Multidrug-Resistant Acinetobacter baumannii Infections in the United Kingdom versus Egypt: Trends and Potential Natural Products Solutions
Abstract
:1. Introduction
2. Methodology
3. Events of Detection of A. baumannii in the United Kingdom and Egypt
3.1. A. baumannii in the United Kingdom
3.2. A. baumannii in Egypt
4. Efforts in the United Kingdom and Egypt to Combat A. baumannii Using Natural Products
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter baumannii: An emerging opportunistic pathogen. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez-López, R.; Solano-Gálvez, S.G.; Juárez Vignon-Whaley, J.J.; Abello Vaamonde, J.A.; Padró Alonzo, L.A.; Rivera Reséndiz, A.; Muleiro Álvarez, M.; Vega López, E.N.; Franyuti-Kelly, G.; Álvarez-Hernández, D.A.; et al. Acinetobacter baumannii resistance: A real challenge for clinicians. Antibiotics 2020, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Alvi, I.A.; Rehman, S.U. Insight into Acinetobacter baumannii: Pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect. Drug Resist. 2018, 11, 1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017.
- Rizk, S.S.; Elwakil, W.H.; Attia, A.S. Antibiotic-Resistant Acinetobacter baumannii in Low-Income Countries (2000–2020): Twenty-One Years and Still below the Radar, Is It Not There or Can They Not Afford to Look for It? Antibiotics 2021, 10, 764. [Google Scholar] [CrossRef]
- Lee, J.S.; Choi, C.H.; Kim, J.W.; Lee, J.C. Acinetobacter baumannii outer membrane protein A induces dendritic cell death through mitochondrial targeting. J. Microbiol. 2010, 48, 387–392. [Google Scholar] [CrossRef]
- Elhosseiny, N.M.; Elhezawy, N.B.; Attia, A.S. Comparative proteomics analyses of Acinetobacter baumannii strains ATCC 17978 and AB5075 reveal the differential role of type II secretion system secretomes in lung colonization and ciprofloxacin resistance. Microb. Pathog. 2019, 128, 20–27. [Google Scholar] [CrossRef]
- Repizo, G.D.; Gagné, S.; Foucault-Grunenwald, M.-L.; Borges, V.; Charpentier, X.; Limansky, A.S.; Gomes, J.P.; Viale, A.M.; Salcedo, S.P. Differential role of the T6SS in Acinetobacter baumannii virulence. PLoS ONE 2015, 10, e0138265. [Google Scholar] [CrossRef] [Green Version]
- Elhosseiny, N.M.; Elhezawy, N.B.; Sayed, R.M.; Khattab, M.S.; El Far, M.Y.; Attia, A.S. γ-Glutamyltransferase as a novel virulence factor of Acinetobacter baumannii inducing alveolar wall destruction and renal damage in systemic disease. J. Infect. Dis. 2020, 222, 871–879. [Google Scholar] [CrossRef]
- Johnson, T.L.; Waack, U.; Smith, S.; Mobley, H.; Sandkvist, M. Acinetobacter baumannii is dependent on the type II secretion system and its substrate LipA for lipid utilization and in vivo fitness. J. Bacteriol. 2016, 198, 711–719. [Google Scholar] [CrossRef] [Green Version]
- Cook-Libin, S.; Sykes, E.M.; Kornelsen, V.; Kumar, A. Iron Acquisition Mechanisms and Their Role in the Virulence of Acinetobacter baumannii. Infect. Immun. 2022, 90, e00223-22. [Google Scholar] [CrossRef]
- Mortensen, B.L.; Skaar, E.P. The contribution of nutrient metal acquisition and metabolism to Acinetobacter baumannii survival within the host. Front. Cell. Infect. Microbiol. 2013, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Mea, H.J.; Yong, P.V.C.; Wong, E.H. An overview of Acinetobacter baumannii pathogenesis: Motility, adherence and biofilm formation. Microbiol. Res. 2021, 247, 126722. [Google Scholar] [CrossRef] [PubMed]
- CDC. Antibiotic Resistance Threats in the United States; Department of Health and Human Services: Atlanta, GA, USA, 2019.
- Ayobami, O.; Willrich, N.; Harder, T.; Okeke, I.N.; Eckmanns, T.; Markwart, R. The incidence and prevalence of hospital-acquired (carbapenem-resistant) Acinetobacter baumannii in Europe, Eastern Mediterranean and Africa: A systematic review and meta-analysis. Emerg. Microbes Infect. 2019, 8, 1747–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manenzhe, R.I.; Zar, H.J.; Nicol, M.P.; Kaba, M. The spread of carbapenemase-producing bacteria in Africa: A systematic review. J. Antimicrob. Chemother. 2015, 70, 23–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, M.L.; Apisarnthanarak, A.; Madriaga, G. The burden of healthcare-associated infections in Southeast Asia: A systematic literature review and meta-analysis. Clin. Infect. Dis. 2015, 60, 1690–1699. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Ezpeleta-Lobato, G.; Han, X.; Carmona-Cartaya, Y.; Quiñones-Pérez, D. Carbapenamase-Producing Acinetobacter baumannii in China, Latin America and the Caribbean: A Systematic Review and Meta-Analysis. MEDICC Rev. 2022, 24, 59–69. [Google Scholar] [CrossRef]
- Vivas, R.; Barbosa, A.A.T.; Dolabela, S.S.; Jain, S. Multidrug-resistant bacteria and alternative methods to control them: An overview. Microb. Drug Resist. 2019, 25, 890–908. [Google Scholar] [CrossRef]
- Tiwari, V.; Roy, R.; Tiwari, M. Antimicrobial active herbal compounds against Acinetobacter baumannii and other pathogens. Front. Microbiol. 2015, 6, 618. [Google Scholar] [CrossRef] [Green Version]
- Paton, R.; Miles, R.; Hood, J.; Amyes, S. ARI 1: β-lactamase-mediated imipenem resistance in Acinetobacter baumannii. Int. J. Antimicrob. Agents 1993, 2, 81–87. [Google Scholar] [CrossRef]
- Crowe, M.; Towner, K.; Humphreys, H. Clinical and epidemiological features of an outbreak of Acinetobacter infection in an intensive therapy unit. J. Med. Microbiol. 1995, 43, 55–62. [Google Scholar] [CrossRef]
- Webster, C.; Crowe, M.; Humphreys, H.; Towner, K. Surveillance of an adult intensive care unit for long-term persistence of a multi-resistant strain of Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Morar, P.; Singh, V.; Jones, A.S.; Hughes, J.; Van Saene, R. Impact of tracheotomy on colonization and infection of lower airways in children requiring long-term ventilation: A prospective observational cohort study. Chest 1998, 113, 77–85. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.; Amyes, S.; Paton, R. The persistence and clonal spread of a single strain of Acinetobacter 13TU in a large Scottish teaching hospital. J. Chemother. 1999, 11, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Henwood, C.J.; Gatward, T.; Warner, M.; James, D.; Stockdale, M.W.; Spence, R.P.; Towner, K.J.; Livermore, D.M.; Woodford, N. Antibiotic resistance among clinical isolates of Acinetobacter in the UK, and in vitro evaluation of tigecycline (GAR-936). J. Antimicrob. Chemother. 2002, 49, 479–487. [Google Scholar] [CrossRef] [Green Version]
- Towner, K.J.; Gee, T.; Boswell, T. An unwanted import to the UK: A carbapenem-resistant clinical isolate of Acinetobacter baumannii producing metallo-β-lactamase. J. Antimicrob. Chemother. 2002, 50, 1092–1093. [Google Scholar] [CrossRef] [Green Version]
- Spence, R.P.; Towner, K.J.; Henwood, C.J.; James, D.; Woodford, N.; Livermore, D.M. Population structure and antibiotic resistance of Acinetobacter DNA group 2 and 13TU isolates from hospitals in the UK. J. Med. Microbiol. 2002, 51, 1107–1112. [Google Scholar] [CrossRef] [Green Version]
- Das, I.; Lambert, P.; Hill, D.; Noy, M.; Bion, J.; Elliott, T. Carbapenem-resistant Acinetobacter and role of curtains in an outbreak in intensive care units. J. Hosp. Infect. 2002, 50, 110–114. [Google Scholar] [CrossRef]
- Spence, R.P.; Towner, K.J. Frequencies and mechanisms of resistance to moxifloxacin in nosocomial isolates of Acinetobacter baumannii. J. Antimicrob. Chemother. 2003, 52, 687–690. [Google Scholar] [CrossRef]
- Theaker, C.; Azadian, B.; Soni, N. The impact of Acinetobacter baumannii in the intensive care unit. Anaesthesia 2003, 58, 271–274. [Google Scholar] [CrossRef]
- Dimopoulou, I.; Kartali, S.; Kartalis, G.; Manolas, K.; Simopoulos, K.; Vargemezis, B.; Theodoropoulou-Rodiou, G.; Bowler, I.; Crook, D. Relationship between nosocomial Acinetobacter species occurring in two geographical areas (Greece and the UK). J. Hosp. Infect. 2003, 54, 207–211. [Google Scholar] [CrossRef]
- Denton, M.; Wilcox, M.; Parnell, P.; Green, D.; Keer, V.; Hawkey, P.; Evans, I.; Murphy, P. Role of environmental cleaning in controlling an outbreak of Acinetobacter baumannii on a neurosurgical intensive care unit. J. Hosp. Infect. 2004, 56, 106–110. [Google Scholar] [CrossRef]
