scholar] [CrossRef]
- Varma, S.R.; Sivaprakasam, T.O.; Arumugam, I.; Dilip, N.; Raghuraman, M.; Pavan, K.B.; Rafiq, M.; Paramesh, R. In vitro anti-inflammatory and skin protective properties of Virgin coconut oil. J. Tradit. Complement. Med. 2019, 9, 5–14. [Google Scholar] [CrossRef]
- dos Santos, L.B.; Favero, F.C.; Conde, M.H.; Freitas, M.G.; Santos-Zanuncio, V.S.; Carollo, C.A.; Borges, F.d.A. Clinical safety of lauric acid for cattle and its in vitro and in vivo efficacy against Rhipicephalus microplus. Vet. Parasitol. 2020, 280, 109095. [Google Scholar] [CrossRef]
- Pupala, S.S.; Rao, S.; Strunk, T.; Patole, S. Topical application of coconut oil to the skin of preterm infants: A systematic review. Eur. J. Pediatr. 2019, 178, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Kawasaki, Y.; Morimoto, K.; Kikuchi, I.; Kawana, S. Treatment for crusted scabies: Limitations and side effects of treatment with ivermectin. J. Nippon. Med. Sch. 2014, 81, 157–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Kilsdonk, J.W.; Bergers, M.; Van Kempen, L.C.; Schalkwijk, J.; Swart, G.W. Keratinocytes drive melanoma invasion in a reconstructed skin model. Melanoma Res. 2010, 20, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Korkina, L. Redox imbalance in T cell-mediated skin diseases. Med. Inflamm. 2010, 2010, 861949. [Google Scholar] [CrossRef]
- Samotij, D.; Nedoszytko, B.; Bartosińska, J.; Batycka-Baran, A.; Czajkowski, R.; Dobrucki, I.; Dobrucki, L.; Górecka-Sokołowska, M.; Janaszak-Jasienicka, A.; Krasowska, D. Pathogenesis of psoriasis in the “omic” era. Part I. Epidemiology, clinical manifestation, immunological and neuroendocrine disturbances. Adv. Dermatol. Allergol. 2020, 37, 135–153. [Google Scholar] [CrossRef]
- Hall, J.M.; Podawiltz, A.; Mummert, D.I.; Jones, H.; Mummert, M.E. Psychological stress and the cutaneous immune response: Roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis. Dermatol. Res. Pract. 2012, 2012, 403908. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Sayama, K.; Tohyama, M.; Shirakata, Y.; Hanakawa, Y.; Tokumaru, S.; Yang, L.; Hirakawa, S.; Hashimoto, K. Mite allergen is a danger signal for the skin via activation of inflammasome in keratinocytes. J. Allerg. Clin. Immunol. 2011, 127, 806–814.e4. [Google Scholar] [CrossRef]
- Rebholz, B.; Haase, I.; Eckelt, B.; Paxian, S.; Flaig, M.J.; Ghoreschi, K.; Nedospasov, S.A.; Mailhammer, R.; Debey-Pascher, S.; Schultze, J.L. Crosstalk between keratinocytes and adaptive immune cells in an IκBα protein-mediated inflammatory disease of the skin. Immunity 2007, 27, 296–307. [Google Scholar] [CrossRef] [Green Version]
- Birukov, K.G. Cyclic stretch, reactive oxygen species, and vascular remodeling. Antioxid. Redox Signal. 2009, 11, 1651–1667. [Google Scholar] [CrossRef] [Green Version]
- Tolstonog, G.V.; Belichenko-Weitzmann, I.V.; Lu, J.-P.; Hartig, R.; Shoeman, R.L.; Taub, U.; Traub, P. Spontaneously immortalized mouse embryo fibroblasts: Growth behavior of wild-type and vimentin-deficient cells in relation to mitochondrial structure and activity. DNA Cell Biol. 2005, 24, 680–709. [Google Scholar] [CrossRef] [PubMed]