- Ng, G.; Sharma, B.; Fox, G. Acinetobacter skin abscess in a neonate. J. Perinatol. 2004, 24, 526–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turton, J.; Kaufmann, M.; Warner, M.; Coelho, J.; Dijkshoorn, L.; Van Der Reijden, T.; Pitt, T. A prevalent, multiresistant clone of Acinetobacter baumannii in Southeast England. J. Hosp. Infect. 2004, 58, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Turton, J.F.; Kaufmann, M.E.; Glover, J.; Coelho, J.M.; Warner, M.; Pike, R.; Pitt, T.L. Detection and typing of integrons in epidemic strains of Acinetobacter baumannii found in the United Kingdom. J. Clin. Microbiol. 2005, 43, 3074–3082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodford, N.; Ellington, M.J.; Coelho, J.M.; Turton, J.F.; Ward, M.E.; Brown, S.; Amyes, S.G.; Livermore, D.M. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 2006, 27, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.M.; Turton, J.F.; Kaufmann, M.E.; Glover, J.; Woodford, N.; Warner, M.; Palepou, M.-F.; Pike, R.; Pitt, T.L.; Patel, B.C. Occurrence of carbapenem-resistant Acinetobacter baumannii clones at multiple hospitals in London and Southeast England. J. Clin. Microbiol. 2006, 44, 3623–3627. [Google Scholar] [CrossRef] [Green Version]
- Pencavel, T.D.; Singh-Ranger, G.; Crinnion, J.N. Conservative treatment of an early aortic graft infection due to Acinetobacter baumanii. Ann. Vasc. Surg. 2006, 20, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Wilks, M.; Wilson, A.; Warwick, S.; Price, E.; Kennedy, D.; Ely, A.; Millar, M.R. Control of an outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus colonization and infection in an intensive care unit (ICU) without closing the ICU or placing patients in isolation. Infect. Control Hosp. Epidemiol. 2006, 27, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Turton, J.F.; Kaufmann, M.E.; Gill, M.J.; Pike, R.; Scott, P.T.; Fishbain, J.; Craft, D.; Deye, G.; Riddell, S.; Lindler, L.E.; et al. Comparison of Acinetobacter baumannii isolates from the United Kingdom and the United States that were associated with repatriated casualties of the Iraq conflict. J. Clin. Microbiol. 2006, 44, 2630–2634. [Google Scholar] [CrossRef] [Green Version]
- Wareham, D.; Bean, D.C.; Khanna, P.; Hennessy, E.; Krahe, D.; Ely, A.; Millar, M. Bloodstream infection due to Acinetobacter spp.: Epidemiology, risk factors and impact of multi-drug resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Bean, D.; Wareham, D. Paradoxical effect of 1-(1-naphthylmethyl)-piperazine on resistance to tetracyclines in multidrug-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 2009, 63, 349–352. [Google Scholar] [CrossRef] [Green Version]
- Enoch, D.; Summers, C.; Brown, N.; Moore, L.; Gillham, M.; Burnstein, R.; Thaxter, R.; Enoch, L.; Matta, B.; Sule, O. Investigation and management of an outbreak of multidrug-carbapenem-resistant Acinetobacter baumannii in Cambridge, UK. J. Hosp. Infect. 2008, 70, 109–118. [Google Scholar] [CrossRef]
- Gordon, N.; Wareham, D. Evaluation of CHROMagar Acinetobacter for detection of enteric carriage of multidrug-resistant Acinetobacter baumannii in samples from critically ill patients. J. Clin. Microbiol. 2009, 47, 2249–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livermore, D.M.; Hill, R.L.; Thomson, H.; Charlett, A.; Turton, J.F.; Pike, R.; Patel, B.C.; Manuel, R.; Gillespie, S.; Balakrishnan, I.; et al. Antimicrobial treatment and clinical outcome for infections with carbapenem-and multiply-resistant Acinetobacter baumannii around London. Int. J. Antimicrob. Agents 2010, 35, 19–24. [Google Scholar] [CrossRef]
- Lewis, T.; Loman, N.; Bingle, L.; Jumaa, P.; Weinstock, G.; Mortiboy, D.; Pallen, M.J. High-throughput whole-genome sequencing to dissect the epidemiology of Acinetobacter baumannii isolates from a hospital outbreak. J. Hosp. Infect. 2010, 75, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Hornsey, M.; Ellington, M.J.; Doumith, M.; Thomas, C.P.; Gordon, N.C.; Wareham, D.W.; Quinn, J.; Lolans, K.; Livermore, D.M.; Woodford, N. AdeABC-mediated efflux and tigecycline MICs for epidemic clones of Acinetobacter baumannii. J. Antimicrob. Chemother. 2010, 65, 1589–1593. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.; Yee, L.; Rimmer, J.-A.; Williams, R.; Martin, H.; Ovington, C. Investigation and management of an A. baumannii outbreak in ICU. Br. J. Nurs. 2011, 20, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.; Gould, I.; Opazo, A.; AMYES, S.B. The resistance profile of Acinetobacter baumannii strains iso-lated from the Aberdeen Royal Infirmary. Int. J. Antimicrob. Agents 2012, 39, 361–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halachev, M.R.; Chan, J.Z.; Constantinidou, C.I.; Cumley, N.; Bradley, C.; Smith-Banks, M.; Oppenheim, B.; Pallen, M.J. Genomic epidemiology of a protracted hospital outbreak caused by multidrug-resistant Acinetobacter baumannii in Birmingham, England. Genome Med. 2014, 6, 70. [Google Scholar] [CrossRef] [Green Version]
- Freeman, R.; Moore, L.; Charlett, A.; Donaldson, H.; Holmes, A. Exploring the epidemiology of carbapenem-resistant Gram-negative bacteria in west London and the utility of routinely collected hospital microbiology data. J. Antimicrob. Chemother. 2015, 70, 1212–1218. [Google Scholar] [CrossRef]
- Hughes, J.; Goldenberg, S.D.; Tosas, O.; Edgeworth, J.D.; Otter, J.A. Recent emergence of carbapenem-resistant organisms in a low prevalence UK setting in London. J. Infect. Prev. 2016, 17, 130–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabayoje, D.A.; NicFhogartaigh, C.; Cherian, B.P.; Tan, M.G.M.; Wareham, D.W. Compassionate use of cefiderocol for carbapenem-resistant Acinetobacter baumannii prosthetic joint infection. JAC-Antimicrob. Resist. 2021, 3, i21–i24. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.; Bal, A.M.; Balakrishnan, I.; Brown, N.M.; Burns, P.; Clark, M.; Diggle, M.; Donaldson, H.; Eltringham, I.; Folb, J. A prospective surveillance study to determine the prevalence of 16S rRNA methyltransferase-producing Gram-negative bacteria in the UK. J. Antimicrob. Chemother. 2021, 76, 2428–2436. [Google Scholar] [CrossRef] [PubMed]
- Gant, V.; Hussain, A.; Bain, M.; Longshaw, C.; Henriksen, A.S. In vitro activity of cefiderocol and comparators against gram-negative bacterial isolates from a series of surveillance studies in England: 2014–2018. J. Glob. Antimicrob. Resist. 2021, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.; Jauneikaite, E.; Sriskandan, S.; Woodford, N.; Hopkins, K.L. Novel 16S rRNA methyltransferase RmtE3 in Acinetobacter baumannii ST79. J. Med. Microbiol. 2022, 71, 001531. [Google Scholar] [CrossRef]
- Szabó, D.; Szentandrássy, J.; Juhász, Z.; Katona, K.; Nagy, K.; Rókusz, L. Imported PER-1 producing Pseudomonas aeruginosa, PER-1 producing Acinetobacter baumanii and VIM-2-producing Pseudomonas aeruginosa strains in Hungary. Ann. Clin. Microbiol. Antimicrob. 2008, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Bogaerts, P.; Naas, T.; El Garch, F.; Cuzon, G.; Deplano, A.; Delaire, T.; Huang, T.-D.; Lissoir, B.; Nordmann, P.; Glupczynski, Y. GES extended-spectrum β-lactamases in Acinetobacter baumannii isolates in Belgium. Antimicrob. Agents Chemother. 2010, 54, 4872–4878. [Google Scholar] [CrossRef] [Green Version]
- Hrabák, J.; Štolbová, M.; Študentová, V.; Fridrichová, M.; Chudáčková, E.; Zemlickova, H. NDM-1 producing Acinetobacter baumannii isolated from a patient repatriated to the Czech Republic from Egypt, July 2011. Eurosurveillance 2012, 17, 20085. [Google Scholar] [CrossRef]
- Kaase, M.; Nordmann, P.; Wichelhaus, T.A.; Gatermann, S.G.; Bonnin, R.A.; Poirel, L. NDM-2 carbapenemase in Acinetobacter baumannii from Egypt. J. Antimicrob. Chemother. 2011, 66, 1260–1262. [Google Scholar] [CrossRef] [Green Version]
- Bonnin, R.A.; Cuzon, G.; Poirel, L.; Nordmann, P. Multidrug-resistant Acinetobacter baumannii clone, France. Emerg. Infect. Dis. 2013, 19, 822. [Google Scholar] [CrossRef]
- Mohamed, N.M.; Youssef, A.A. In vitro activity of tigecycline and comparators against Gram-negative bacteria isolated from a tertiary hospital in Alexandria, Egypt. Microb. Drug Resist. 2011, 17, 489–495. [Google Scholar] [CrossRef] [PubMed]