- van Buul, J.D. Signaling in Leukocyte Transendothelial Migration: A Roadmap for Homing of Progenitor Cells; Universiteit van Amsterdam [Host]: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Al-Warhi, T.; Zahran, E.M.; Selim, S.; Al-Sanea, M.M.; Ghoneim, M.M.; Maher, S.A.; Mostafa, Y.A.; Alsenani, F.; Elrehany, M.A.; Almuhayawi, M.S. Antioxidant and Wound Healing Potential of Vitis vinifera Seeds Supported by Phytochemical Characterization and Docking Studies. Antioxidants 2022, 11, 881. [Google Scholar] [CrossRef] [PubMed]
- Kanta, J. The role of hydrogen peroxide and other reactive oxygen species in wound healing. Acta Med. 2011, 54, 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsunaga, T.; Katayama, I.; Yokozeki, H.; Nishioka, K. ICAM-1 expression on keratinocytes in mechanically-injured skin of a patient with atopic dermatitis. J. Dermatol. Sci. 1996, 12, 219–226. [Google Scholar] [CrossRef]
- Reale, M.; Frydas, S.; Barbacane, R.C.; Placido, F.C.; Cataldo, I.; Vacalis, D.; Trakatellis, A.; Anogianakis, G.; Felaco, M.; Di Gioacchino, M.; et al. Induction of monocyte chemotactic protein-1 (MCP-1) and TNF alpha by Trichinella spiralis in serum of mice in vivo. Mol. Cell. Biochem. 1998, 179, 1–5. [Google Scholar] [CrossRef]
- Zahran, E.; Sayed, A.; Alaaeldin, R.; El-Rehany, M.; Khattab, A.; Abdelmohsen, U. Bioactives and functional food ingredients with promising potential for management of cerebral and myocardial ischemia: A comprehensive mechanistic review. Food Funct. 2022. [Google Scholar] [CrossRef]
- Livshits, G.; Kalinkovich, A. Hierarchical, imbalanced pro-inflammatory cytokine networks govern the pathogenesis of chronic arthropathies. Osteoarthr. Cartil. 2018, 26, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Takeshita, S.; Tokutomi, T.; Kawase, H.; Nakatani, K.; Tsujimoto, H.; Kawamura, Y.; Sekine, I. Elevated serum levels of matrix metalloproteinase-9 (MMP-9) in Kawasaki disease. Clin. Exp. Immunol. 2001, 125, 340–344. [Google Scholar] [CrossRef]
- Han, S.M.; Kim, S.G.; Jang, H.R.; Woo, S.O.; Pak, S.C. Anti-Atopic Dermatitis of Purified Bee Venom on Keratinocytes Via Suppression of PAR2, ICAM-1, and IL-6 Expression. J. Apicult. Sci. 2018, 62, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Jeffris, R.A.; Beers, E.H.; Smytheman, P.; Rehfield-Ray, L. Erythritol, an Artificial Sweetener, Is Acaricidal Against Pest Mites and Minimally Harmful to a Predatory Mite. J. Econ. Entomol. 2021, 114, 1701–1708. [Google Scholar] [CrossRef]
- Farag, M.A.; Gad, M.Z. Omega-9 fatty acids: Potential roles in inflammation and cancer management. J. Gen. Eng. Biotechnol. 2022, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Solikhah, T. Aloe vera and Virgin Coconut Oil (VCO) accelerate healing process in domestic cat (Felis domesticus) suffering from scabies. Iraqi J. Vet. Sci. 2021, 35, 699–704. [Google Scholar] [CrossRef]
- Burusapat, C.; Supawan, M.; Pruksapong, C.; Pitiseree, A.; Suwantemee, C. Topical Aloe vera gel for accelerated wound healing of split-thickness skin graft donor sites: A double-blind, randomized, controlled trial and systematic review. Plast. Reconstr. Surg. 2018, 142, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Tojima, H.; Haruta, T.; Suzuki, M.; Kakemi, M. Enhancing effects of unsaturated fatty acids with various structures on the permeation of indomethacin through rat skin. J. Pharm. Pharm. 1996, 48, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Tanojo, H.; Bouwstra, J.A.; Junginger, H.E.; Boddé, H.E. In vitro human skin barrier modulation by fatty acids: Skin permeation and thermal analysis studies. Pharm. Res. 1997, 14, 42–49. [Google Scholar] [CrossRef]
- Ghonaim, H.; Noro, M.G.; Anwar, J. Effect of natural sunflower oil and its components on the skin permeability to water and some drugs. Int. J. Pharm. Pharm. Sci. 2014, 6, 630–636. [Google Scholar]
- Alzarea, S.I.; Elmaidomy, A.H.; Saber, H.; Musa, A.; Al-Sanea, M.M.; Mostafa, E.M.; Hendawy, O.M.; Youssif, K.A.; Alanazi, A.S.; Alharbi, M. Potential anticancer lipoxygenase inhibitors from the red sea-derived brown algae Sargassum cinereum: An in-silico-supported In-Vitro Study. Antibiotics 2021, 10, 416. [Google Scholar] [CrossRef]
- Shamikh, Y.I.; El Shamy, A.A.; Gaber, Y.; Abdelmohsen, U.R.; Madkour, H.A.; Horn, H.; Hassan, H.M.; Elmaidomy, A.H.; Alkhalifah, D.H.M.; Hozzein, W.N. Actinomycetes from the Red Sea sponge Coscinoderma mathewsi: Isolation, diversity, and potential for bioactive compounds discovery. Microorganisms 2020, 8, 783. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Abdelmohsen, U.R.; Alsenani, F.; Aly, H.F.; Shams, S.G.E.; Younis, E.A.; Ahmed, K.A.; Sayed, A.M.; Owis, A.I.; Afifi, N. The anti-Alzheimer potential of Tamarindus indica: An in vivo investigation supported by in vitro and in silico approaches. RSC Adv. 2022, 12, 11769–11785. [Google Scholar] [CrossRef] [PubMed]
- Bagalagel, A.A.; El-hawary, S.S.; Alaaeldin, R.; Elmaidomy, A.H.; Altemani, F.H.; Waggas, D.S.; Algehainy, N.A.; Saeedi, N.H.; Alsenani, F.; Mokhtar, F.A. The Protective and Therapeutic Anti-Alzheimer Potential of Olea europaea L. cv. Picual: An In Silico and In Vivo Study. Metabolites 2022, 12, 1178. [Google Scholar] [CrossRef]
- Ahmed, W.; Ibrahim, M.A.; Helmy, N.A.; ElKashlan, A.M.; Elmaidomy, A.H.; Zaki, A.R. Amelioration of aluminum-induced hepatic and nephrotoxicity by Premna odorata extract is mediated by lowering MMP9 and TGF-β gene alterations in Wistar rat. Environ. Sci. Pollut. Res. 2022, 29, 72827–72838. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Zahran, E.M.; Soltane, R.; Alasiri, A.; Saber, H.; Ngwa, C.J.; Pradel, G.; Alsenani, F.; Sayed, A.M.; Abdelmohsen, U.R. New Halogenated Compounds from Halimeda macroloba Seaweed with Potential Inhibitory Activity against Malaria. Molecules 2022, 27, 5617. [Google Scholar] [CrossRef] [PubMed]
- Al-Warhi, T.; Elmaidomy, A.H.; Maher, S.A.; Abu-Baih, D.H.; Selim, S.; Albqmi, M.; Al-Sanea, M.M.; Alnusaire, T.S.; Ghoneim, M.M.; Mostafa, E.M. The Wound-Healing Potential of Olea europaea L. Cv. Arbequina Leaves Extract: An Integrated In Vitro, In Silico, and In Vivo Investigation. Metabolites 2022, 12, 791. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Mohammed, R.; Owis, A.I.; Hetta, M.H.; AboulMagd, A.M.; Siddique, A.B.; Abdelmohsen, U.R.; Rateb, M.E.; El Sayed, K.A.; Hassan, H.M. Triple-negative breast cancer suppressive activities, antioxidants and pharmacophore model of new acylated rhamnopyranoses from Premna odorata. RSC Adv. 2020, 10, 10584–10598. [Google Scholar] [CrossRef] [Green Version]