- El-Kholy, A.; Saied, T.; Gaber, M.; Younan, M.A.; Haleim, M.M.; El-Sayed, H.; Bazara’a, H.; Talaat, M. Device-associated nosocomial infection rates in intensive care units at Cairo University hospitals: First step toward initiating surveillance programs in a resource-limited country. Am. J. Infect. Control 2012, 40, e216–e220. [Google Scholar] [CrossRef] [PubMed]
- Soliman, I.S.; Ahmed, A.S.; Deaf, E.A.-E.-M.; Sayed, I.M. Nosocomial Acinetobacter infection in Assiut University Hospital. Bull. Pharm. Sci. Assiut 2012, 35, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Al-Hassan, L.; El Mehallawy, H.; Amyes, S. Diversity in Acinetobacter baumannii isolates from paediatric cancer patients in Egypt. Clin. Microbiol. Infect. 2013, 19, 1082–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fouad, M.; Attia, A.S.; Tawakkol, W.M.; Hashem, A.M. Emergence of carbapenem-resistant Acinetobacter baumannii harboring the OXA-23 carbapenemase in intensive care units of Egyptian hospitals. Int. J. Infect. Dis. 2013, 17, e1252–e1254. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.; Rashad, A.; Fouad, A.; Azeem, A.A. Re-emerging of colistin for treatment of nosocomial pneumonia due to Gram negative multi-drug resistant pathogens in critically ill patients. Egypt. J. Chest Dis. Tuberc. 2013, 62, 447–451. [Google Scholar] [CrossRef] [Green Version]
- Nageeb, W.; Kamel, M.; Zakaria, S.; Metwally, L. Phenotypic characterization of Acinetobacter baumannii isolates from intensive care units at a tertiary-care hospital in Egypt. East. Mediterr. Health J. 2014, 20, 203–211. [Google Scholar] [CrossRef]
- Al-Agamy, M.H.; Khalaf, N.G.; Tawfick, M.M.; Shibl, A.M.; El Kholy, A. Molecular characterization of carbapenem-insensitive Acinetobacter baumannii in Egypt. Int. J. Infect. Dis. 2014, 22, 49–54. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed-Ahmed, M.A.E.-G.; Amin, M.A.; Tawakol, W.M.; Loucif, L.; Bakour, S.; Rolain, J.-M. High prevalence of blaNDM-1 carbapenemase-encoding gene and 16S rRNA armA methyltransferase gene among Acinetobacter baumannii clinical isolates in Egypt. Antimicrob. Agents Chemother. 2015, 59, 3602–3605. [Google Scholar] [CrossRef] [Green Version]
- Helal, S.; El Anany, M.; Ghaith, D.; Rabeea, S. The Role of MDR-Acinetobacter baumannii in orthopedic surgical site infections. Surg. Infect. 2015, 16, 518–522. [Google Scholar] [CrossRef]
- Ghaith, D.M.; Hassan, R.M.; Hasanin, A.M. Rapid identification of nosocomial Acinetobacter baumannii isolated from a surgical intensive care unit in Egypt. Ann. Saudi Med. 2015, 35, 440–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Mahallawy, H.A.; Hassan, S.S.; El-Wakil, M.; Moneer, M.M. Bacteremia due to ESKAPE pathogens: An emerging problem in cancer patients. J. Egypt. Natl. Cancer Inst. 2016, 28, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanin, A.; Mukhtar, A.; El-adawy, A.; Elazizi, H.; Lotfy, A.; Nassar, H.; Ghaith, D. Ventilator associated pneumonia caused by extensive-drug resistant Acinetobacter species: Colistin is the remaining choice. Egypt. J. Anaesth. 2016, 32, 409–413. [Google Scholar] [CrossRef] [Green Version]
- El-Kholy, I.M.; Abdelaziz, M.; Abdelsalam, S. Prevalence of Bacterial Infection in Patients with Chronic Hepatitis C Treated and not Treated with Interferon. Egypt. J. Bot. 2016, 56, 269–282. [Google Scholar] [CrossRef]
- Abouseada, N.; Raouf, M.; El-Attar, E.; Moez, P. Matrix-assisted laser desorption ionisation time-of-flight mass spectrometry rapid detection of carbapenamase activity in Acinetobacter baumannii isolates. Indian J. Med. Microbiol. 2017, 35, 85–89. [Google Scholar] [CrossRef]
- Alkasaby, N.M.; Zaki, M.E.S. Molecular study of Acinetobacter baumannii isolates for metallo-β-lactamases and extended-spectrum-β-lactamases genes in intensive care unit, Mansoura University Hospital, Egypt. Int. J. Microbiol. 2017, 2017, 3925868. [Google Scholar] [CrossRef] [Green Version]
- Gomaa, F.A.M.; Helal, Z.H.; Khan, M.I. High Prevalence of blaNDM-1, blaVIM, qacE, and qacEΔ1 genes and their association with decreased susceptibility to antibiotics and common hospital biocides in clinical isolates of Acinetobacter baumannii. Microorganisms 2017, 5, 18. [Google Scholar] [CrossRef]
- Ghaith, D.M.; Zafer, M.M.; Al-Agamy, M.H.; Alyamani, E.J.; Booq, R.Y.; Almoazzamy, O. The emergence of a novel sequence type of MDR Acinetobacter baumannii from the intensive care unit of an Egyptian tertiary care hospital. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 34. [Google Scholar] [CrossRef] [Green Version]
- Montasser, M.F.; Abdelkader, N.A.; Abdelhakam, S.M.; Dabbous, H.; Montasser, I.F.; Massoud, Y.M.; Abdelmoaty, W.; Saleh, S.A.; Bahaa, M.; Said, H.; et al. Bacterial infections post-living-donor liver transplantation in Egyptian hepatitis C virus-cirrhotic patients: A single-center study. World J. Hepatol. 2017, 9, 896. [Google Scholar] [CrossRef]
- Helmy, O.M.; Kashef, M.T. Different phenotypic and molecular mechanisms associated with multidrug resistance in Gram-negative clinical isolates from Egypt. Infect. Drug Resist. 2017, 10, 479. [Google Scholar] [CrossRef]
- Abdelkader, M.M.; Aboshanab, K.M.; El-Ashry, M.A.; Aboulwafa, M.M. Prevalence of MDR pathogens of bacterial meningitis in Egypt and new synergistic antibiotic combinations. PLoS ONE 2017, 12, e0171349. [Google Scholar] [CrossRef] [PubMed]
- Nour, I.; Eldegla, H.E.; Nasef, N.; Shouman, B.; Abdel-Hady, H.; Shabaan, A.E. Risk factors and clinical outcomes for carbapenem-resistant Gram-negative late-onset sepsis in a neonatal intensive care unit. J. Hosp. Infect. 2017, 97, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Todary, A.; El-Attar, M.; Zaghloul, M.Z.; Galal, Z. Quantitative culture of endotracheal aspirate in respiratory ICU. Egypt. J. Chest Dis. Tuberc. 2018, 67, 237. [Google Scholar] [CrossRef]
- Sultan, A.M.; Seliem, W.A. Identifying risk factors for healthcare-associated infections caused by carbapenem-resistant Acinetobacter baumannii in a neonatal intensive care unit. Sultan Qaboos Univ. Med. J. 2018, 18, e75. [Google Scholar] [CrossRef]
- Hassan, R.; Mukhtar, A.; Hasanin, A.; Ghaith, D. Role of insertion sequence aba-1 and adeS in reduced tigecycline susceptibility in MDR-Acinetobacter baumannii clinical isolates from Cairo, Egypt. J. Chemother. 2018, 30, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.E.S.; Abou ElKheir, N.; Mofreh, M. Molecular Study of Quinolone Resistance Determining Regions of Gene and Gene Clinical Isolates of to Fluoroquinolone. Open Microbiol. J. 2018, 12, 116–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tohamy, S.T.; Aboshanab, K.M.; El-Mahallawy, H.A.; El-Ansary, M.R.; Afifi, S.S. Prevalence of multidrug-resistant Gram-negative pathogens isolated from febrile neutropenic cancer patients with bloodstream infections in Egypt and new synergistic antibiotic combinations. Infect. Drug Resist. 2018, 11, 791. [Google Scholar] [CrossRef] [Green Version]
- El-Kholy, A.A.; Elanany, M.G.; Sherif, M.M.; Gad, M.A. High prevalence of VIM, KPC, and NDM expression among surgical site infection pathogens in patients having emergency surgery. Surg. Infect. 2018, 19, 629–633. [Google Scholar] [CrossRef]
- Ramadan, R.A.; Gebriel, M.G.; Kadry, H.M.; Mosallem, A. Carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa: Characterization of carbapenemase genes and E-test evaluation of colistin-based combinations. Infect. Drug Resist. 2018, 11, 1261. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elmonsef, M.M.; Elsharawy, D.; Abd-Elsalam, A.S. Mechanical ventilator as a major cause of infection and drug resistance in intensive care unit. Environ. Sci. Pollut. Res. 2018, 25, 30787–30792. [Google Scholar] [CrossRef]
- Abdulzahra, A.T.; Khalil, M.A.F.; Elkhatib, W.F. First report of colistin resistance among carbapenem-resistant Acinetobacter baumannii isolates recovered from hospitalized patients in Egypt. New Microbes New Infect. 2018, 26, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Said, H.S.; Benmahmod, A.B.; Ibrahim, R.H. Co-production of AmpC and extended spectrum beta-lactamases in cephalosporin-resistant Acinetobacter baumannii in Egypt. World J. Microbiol. Biotechnol. 2018, 34, 189. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, N.T.; El-gendy, A.O.; Saafan, A.E.; Tawakkol, W.M. Phenotypic detection of efflux mechanism in panaminoglycoside resistant Acinetobacter baumannii from Egyptian clinical isolates. Jundishapur J. Microbiol. 2018, 11, e69541. [Google Scholar] [CrossRef] [Green Version]