- Elmaidomy, A.H.; Alhadrami, H.A.; Amin, E.; Aly, H.F.; Othman, A.M.; Rateb, M.E.; Hetta, M.H.; Abdelmohsen, U.R.; Hassan, H.M. Anti-inflammatory and antioxidant activities of terpene-and polyphenol-rich Premna odorata leaves on alcohol-inflamed female wistar albino rat liver. Molecules 2020, 25, 3116. [Google Scholar] [CrossRef] [PubMed]
- Al-Warhi, T.; Elmaidomy, A.H.; Selim, S.; Al-Sanea, M.M.; Albqmi, M.; Mostafa, E.M.; Ibrahim, S.; Ghoneim, M.M.; Sayed, A.M.; Abdelmohsen, U.R. Bioactive Phytochemicals of Citrus reticulata Seeds—An Example of Waste Product Rich in Healthy Skin Promoting Agents. Antioxidants 2022, 11, 984. [Google Scholar] [CrossRef] [PubMed]
- Alnusaire, T.S.; Sayed, A.M.; Elmaidomy, A.H.; Al-Sanea, M.M.; Albogami, S.; Albqmi, M.; Alowaiesh, B.F.; Mostafa, E.M.; Musa, A.; Youssif, K.A. An In Vitro and In Silico Study of the Enhanced Antiproliferative and Pro-Oxidant Potential of Olea europaea L. cv. Arbosana Leaf Extract via Elastic Nanovesicles (Spanlastics). Antioxidants 2021, 10, 1860. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Sayed, A.M.; Elmaidomy, A.H. Specialty Grand Challenge: Natural Products Extraction and Isolation-Between Conventional and Modern Techniques. Front. Nat. Prod. 2022, 1, 1–4. [Google Scholar]
- Alsenani, F.; Ashour, A.M.; Alzubaidi, M.A.; Azmy, A.F.; Hetta, M.H.; Abu-Baih, D.H.; Elrehany, M.A.; Zayed, A.; Sayed, A.M.; Abdelmohsen, U.R. Wound Healing Metabolites from Peters’ Elephant-Nose Fish Oil: An In Vivo Investigation Supported by In Vitro and In Silico Studies. Mar. Drugs 2021, 19, 605. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Abdel-Rahman, I.A.; Saber, H.; Attia, E.Z.; Abdelraheem, W.M.; Madkour, H.A.; Hassan, H.M.; Elmaidomy, A.H.; Abdelmohsen, U.R. Pharmacological and natural products diversity of the brown algae genus Sargassum. RSC Adv. 2020, 10, 24951–24972. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Mohamed, E.M.; Aly, H.F.; Younis, E.A.; Shams, S.G.E.; Altemani, F.H.; Alzubaidi, M.A.; Almaghrabi, M.; Harbi, A.A.; Alsenani, F. Anti-Inflammatory and Antioxidant Properties of Malapterurus electricus Skin Fish Methanolic Extract in Arthritic Rats: Therapeutic and Protective Effects. Mar. Drugs 2022, 20, 639. [Google Scholar] [CrossRef] [PubMed]
- Elmaidomy, A.H.; Hassan, H.M.; Amin, E.; Mohamed, W.; Hetta, M.H. Premna odorata volatile oil as a new mycobacterium tuberculosis growth inhibitor for the control of tuberculosis disease. Eur. J. Med. Plants 2017, 21, 1–11. [Google Scholar] [CrossRef]
- Andriantsoanirina, V.; Guillot, J.; Ratsimbason, M.; Mekhloufi, G.; Randriamialinoro, F.; Ranarivelo, L.; Ariey, F.; Durand, R. In vitro efficacy of essential oils against Sarcoptes scabiei. Sci. Rep. 2022, 12, 7176. [Google Scholar] [CrossRef] [PubMed]
- Aboelhadid, S.; Mahrous, L.N.; Hashem, S.A.; Abdel-Kafy, E.; Miller, R.J. In vitro and in vivo effect of Citrus limon essential oil against sarcoptic mange in rabbits. Parasitol. Res. 2016, 115, 3013–3020. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.M.; Abdelmohsen, U.R.; Ayoub, A.T.; Salem, M.A.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. Metabolic profiling, histopathological anti-ulcer study, molecular docking and molecular dynamics of ursolic acid isolated from Ocimum forskolei Benth.(family Lamiaceae). S. Afr. J. Bot. 2020, 131, 311–319. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Abdelgawad, M.A.; Alaaeldin, R.; Fathalla, Z.; Moharram, H.; Abdallah, R.M.A.; Abdel-Rahman, I.M.; Abdel-Aziz, M.; Abuo-Rahma, G.E.-D.A.; Ghoneim, M.M.; et al. Solulan C24- and Bile Salts-Modified Niosomes for New Ciprofloxacin Mannich Base for Combatting Pseudomonas-Infected Corneal Ulcer in Rabbits. Pharmaceuticals 2022, 15, 44. [Google Scholar] [CrossRef]