- Hamed, S.M.; Elkhatib, W.F.; El-Mahallawy, H.A.; Helmy, M.M.; Ashour, M.S.; Aboshanab, K. Multiple mechanisms contributing to ciprofloxacin resistance among Gram negative bacteria causing infections to cancer patients. Sci. Rep. 2018, 8, 12268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benmahmod, A.B.; Said, H.S.; Ibrahim, R.H. Prevalence and Mechanisms of Carbapenem Resistance Among Acinetobacter baumannii Clinical Isolates in Egypt. Microb. Drug Resist. 2019, 25, 480–488. [Google Scholar] [CrossRef]
- Al-Hassan, L.; Zafer, M.M.; El-Mahallawy, H. Multiple sequence types responsible for healthcare-associated Acinetobacter baumannii dissemination in a single centre in Egypt. BMC Infect. Dis. 2019, 19, 829. [Google Scholar] [CrossRef] [Green Version]
- Abouelfetouh, A.; Torky, A.S.; Aboulmagd, E. Phenotypic and genotypic characterization of carbapenem-resistant Acinetobacter baumannii isolates from Egypt. Antimicrob. Resist. Infect. Control 2019, 8, 185. [Google Scholar] [CrossRef] [Green Version]
- Attia, N.M.; Elbaradei, A. Fluoroquinolone resistance conferred by gyrA, parC mutations, and AbaQ efflux pump among Acinetobacter baumannii clinical isolates causing ventilator-associated pneumonia. Acta Microbiol. Immunol. Hung. 2020, 67, 234–238. [Google Scholar] [CrossRef]
- Miran, Y.; El-Mahallawy, H.A.; Attia, A.S. Tracing the dissemination of the international clones of multidrug-resistant Acinetobacter baumannii among cancer patients in Egypt using the PCR-based open reading frame typing (POT) method. J. Glob. Antimicrob. Resist. 2019, 19, 210–215. [Google Scholar] [CrossRef]
- Tolba, S.; El Shatoury, E.H.; Abo AlNasr, N.M. Prevalence of carbapenem resistant Acinetobacter baumannii (CRAB) in some Egyptian hospitals: Evaluation of the use of blaOXA-51-like gene as species specific marker for CRAB. Egypt. J. Bot. 2019, 59, 723–733. [Google Scholar] [CrossRef]
- Elbrolosy, A.M.; Labeeb, A.Z.; Hassan, D.M. New Delhi metallo-β-lactamase-producing Acinetobacter isolates among late-onset VAP patients: Multidrug-resistant pathogen and poor outcome. Infect. Drug Resist. 2019, 12, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanem, M.K.; Makhlouf, H.A.; Hasan, A.A.; Rashed, H.G.; Khalifa, H.S. Bacteriological profile of critically ill patients with chronic obstructive pulmonary disease in respiratory intensive care unit in Assuit University Hospital. Egypt. J. Bronchol. 2019, 13, 343–348. [Google Scholar] [CrossRef]
- Wassef, M.; Mukhtar, A.; Nabil, A.; Ezzelarab, M.; Ghaith, D. Care bundle approach to reduce surgical site infections in acute surgical intensive care unit, Cairo, Egypt. Infect. Drug Resist. 2020, 13, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsayed, E.; Elarabi, M.A.; Sherif, D.A.; Elmorshedi, M.; El-Mashad, N. Extensive drug resistant Acinetobacter baumannii: A comparative study between non-colistin based combinations. Int. J. Clin. Pharm. 2020, 42, 80–88. [Google Scholar] [CrossRef]
- Farag, A.M.; Tawfick, M.M.; Abozeed, M.Y.; Shaban, E.A.; Abo-Shadi, M.A. Microbiological profile of ventilator-associated pneumonia among intensive care unit patients in tertiary Egyptian hospitals. J. Infect. Dev. Ctries 2020, 14, 153–161. [Google Scholar] [CrossRef]
- Al-Hassan, L.L.; Al-Madboly, L.A. Molecular characterisation of an Acinetobacter baumannii outbreak. Infect. Prev. Pract. 2020, 2, 100040. [Google Scholar] [CrossRef]
- Mabrouk, S.S.; Abdellatif, G.R.; El-Ansary, M.R.; Aboshanab, K.M.; Ragab, Y.M. Carbapenemase producers Among extensive drug-resistant gram-negative pathogens recovered from febrile neutrophilic patients in Egypt. Infect. Drug Resist. 2020, 13, 3113. [Google Scholar] [CrossRef]
- El-Kazzaz, W.; Metwally, L.; Yahia, R.; Al-Harbi, N.; El-Taher, A.; Hetta, H.F. Antibiogram, prevalence of OXA carbapenemase encoding genes, and RAPD-genotyping of multidrug-resistant Acinetobacter baumannii incriminated in hidden community-acquired infections. Antibiotics 2020, 9, 603. [Google Scholar] [CrossRef]
- Ramadan, H.K.-A.; Mahmoud, M.A.; Aburahma, M.Z.; Elkhawaga, A.A.; El-Mokhtar, M.A.; Sayed, I.M.; Hosni, A.; Hassany, S.M.; Medhat, M.A. Predictors of severity and co-infection resistance profile in COVID-19 patients: First report from upper Egypt. Infect. Drug Resist. 2020, 13, 3409. [Google Scholar] [CrossRef]
- Makharita, R.R.; El-Kholy, I.; Hetta, H.F.; Abdelaziz, M.H.; Hagagy, F.I.; Ahmed, A.A.; Algammal, A.M. Antibiogram and genetic characterization of carbapenem-resistant Gram-negative pathogens incriminated in healthcare-associated infections. Infect. Drug Resist. 2020, 13, 3991. [Google Scholar] [CrossRef]
- Fam, N.S.; Gamal, D.; Mohamed, S.H.; Wasfy, R.M.; Soliman, M.S.; El-Kholy, A.A.; Higgins, P.G. Molecular characterization of Carbapenem/Colistin-resistant Acinetobacter baumannii clinical isolates from Egypt by whole-genome sequencing. Infect. Drug Resist. 2020, 13, 4487. [Google Scholar] [CrossRef] [PubMed]
- Fam, N.S.; Mohamed, S.H.; Gamal, D.; Wasfy, R.M.; Soliman, M.S.; El-Kholy, A.A. Reliability of phenotypic methods for detection of colistin resistance among carbapenem-resistant Acinetobacter baumannii clinical isolates from Egypt. Germs 2020, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Khodier, A.A.; Saafan, A.; Bakeer, W.; Khairalla, A.S. Molecular Characterization of Multiple Antibiotic-Resistant Acinetobacter baumannii Isolated from Egyptian Patients. J. Pure Appl. Microbiol. 2020, 14, 2399–2405. [Google Scholar] [CrossRef]
- Abd El-Baky, R.M.; Farhan, S.M.; Ibrahim, R.A.; Mahran, K.M.; Hetta, H.F. Antimicrobial resistance pattern and molecular epidemiology of ESBL and MBL producing Acinetobacter baumannii isolated from hospitals in Minia, Egypt. Alex. J. Med. 2020, 56, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Shabban, M.; Fahim, N.A.E.; Montasser, K.; El Magd, N.M.A. Resistance to colistin mediated by mcr-1 among multidrug resistant Gram negative pathogens at a tertiary care hospital, Egypt. J. Pure Appl. Microbiol. 2020, 14, 1125–1132. [Google Scholar] [CrossRef]
- ELsheredy, A.; Yousif, Z.; Elghazzawi, E.; Elmenshawy, A.; Ghazal, A. Prevalence of genes encoding aminoglycoside-modifying enzymes and armA among Acinetobacter baumannii clinical isolates in Alexandria, Egypt. Infect. Disord.-Drug Targets 2021, 21, 49–57. [Google Scholar] [CrossRef]
- Kishk, R.; Soliman, N.; Nemr, N.; Eldesouki, R.; Mahrous, N.; Gobouri, A.; Azab, E.; Anani, M. Prevalence of aminoglycoside resistance and aminoglycoside modifying enzymes in Acinetobacter baumannii among intensive care unit patients, Ismailia, Egypt. Infect. Drug Resist. 2021, 14, 143. [Google Scholar] [CrossRef]
- Asaad, A.M.; Ansari, S.; Ajlan, S.E.; Awad, S.M. Epidemiology of biofilm producing Acinetobacter baumannii nosocomial isolates from a tertiary care hospital in Egypt: A cross-sectional study. Infect. Drug Resist. 2021, 14, 709. [Google Scholar] [CrossRef]
- Wasfi, R.; Rasslan, F.; Hassan, S.S.; Ashour, H.M.; El-Rahman, A.; Ola, A. Co-existence of carbapenemase-encoding genes in Acinetobacter baumannii from cancer patients. Infect. Dis. Ther. 2021, 10, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.M.; Salem, S.T.; Hassan, S.I.M.; Hegab, A.S.; Elkholy, Y.S. Molecular characterization of carbapenem-resistant Acinetobacter baumannii clinical isolates from Egyptian patients. PLoS ONE 2021, 16, e0251508. [Google Scholar] [CrossRef] [PubMed]
- Meawed, T.E.; Ahmed, S.M.; Mowafy, S.M.; Samir, G.M.; Anis, R.H. Bacterial and fungal ventilator associated pneumonia in critically ill COVID-19 patients during the second wave. J. Infect. Public Health 2021, 14, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Salim, M.T.; Anwer, B.E.; Aboshanab, K.M.; Aboulwafa, M.M. Impact of target site mutations and plasmid associated resistance genes acquisition on resistance of Acinetobacter baumannii to fluoroquinolones. Sci. Rep. 2021, 11, 20136. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.A.; Ahmed, F.A.; Elkhateeb, A.F.; Mahmoud, E.E.; Ahmed, M.I.; Ahmed, R.I.; Hosni, A.; Alghamdi, S.; Kabrah, A.; Dablool, A.S.; et al. Virulence characteristics of biofilm-forming Acinetobacter baumannii in clinical isolates using a Galleria Mellonella Model. Microorganisms 2021, 9, 2365. [Google Scholar] [CrossRef]
- Zafer, M.M.; Hussein, A.F.; Al-Agamy, M.H.; Radwan, H.H.; Hamed, S.M. Genomic Characterization of Extensively Drug-Resistant NDM-Producing Acinetobacter baumannii Clinical Isolates With the Emergence of Novel bla ADC-257. MSAR 2021, 12, 736982. [Google Scholar] [CrossRef]
- Jalal, D.; Elzayat, M.G.; Diab, A.A.; El-Shqanqery, H.E.; Samir, O.; Bakry, U.; Hassan, R.; Elanany, M.; Shalaby, L.; Sayed, A.A. Deciphering multidrug-resistant Acinetobacter baumannii from a pediatric cancer hospital in Egypt. Msphere 2021, 6, e00725-21. [Google Scholar] [CrossRef] [PubMed]
- Abouelfetouh, A.; Mattock, J.; Turner, D.; Li, E.; Evans, B.A. Diversity of carbapenem-resistant Acinetobacter baumannii and bacteriophage-mediated spread of the Oxa23 carbapenemase. Microb. Genom. 2022, 8, 000752. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.M.; Hussein, A.F.; Al-Agamy, M.H.; Radwan, H.H.; Zafer, M.M. Genetic Configuration of Genomic Resistance Islands in Acinetobacter baumannii Clinical Isolates From Egypt. Front. Microbiol. 2022, 13, 878912. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.H.; El-Sayed, A.K. Traditional and Molecular Gene Detection (blaIMP-1 and blaIMP) of multi-drug resistant Acinetobacter baumannii. Catrina Int. J. Environ. Sci. 2021, 24, 75–80. [Google Scholar] [CrossRef]
- Betts, J.W.; Wareham, D.W. In vitro activity of curcumin in combination with epigallocatechin gallate (EGCG) versus multidrug-resistant Acinetobacter baumannii. BMC Microbiol. 2014, 14, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betts, J.W.; Sharili, A.S.; La Ragione, R.M.; Wareham, D.W. In vitro antibacterial activity of curcumin–polymyxin B combinations against multidrug-resistant bacteria associated with traumatic wound infections. J. Nat. Prod. 2016, 79, 1702–1706. [Google Scholar] [CrossRef]
- Halstead, F.; Webber, M.; Oppenheim, B. Use of an engineered honey to eradicate preformed biofilms of important wound pathogens: An in vitro study. J. Wound Care 2017, 26, 442–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betts, J.W.; Hornsey, M.; Wareham, D.W.; La Ragione, R.M. In vitro and in vivo activity of theaflavin–epicatechin combinations versus multidrug-resistant Acinetobacter baumannii. Infect. Dis. Ther. 2017, 6, 435–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd El-Malek, F.F.; Yousef, A.S.; El-Assar, S.A. Hydrogel film loaded with new formula from manuka honey for treatment of chronic wound infections. J. Glob. Antimicrob. Resist. 2017, 11, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.K.; Mattar, Z.A.; Abdel-Khalek, H.H.; Azzam, Y.M. Evaluation of antibacterial efficacy of anise wastes against some multidrug resistant bacterial isolates. J. Radiat. Res. Appl. Sci. 2017, 10, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Salem, M.; El-Hefny, M.; Ali, H.; Elansary, H.; Nasser, R.; El-Settawy, A.; El Shanhorey, N.; Ashmawy, N.; Salem, A. Antibacterial activity of extracted bioactive molecules of Schinus terebinthifolius ripened fruits against some pathogenic bacteria. Microb. Pathog. 2018, 120, 119–127. [Google Scholar] [CrossRef]
- Salam, A.S.; Ayaat, N.; Amer, M.; Shahin, A.; Amin, H. Antibacterial activity of some medicinal plants oils against multiresistant Acinetobacter strains isolated from nosocomial infections. Res. J. Pharm. Biol. Chem. Sci. 2018, 9, 337–348. [Google Scholar]
- Ahmed, F.A.; Ouda, N.H.S.E.-D.; Husseiny, S.M.; Adel, A. Antibacterial activity and composition of essential oils extracted from some plants belonging to family Lamiaceae against some multidrug resistant gram negative bacteria. Indo Am. J. Pharm. Sci. 2018, 5, 463–475. [Google Scholar] [CrossRef]
- Elamary, R.B.; Albarakaty, F.M.; Salem, W.M. Efficacy of Acacia nilotica aqueous extract in treating biofilm-forming and multidrug resistant uropathogens isolated from patients with UTI syndrome. Sci. Rep. 2020, 10, 11125. [Google Scholar] [CrossRef]
- Ismail, M.M.; Samir, R.; Saber, F.R.; Ahmed, S.R.; Farag, M.A. Pimenta oil as a potential treatment for Acinetobacter baumannii wound infection: In vitro and in vivo bioassays in relation to its chemical composition. Antibiotics 2020, 9, 679. [Google Scholar] [CrossRef]
- Salem, M.A.; El-Shiekh, R.A.; Hashem, R.A.; Hassan, M. In vivo antibacterial activity of star anise (Illicium verum Hook.) Extract Using Murine MRSA skin infection model in relation to its metabolite profile. Infect. Drug Resist. 2021, 14, 33. [Google Scholar] [CrossRef]
- El-Shiekh, R.A.; Hassan, M.; Hashem, R.A.; Abdel-Sattar, E. Bioguided isolation of antibiofilm and antibacterial pregnane glycosides from Caralluma quadrangula: Disarming multidrug-resistant pathogens. Antibiotics 2021, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Gaber, S.N.; Hemeda, E.E.M.; Elsayeh, H.-A.S.; Wahed, W.Y.A.; Khalil, M.A.; Ibrahim, E.G. Propolis extract: A possible antiseptic oral care against multidrug-resistant non-fermenting bacteria isolated from non-ventilator hospital-acquired pneumonia. J. Pure Appl. Microbiol. 2020, 14, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Wahed, A.A.; Farag, M.A.; Eraqi, W.A.; Mersal, G.A.; Zhao, C.; Khalifa, S.A.; El-Seedi, H.R. Unravelling the beehive air volatiles profile as analysed via solid-phase microextraction (SPME) and chemometrics. J. King Saud Univ.-Sci. 2021, 33, 101449. [Google Scholar] [CrossRef]
- Mahmoud, A.; Afifi, M.M.; El Shenawy, F.; Salem, W.; Elesawy, B.H. Syzygium aromaticum Extracts as a Potential Antibacterial Inhibitors against Clinical Isolates of Acinetobacter baumannii: An In-Silico-Supported In-Vitro Study. Antibiotics 2021, 10, 1062. [Google Scholar] [CrossRef] [PubMed]
- Sherif, M.M.; Elkhatib, W.F.; Khalaf, W.S.; Elleboudy, N.S.; Abdelaziz, N.A. Multidrug Resistant Acinetobacter baumannii Biofilms: Evaluation of Phenotypic–Genotypic Association and Susceptibility to Cinnamic and Gallic Acids. Front. Microbiol. 2021, 12, 716627. [Google Scholar] [CrossRef]
- Abdelaziz, N.A.; Elkhatib, W.F.; Sherif, M.M.; Abourehab, M.A.; Al-Rashood, S.T.; Eldehna, W.M.; Mostafa, N.M.; Elleboudy, N.S. In Silico Docking, Resistance Modulation and Biofilm Gene Expression in Multidrug-Resistant Acinetobacter baumannii via Cinnamic and Gallic Acids. Antibiotics 2022, 11, 870. [Google Scholar] [CrossRef]
- Amer, A.M.; El-snosi, Y.; Naaom, S.S.; Elariny, E.Y. Antimicrobial Activity of Some Plant Extracts and Plant Nanoparticles Against Gram Negative Bacteria Isolated from Clinical Samples. Egypt. J. Chem. 2021, 64, 5127–5136. [Google Scholar] [CrossRef]
- Elhosseiny, N.M.; Attia, A.S. Acinetobacter: An emerging pathogen with a versatile secretome. Emerg. Microbes Infect. 2018, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.; Morgan, D.; Walsh, A.; Turton, J.; Livermore, D.; Pitt, T.; Green, A.; Gill, M.; Mortiboy, D. Importation of multidrug-resistant Acinetobacter spp. infections with casualties from Iraq. Lancet Infect. Dis. 2006, 6, 317–318. [Google Scholar] [CrossRef]
- Scott, P.; Deye, G.; Srinivasan, A.; Murray, C.; Moran, K.; Hulten, E.; Fishbain, J.; Craft, D.; Riddell, S.; Lindler, L.; et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin. Infect. Dis. 2007, 44, 1577–1584. [Google Scholar] [CrossRef] [Green Version]
- HM-Government. Tackling Antimicrobial Resistance 2019–2024—The UK’s Five-Year National Action Plan. 2019. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/773130/uk-amr-5-year-national-action-plan.pdf (accessed on 18 December 2022).
- WHO. Global Action Plan on Antimicrobial Resistance. 2015. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 18 December 2022).
- Courtenay, M.; Castro-Sanchez, E.; Fitzpatrick, M.; Gallagher, R.; Lim, R.; Morris, G. Tackling antimicrobial resistance 2019–2024–the UK’s five-year national action plan. J. Hosp. Infect. 2019, 101, 426–427. [Google Scholar] [CrossRef] [PubMed]
- MOH-Egypt. Egypt National Action Plan for Antimicrobial Resistance. 2018. Available online: https://www.who.int/publications/m/item/egypt-national-action-plan-for-antimicrobial-resistance (accessed on 18 December 2022).
- Hicks, L.A.; Talaat, M. Protecting the Power of Antibiotics: Lessons from Egypt. 2015. Available online: https://www.cdc.gov/globalhealth/stories/protecting_power_antibiotics.htm (accessed on 18 December 2022).