- Albohy, A.; Zahran, E.M.; Abdelmohsen, U.R.; Salem, M.A.; Al-Warhi, T.; Al-Sanea, M.M.; Abelyan, N.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A. Multitarget in silico studies of Ocimum menthiifolium, family Lamiaceae against SARS-CoV-2 supported by molecular dynamics simulation. J. Biomol. Struct. Dyn. 2022, 40, 4062–4072. [Google Scholar] [CrossRef] [PubMed]
- El-Sharawy, D.M.; Khater, S.; HM, E.; Sherif, N.H.; Hassan, H.M.; Elmaidomy, A.H. 99mTc-Luteolin: Radiolabeling, In Silico ADMET and Biological Evaluation as a Natural Tracer Tumor imaging. J. Radiat. Res. Appl. Sci. 2021, 14, 125–132. [Google Scholar] [CrossRef]
- Dworzanski, J.P.; Berwald, L.; Meuzelaar, H.L. Pyrolytic methylation-gas chromatography of whole bacterial cells for rapid profiling of cellular fatty acids. Appl. Environ. Microbiol. 1990, 56, 1717–1724. [Google Scholar] [CrossRef] [Green Version]
- Hassan, H.; Abdel-Aziz, A. Evaluation of free radical-scavenging and anti-oxidant properties of black berry against fluoride toxicity in rats. Food Chem. Toxicol. 2010, 48, 1999–2004. [Google Scholar] [CrossRef]
- Sreenivasan, S.; Ibrahim, D.; MOHD KASSIM, M.J.N. Free radical Scavenging Activity and Total Phenolic Compounds of Gracilaria changii. Int. J. Nat. Eng. Sci. 2007, 1, 115–117. [Google Scholar]











| No. | Identified Compound | MF | Area % | RT | RI |
|---|---|---|---|---|---|
| 1 | Dodecane | C12H26 | 0.09 | 9.77 | 928 |
| 2 | Capric acid | C10H20O2 | 2.56 | 12.79 | 903 |
| 3 | Tetradecane | C14H30 | 0.54 | 14.18 | 973 |
| 4 | Lauric acid | C12H24O2 | 12.22 | 17.04 | 911 |
| 5 | Benzene, (1-butylhexyl)- | C16H26 | 3.47 | 18.13 | 920 |
| 6 | Benzene, (1-ethylnonyl)- | C17H28 | 6.57 | 18.62 | 910 |
| 7 | Benzene, (1-propyloctyl)- | C17H28 | 6.96 | 19.83 | 974 |
| 8 | Myristic acid | C14H28O2 | 9.24 | 21.51 | 901 |
| 9 | Benzene, (1-ethyldecyl)- | C18H30 | 14.39 | 22.06 | 932 |
| 10 | Benzene, (1-ethylundecyl)- | C19H32 | 6.17 | 23.71 | 904 |
| 11 | Palmitic acid | C16H32O2 | 8.01 | 24.73 | 900 |
| 12 | 10,13-Octadecadienoic acid | C18H32O2 | 0.18 | 24.88 | 901 |
| 13 | 1-Docosene | C22H44 | 0.79 | 25.35 | 956 |
| 14 | Oleic acid | C18H34O2 | 19.09 * | 27.45 | 939 |
| 15 | Stearic acid | C18H36O2 | 6.82 | 27.74 | 921 |
| 16 | Gondoic acid | C20H38O2 | 0.45 | 29.90 | 929 |
| 17 | 1-Docosanol | C22H46O | 0.14 | 31.08 | 905 |
| Total | 97.69% | ||||
| Cpd. | S a kcal/mole | RMSD_Refine b | Amino Acid Bond | Distance Å | E (kcal/mol) |
|---|---|---|---|---|---|
| Gondoic acid | −5.817 | 1.684 | MET 148/H-acceptor | 3.19 | −1.00 |
| Ligand | −5.87 | 1.311 | MET 148/H-donor | 3.04 | −2.90 |
| MET 148/H-acceptor | 3.08 | −0.80 | |||
| THR 147/H-acceptor | 2.74 | −5.00 | |||
| GLN 149/H-acceptor | 2.98 | −3.50 | |||
| ARG 11/H-acceptor | 3.15 | −5.00 | |||
| Arg 11/Ionic | 2.98 | −4.60 |
| Cpd. | S a kcal/mole | RMSD_Refine b | Amino Acid Bond | Distance Å | E (kcal/mol) |