- Ombarak, R.A.; Hinenoya, A.; Elbagory, A.-R.M.; Yamasaki, S. Prevalence and molecular characterization of antimicrobial resistance in Escherichia coli isolated from raw milk and raw milk cheese in Egypt. J. Food Prot. 2018, 81, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.; Towner, K.; Saunders, G.; Crewe-Brown, H.; Humphreys, H. Molecular and antibiogram relationships of Acinetobacter isolates from two contrasting hospitals in the United Kingdom and South Africa. Eur. J. Clin. Microbiol. Infect. Dis. 1999, 18, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Dooling, K.L.; Kandeel, A.; Hicks, L.A.; El-Shoubary, W.; Fawzi, K.; Kandeel, Y.; Etman, A.; Lohiniva, A.L.; Talaat, M. Understanding antibiotic use in Minya District, Egypt: Physician and pharmacist prescribing and the factors influencing their practices. Antibiotics 2014, 3, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, R.P. The antibiotic pipeline—Challenges, costs, and values. N. Engl. J. Med. 2004, 351, 523–526. [Google Scholar] [CrossRef]
- Molinari, G. Natural Products in Drug Discovery: Present Status and Perspectives. In Pharmaceutical Biotechnology; Guzmán, C.A., Feuerstein, G.Z., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2009; pp. 13–27. [Google Scholar]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Osterburg, A.; Gardner, J.; Hyon, S.; Neely, A.; Babcock, G. Highly antibiotic-resistant Acinetobacter baumannii clinical isolates are killed by the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG). Clin. Microbiol. Infect. 2009, 15, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Montagu, A.; Saulnier, P.; Cassisa, V.; Rossines, E.; Eveillard, M.; Joly-Guillou, M.-L. Aromatic and Terpenic Compounds Loaded in Lipidic Nanocapsules: Activity against Multi-drug Resistant Acinetobacter baumannii Assessed in vitro and in a Murine Model of Sepsis. J. Nanomed. Nanotechnol. 2014, 5, 206. [Google Scholar]
- Miyasaki, Y.; Rabenstein, J.D.; Rhea, J.; Crouch, M.-L.; Mocek, U.M.; Kittell, P.E.; Morgan, M.A.; Nichols, W.S.; Van Benschoten, M.; Hardy, W.D. Isolation and characterization of antimicrobial compounds in plant extracts against multidrug-resistant Acinetobacter baumannii. PLoS ONE 2013, 8, e61594. [Google Scholar] [CrossRef] [Green Version]
- Masoud, E.; Gouda, H. Effect of some natural plant extracts against gram negative bacteria in Njran Area, Saudi Arabia. Egypt. Acad. J. Biol. Sci. 2012, 4, 85–92. [Google Scholar]
- Seeff, L.B.; Bonkovsky, H.L.; Navarro, V.J.; Wang, G. Herbal products and the liver: A review of adverse effects and mechanisms. Gastroenterology 2015, 148, 517-532.e3. [Google Scholar] [CrossRef] [PubMed]
- Vostinaru, O.; Heghes, S.C.; Filip, L. Safety Profile of Essential Oils. In Essential Oils-Bioactive Compounds, New Perspectives and Applications; de Oliveira, M.S., Silva, S., Da Costa, W.A., Eds.; BoD–Books on Demand: Norderstedt, Germany, 2020; pp. 1–13. [Google Scholar]
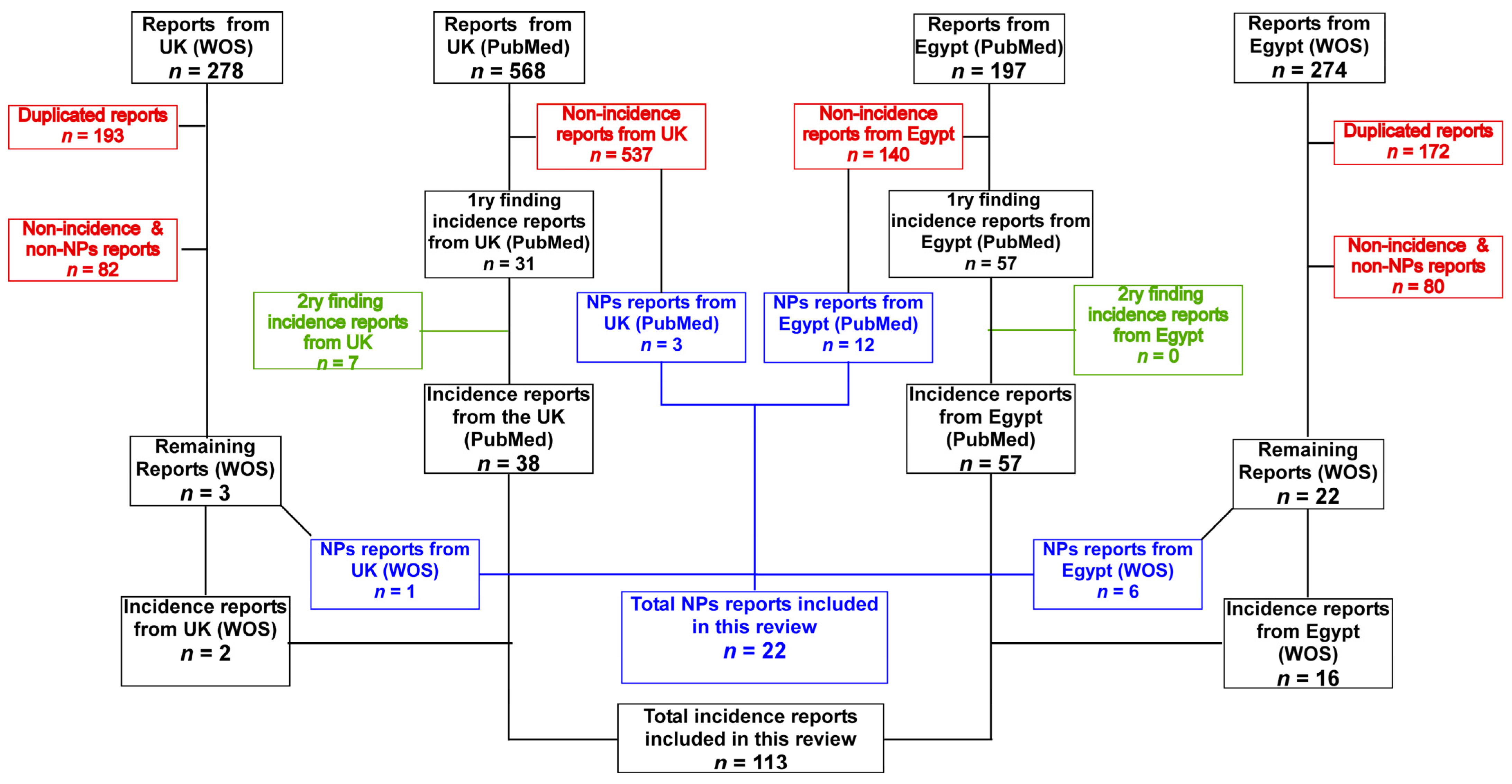
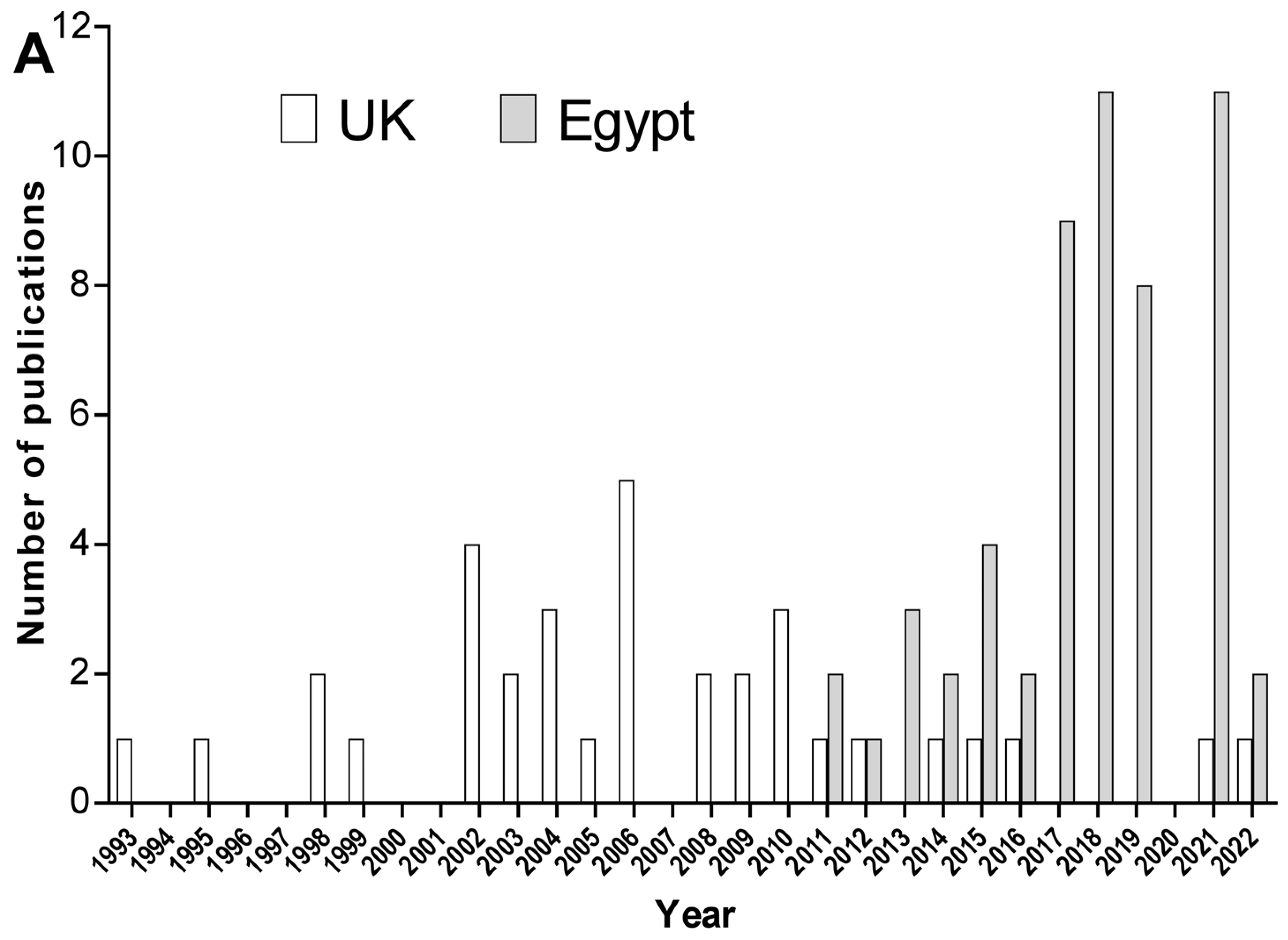

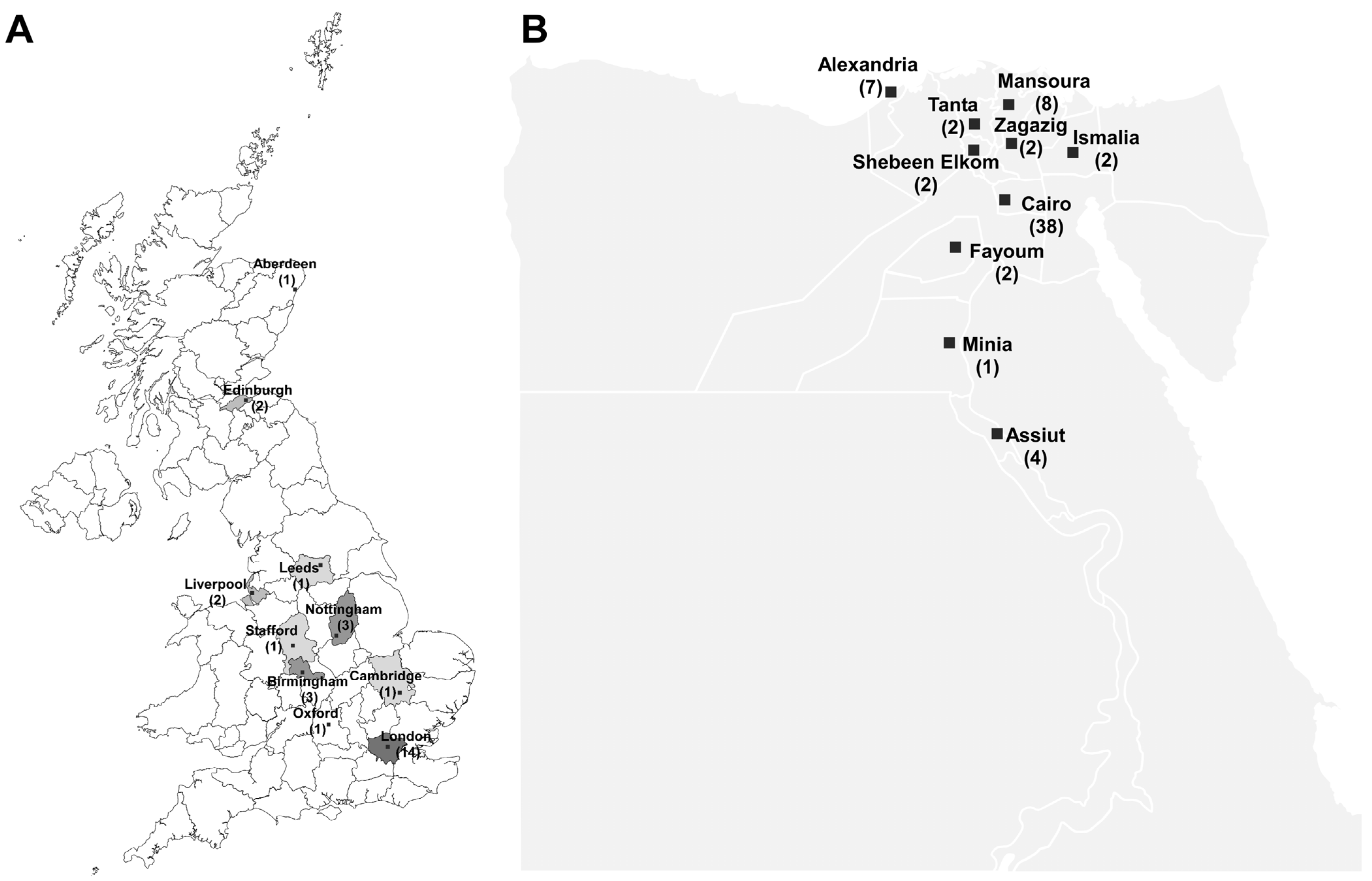
| Study | Isolates (n) | MDR% | CRAB % | Isolates Characterization | Genes | Reference |
|---|---|---|---|---|---|---|
| Paton et al., 1993 | 1 | 100 | 100 | AST by Stokes | - | [21] |
| Crowe et al., 1995 | 11 | 100 | - | API 20NE, PFGE, ribotyping | - | [22] |
| Webster et al., 1998 | 6 | 100 | - | API 20NE, RAPD | - | [23] |
| Morar et al., 1998 | - | - | - | - | - | [24] |
| McDonald et al., 1999 | 18 | - | - | API 20NE, PFGE | - | [25] |
| Henwood et al., 2002 | 443 | - | 2 | PCR, tRNA spacer fingerprinting | blaIMP, blaVIM, blaOXA-23, blaOXA-24 | [26] |
| Towner et al., 2002 | 1 | 100 | 100 | tRNA spacer fingerprinting, Etest, PCR | blaIMP | [27] |
| Spence et al., 2002 | 287 | - | 1.8 | tDNA fingerprinting, RAPD | - | [28] |
| Das et al., 2002 | 13 | 100 | 100 | API 20NE, PFGE | - | [29] |
| Spence et al., 2003 | 226 | - | - | tDNA and AFLP fingerprinting, Etest. PCR | gyrA, parC | [30] |
| Theaker et al., 2003 | 27 | - | - | PCR, DNA sequencing | - | [31] |
| Dimopoulou et al., 2003 | 17 | 12 | - | PCR, DNA sequencing, REP-PCR | 16S rDNA | [32] |
| Denton et al., 2004 | 27 | 0 | 0 | API 20NE, AST by Stokes, PFGE | - | [33] |
| Ng et al., 2004 | 1 | 100 | 0 | AST | - | [34] |
| Turton et al., 2004 | 375 * | 100 | 67 | API 20NE, tDNA fingerprinting, PFGE, Etest, PCR | blaIMP, blaVIM, blaOXA-23, blaOXA-24 | [35] |
| Turton et al., 2005 | - | - | - | PCR, PFGE, DNA sequencing | blaOXA-23, Class 1 and 2 integron cassettes | [36] |
| Woodford et al., 2006 | 168 | - | - | Multiplex PCR | blaOXA-51, blaOXA-23, blaOXA-58, blaOXA-24 | [37] |
| Coelho et al., 2006 | 627 | - | - | PFGE, PCR | blaOXA-51, blaOXA-23 | [38] |
| Pencavel et al., 2006 | 1 | - | - | - | - | [39] |
| Wilks et al., 2006 | 136 | 100 | 0 | API 20NE, PFGE | - | [40] |
| Turton et al., 2006 | 25 | - | - | PFGE | Integron cassettes | [41] |
| Wareham et al., 2008 | 187 | 34 | 52 | API 20NE, AST by BSAC DD | - | [42] |
| Bean et al., 2009 | 104 | - | - | API 20NE, AST by BSAC DD, PCR | blaOXA-23 | [43] |
| Enoch et al., 2008 | 19 | 100 | 100 | PFGE, AST by BSAC DD | - | [44] |
| Gordon and Wareham, 2009 | 34 | 100 | - | CHROMagar, PCR | blaOXA-51, csuE, ompA | [45] |
| Livermore et al., 2010 | 166 | 100 | 100 | PFGE | blaOXA-23 | [46] |