|---|---|---|---|---|---|
| Gondoic acid | −5.291 | 1.31 | Asp 34/H-donor | 3.22 | −3.70 |
| Asp 34/H-donor | 3.18 | −0.60 | |||
| Arg 30/H-acceptor | 3.05 | −1.50 | |||
| Ligand | −4.191 | 1.758 | Gln 175/H-donor | 2.78 | −1.60 |
| Arg 182/H-acceptor | 2.49 | −3.50 | |||
| Arg 182/Ionic | 3.06 | −4.10 | |||
| Arg 179/H-acceptor | 2.61 | −4.30 | |||
| Arg 179/Ionic | 2.51 | −8.70 |
| Cpd. | S a kcal/mole | RMSD_Refine b | Amino Acid Bond | Distance Å | E (kcal/mol) |
|---|---|---|---|---|---|
| Gondoic acid | −8.362 | 1.67 | LEU 840/H-donor | 2.83 | −6.30 |
| ASN 923/H-acceptor | 3.28 | −1.40 | |||
| ASN 923/H-acceptor | 3.05 | −2.80 | |||
| Ligand | −7.97 | 0.9175 | Asn 923/H-acceptor | 3.40 | −1.20 |
| Cys 919/H-acceptor | 3.55 | −1.10 | |||
| Leu 840/pi-H | 3.76 | −1.60 | |||
| Leu 840/pi-H | 4.00 | −0.50 |
| Cpd. | S a kcal/mole | RMSD_Refine b | Amino Acid Bond | Distance Å | E (kcal/mol) |
|---|---|---|---|---|---|
| Oleic acid | −6.764 | 2.023 | ARG 67/H-acceptor | 3.08 | −3.80 |
| 3″(1‴-O-β-D-glucopyranosyl)-sucrose | −7.24 | 1.769 | Glu 65/H-donor | 3.00 | −1.50 |
| SER 10/H-acceptor | 3.19 | −0.60 | |||
| Arg 67/H-acceptor | 3.08 | −1.10 | |||
| Ser 66/H-acceptor | 3.27 | −1.20 | |||
| Ligand | −5.945 | 1.405 | Glu 65/H-donor | 3.03 | −1.40 |
| Ile 53/H-donor | 3.04 | −3.40 | |||
| Arg 67/H-acceptor | 2.85 | −8.30 | |||
| Ser 66/H-acceptor | 2.89 | −3.60 | |||
| Arg 67/Ionic | 2.85 | −5.50 | |||
| Glu 65/Ionic | 3.03 | −4.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahran, E.M.; Abdel-Maqsoud, N.M.R.; Tammam, O.Y.; Abdel-Rahman, I.M.; Elrehany, M.A.; Bakhsh, H.T.; Altemani, F.H.; Algehainy, N.A.; Alzubaidi, M.A.; Abdelmohsen, U.R.; et al. Scabicidal Potential of Coconut Seed Extract in Rabbits via Downregulating Inflammatory/Immune Cross Talk: A Comprehensive Phytochemical/GC-MS and In Silico Proof. Antibiotics 2023, 12, 43. https://doi.org/10.3390/antibiotics12010043
Zahran EM, Abdel-Maqsoud NMR, Tammam OY, Abdel-Rahman IM, Elrehany MA, Bakhsh HT, Altemani FH, Algehainy NA, Alzubaidi MA, Abdelmohsen UR, et al. Scabicidal Potential of Coconut Seed Extract in Rabbits via Downregulating Inflammatory/Immune Cross Talk: A Comprehensive Phytochemical/GC-MS and In Silico Proof. Antibiotics. 2023; 12(1):43. https://doi.org/10.3390/antibiotics12010043
Chicago/Turabian StyleZahran, Eman Maher, Nehad M. Reda Abdel-Maqsoud, Omar. Y. Tammam, Islam M. Abdel-Rahman, Mahmoud A. Elrehany, Hussain T. Bakhsh, Faisal H. Altemani, Naseh A. Algehainy, Mubarak A. Alzubaidi, Usama Ramadan Abdelmohsen, and et al. 2023. "Scabicidal Potential of Coconut Seed Extract in Rabbits via Downregulating Inflammatory/Immune Cross Talk: A Comprehensive Phytochemical/GC-MS and In Silico Proof" Antibiotics 12, no. 1: 43. https://doi.org/10.3390/antibiotics12010043
APA StyleZahran, E. M., Abdel-Maqsoud, N. M. R., Tammam, O. Y., Abdel-Rahman, I. M., Elrehany, M. A., Bakhsh, H. T., Altemani, F. H., Algehainy, N. A., Alzubaidi, M. A., Abdelmohsen, U. R., & Elmaidomy, A. H. (2023). Scabicidal Potential of Coconut Seed Extract in Rabbits via Downregulating Inflammatory/Immune Cross Talk: A Comprehensive Phytochemical/GC-MS and In Silico Proof. Antibiotics, 12(1), 43. https://doi.org/10.3390/antibiotics12010043