| Lewis et al., 2010 | 6 | 100 | 100 | Vitek 2, PCR, VNTR, PFGE, WGS | Whole genome | [47] |
| Hornsey et al., 2010 | 9 | 100 | 100 | API 20NE, PCR, Etest, RT–PCR | blaOXA-51, blaOXA-23, adeAB | [48] |
| Adams et al., 2011 | 3 | 100 | - | PFGE | blaOXA-51 | [49] |
| Lopes et al., 2012 | 9 | - | 11 | PCR, DNA sequencing, PFGE | blaOXA-51, blaOXA-23-like, blaOXA-40-like, blaOXA-58-like, blaOXA-143-like, blaADC, gyrA, parC, Class I integrons | [50] |
| Halachev et al., 2014 | 112 | 100 | - | Vitek 2, PFGE, WGS | Whole genome | [51] |
| Freeman et al., 2015 | 196 | 100 | 65.8 | - | - | [52] |
| Hughes et al., 2016 | 16 | - | 31.2 | Vitek 2 | - | [53] |
| Mabayoje et al., 2021 | 1 | 100 | 100 | WGS | Whole genome | [54] |
| Taylor et al., 2021 | 16 | 100 | 69 | MALDI-TOF-MS, PFGE, WGS | armA, blaOXA-23, blaNDM-1 | [55] |
| Gant et al., 2021 | 70 | - | 13 | MALDI-TOF-MS | - | [56] |
| Taylor et al., 2022 | 1 | 100 | 100 | PCR, WGS | rmtE3, blaOXA-65, blaOXA-72 | [57] |
| Study | Isolates (n) | MDR% | CRAB% | Isolates Characterization | Genes | Reference |
|---|---|---|---|---|---|---|
| Szabó et al., 2008 | 1 | 100 | 0 | VITEK 2, PCR, IEF | blaPER-1, blaTEM-1 | [58] |
| Bogaerts, et al., 2010 | 2 | 100 | 100 | VITEK 2, MALDI-TOF-MS, AST by KB, PCR | blaGES-11, blaGES-12, blaOXA-82, blaOXA-94 | [59] |
| Hrabák et al., 2012 | 2 | 100 | 100 | API ID32 GN, MALDI-TOF-MS | blaNDM-1 | [60] |
| Kaase et al., 2011 | 1 | 100 | 100 | API 20NE, AST by KB, PCR, MLST | blaNDM-2 | [61] |
| Bonnin et al., 2013 | 1 | 100 | 100 | 16S rRNA gene sequencing, PCR, MLST | blaNDM-1 | [62] |
| Mohamed and Youssef, 2011 | 15 | - | 13 | API 20NE | - | [63] |
| El-Kholy et al., 2011 | 26 | - | 76.9 | Conventional methods | - | [64] |
| Soliman et al., 2012 | 51 | 61 | 31.2 | API 20 NE, AST by KB, CDT, PCR | blaOXA-51, Class I integrase | [65] |
| Al-Hassan et al., 2013 | 34 | - | 73 | VITEK 2, Phoenix, PCR, DNA sequencing, PFGE, MLST | blaOXA-51, blaOXA-23, blaOXA-40, blaOXA-58, blaOXA-64blaOXA-65, blaOXA-66, blaOXA-69, blaOXA-71, blaOXA-78, blaOXA-94, blaOXA-89 | [66] |
| Fouad et al., 2013 | 39 | 80 | 74 | MHT, IPD, PCR, ERIC-PCR | blaOXA-51, blaOXA-23, blaVIM, int1 | [67] |
| Amin et al., 2013 | 40 | 100 | 100 | AST by KB, Etest | - | [68] |
| Nageeb et al., 2014 | 10 | 100 | 60 | API 20NE, AST by KB, MHT | - | [69] |
| Al-Agamy et al., 2014 | 40 | 100 | 70 | API 20NE, PCR | blaTEM, blaPER, blaGES | [70] |
| El-Sayed-Ahmed et al., 2015 | 150 | - | 87.3 | MALDI-TOF-MS, PCR, AST by KB, Vitek 2, MLST | blaOXA-51, blaOXA-23, blaNDM-1, armA | [71] |
| Helal et al., 2015 | 15 | 100 | 100 | CHROMagar, PCR | blaOXA-51 | [72] |
| Ghaith et al., 2015 | 54 | - | - | CHROMagar, PCR, MALDI-TOF-MS | blaOXA-51 | [73] |
| El-Mahallawy et al., 2015 | 8 | - | - | Microscan, AST by KB | - | [74] |
| Hasanin et al., 2016 | 30 | 100 | 100 | API 20NE, E test | - | [75] |
| El-Kholy et al., 2016 | 22 | 100 | 100 | MicroScan, Biolog Microlog, AST by KB | - | [76] |
| Abouseada et al., 2017 | 50 | - | 78 | PCR, MALDI-TOF-MS | - | [77] |
| Alkasaby and Zaki, 2017 | 280 | 100 | 95.7 | Etest, PCR | blaOXA-51, blaTEM, blaSHV, blaCTX-M, blaIMP, blaSIM, blaGIM, | [78] |
| Gomaa et al., 2017 | 56 | 88 | 71.4 | Vitek, PCR | blaOXA51, intl1, blaVIM, blaNDM-1, qacE, qacEΔ1 | [79] |
| Ghaith et al., 2017 | 50 | 100 | 100 | PCR, MLST | blaOXA-51, blaOXA-23 | [80] |
| Montasser et al., 2017 | 19 | 100 | 60 | - | - | [81] |
| Helmy and Kashef 2017 | 15 | 86.6 | 66.7 | API 20NE, PCR | blaOXA-23, aac-Ib, blaTEM-1, blaCTX-M-15 | [82] |
| Abdelkader et al., 2017 | 7 | 100 | 0 | AST by KB, PCR | blaCTX-M, blaSHV, blaTEM, qnr | [83] |
| Nour et al., 2017 | 6 | 17 | AST by KB, CNPt | [84] | ||
| Todary et al., 2017 | 16 | - | - | API 20NE, Vitek 2 | - | [85] |
| Sultan and Selim, 2018 | 124 | 94.5 | 73.4 | API 20NE, AST by KB | - | [86] |
| Hassan et al., 2018 | 63 | 100 | - | PCR, AST by KB, Etest | blaOXA-51, adeR, adeS, adeB | [87] |
| Zaki et al., 2018 | 140 | 100 | 100 | RFLP-PCR | gyrA, parC | [88] |
| Tohamy et al., 2018 | 12 | 100 | 83 | Microscan, AST by KB, PCR | - | [89] |
| El-Kholy et al., 2018 | 6 | 100 | 100 | VITEK 2, Etest, MALDI-TOF-MS, PCR | blaVIM | [90] |
| Ramadan et al., 2018 | 50 | - | 60 | VITEK 2, AST by modified KB, PCR | blaOXA-23, blaNDM, blaGES | [91] |
| Abd-Elmonsef et al., 2018 | 9 | 100 | 33 | AST by KB | - | [92] |
| Abdulzahra et al., 2018 | 40 | 100 | 100 | Vitek 2, PCR | blaOXA-51, blaOXA-23, pmrCAB | [93] |
| Said et al., 2018 | 50 | 98 | 98 | AST by KB, PCR, ERIC-PCR | blaTEM, blaPER, blaSHV, blaVEB, blaADC | [94] |
| Moustafa et al., 2018 | 57 | - | - | PCR, AST by KB | - | [95] |
| Hamed et al., 2018 | 23 | - | - | Vitek 2, PCR, DNA sequencing, ERIC-PCR | gyrA, parC, qnrA, qnrB, qnrS, aac(6′)-Ib | [96] |
| Benmahmod et al., 2019 | 50 | - | 98 | AST by KB, PCR, RAPD | blaOXA-51, blaOXA-23, blaOXA-58, blaOXA-24, blaSIM, blaNDM, blaVIM, blaIMP, blaKPC, blaGES | [97] |
| Al-Hassan et al., 2019 | 59 | - | 93 | Vitek 2, MLST | blaOXA-51, blaOXA-23, blaOXA-58, blaNDM-1blaVIM-1 | [98] |
| Abouelfetouh et al., 2019 | 74 | - | 100 | AST by KB, PCR | blaOXA-51, blaOXA-23, blaOXA-58, blaNDM,blaVIM | [99] |
| Attia and Elbaradei, 2019 | 21 | 76 | 100 | MALDI-TOF-MS, PCR | blaOXA-51, gyrA, parC | [100] |
| El-Far et al., 2019 | 160 | 73 | 89 | Vitek 2, POT | - | [101] |
| Tolba et al., 2019 | 45 | 100 | 89 | PCR, Vitek 2 | blaOXA-51-like, blaOXA-23-like, blaOXA-24-like, blaOXA-58-like | [102] |
| Ghanema et al., 2019 | 7 | 100 | 86 | Vitek 2 | - | [104] |
| Elbrolosy et al., 2019 | 64 | 100 | 84 | Vitek 2, MHT, CDT, PCR | blaNDM-1 | [103] |
| Wassef et al., 2020 | 12 | - | - | Chromagar, Vitek 2 | - | [105] |
| Elsayed et al., 2020 | 30 | 100 | 84 | AST by KB, Vitek 2 | - | [106] |
| Farag et al., 2020 | 6 | 100 | 83 | Chromagar, Vitek 2 | - | [107] |
| Al-Hassan and Al-Madboly, 2020 | 54 | 100 | 81 | API 20 NE, MALDI-TOF-MS, PCR, MLST | blaOXA-23, blaNDM,, blaVIM-2 | [108] |
| Mabrouk et al., 2020 | 129 | 95.3 | 98 | AST by KB, mCIM, CDT, BCT, PCR | blaIMP, blaKPC, blaNDM, blaOXA-48, blaVIM | [109] |
| El-Kazzaz et al., 2020 | 23 | 100 | 50 | API 20NE, PCR, DNA sequencing, MHT, RAPD-PCR | blaOXA-23, blaOXA-24, blaOXA-51, blaOXA-58, blaIMP, blaKPC, blaNDM, blaGES, blaVIM | [110] |
| Ramadan et al., 2020 | 7 | 100 | 71.4 | VITEK 2, PCR | blaNDM, blaTEM, blaCTX-M | [111] |
| Makharita et al., 2020 | 39 | 82 | 48.7 | AST by KB, PCR, MHT, CDT, DNA sequencing | blaKPC, blaGES | [112] |
| Fam et al., 2020 | 17 | - | 100 | Vitek 2, PCR, WGS | blaOXA-51, blaOXA-23, blaNDM, blaGES | [113] |
| Fam et al., 2020 | 22 | - | 100 | API 20NE, Vitek 2, AST by KB | - | [114] |
| Khodier et al., 2020 | 48 | - | 63 | Vitek 2, PCR | blaOXA-51-like, blaOXA-23-like, blaOXA-24-like, blaOXA-58-like, class 1 integrons | [115] |
| Abd El-Baky et al., 2020 | 20 | 60 | 20 | PCR, DDST, MHT | blaCTXM-15, blaOxa-51, blaOxa-23, blaOxa-143 | [116] |
| M Shabban et al., 2020 | 14 | 100 | 85 | Real-time PCR, Etest | mcr-1 | [117] |
| ELsheredy et al., 2021 | 100 | - | - | Vitek 2, AST by KB, PCR | blaOXA-51, aphA6, aphA1, armA | [118] |
| Kishk et al., 2021 | 52 | 75 | 36 | Vitek 2, PCR | aacC1, aphA6, addA1 | [119] |
| Asaad et a, 2021 | 94 | 100 | 75 | API 20NE, Vitek 2, Etest, PCR | bap, ompA, blaPER-1 | [120] |
| Wasfi et al., 2021 | 48 | - | 70.8 | CHROMagar, MALDI-TOF-MS, Vitek 2, PCR, ERIC-PCR, MLST | blaOXA-51, blaOXA-23, blaNDM, blaKPC, blaGIM, blaSPM, blaSIM, blaIMP, blaOXA-58, blaOXA-23/24 | [121] |
| Hassan et al., 2021 | 206 | - | 100 | PCR, AST by KB, PCR, DNA sequencing | blaOXA-51, blaOXA-23, blaOXA-58, blaNDM-1, blaSPM, blaVIM, blaSIM-1, blaKPC | [122] |
| Meawed et al., 2021 | 54 | - | - | Vitek 2, AST by KB | - | [123] |
| Mohammed et al., 2021 | 100 | 100 | - | PCR, AST by KB, DNA sequencing, ERIC-PCR | gyrA, parC | [124] |
| Khalil et al., 2021 | 54 | - | 97 | API 20NE, Vitek 2, AST by KB, THT, PCR, | blaOXA-23, blaNDM, blaPER-1, bap | [125] |
| Zafer et al., 2021 | 20 | 100 | 100 | Vitek 2, PCR, AST by KB, MLST, WGS | blaNDM, blaVIM, blaIMP | [126] |
| Jalal et al., 2021 | 31 | 100 | 100 | Vitek 2, WGS | - | [127] |
| Saleh and El-Sayed, 2021 | 52 | 98 | 100 | Vitek 2, MHT, CDDT, PCR | blaIMP-1 | [130] |
| Abouelfetouh, 2022 | 54 | - | 100 | Vitek 2, MALDI-TOF-MS, WGS | blaOXA-23, blaNDM-1, blaNDM-2 | [128] |
| Hamed et al., 2022 | 20 | 100 | 100 | Vitek 2, AST by KB, PCR, WGS | blaOXA-51 | [129] |
| Study | Natural Product (NP) | Specifications of NP | Observed Activity | Ref. |
|---|---|---|---|---|
| United Kingdom: | ||||
| Betts and Wareham, 2014 | Curcumin andand EGCG | Pure compounds with purity over 90% were commercially purchased | Enhanced antibacterial activity | [131] |
| Betts et al., 2016 | Curcumin | Curcumin >95% purity was commercially purchased | Synergism with polymyxin B | [132] |
| Halstead et al., 2017 | Engineered honey SHRO | SurgihoneyRO (SHRO) (Matoke Holdings, UK) is a licensed CE marked sterile topical engineered honey. It exerts its effect through producing higher ROS such as H2O2. | Biofilm detachment | [133] |
| Betts et al., 2017 | Theaflavin and epicatechin | Epicatechin >90% and theaflavin >95% purity, were commercially purchased | Enhanced antibacterial activity | [134] |
| Egypt: | ||||
| Abd El-Malek et al., 2017 | Manuka honey | Manuka honey obtained from Australian company, was dissolved in acidulated water, pH = 2. Fractionated using liquid chromatography and the diethyl ether fraction was rich in phenolics such as isoferulic acid, luteolin, andand chrysin | Antibacterial activity | [135] |
| Ibrahim et al., 2017 | Aniseeds waste residue and Star anise waste residue extracts | Anise and star anise fruits were hydrodistilled to obtain the VOs. Also, the post-distillation remaining water was freeze dried and tested | Synergistic activity with antibiotics | [136] |
| Salem et al., 2018 | Schinus terebinthifolius | VOs were obtained by hydro distillation and its components were identified using GCMS. Other extracts were extracted with acetone and n-hexane, separately, and their components were identified by GCMS and spectroscopic determination of phenolics. | Antibacterial activity | [137] |
| Ahmed et al., 2018 | Aerial part of some medicinal plants from family Lamiaceae | Essential oils from the shoots of all plants were obtained by hydro distillation. The composition of the oils was identified using GCMS. | Antimicrobial activity | [139] |
| Salem et al., 2018 | Essential oils | Essential oils of 10 plants commonly used in Egypt were purchased. The characterization of oil was not discussed | Antibacterial activity | [138] |
| Elamary et al., 2020 | Acacia nilotica | Aqueous extract was obtained by macerating the plant into hot distilled water and filtered on 0.45 µm disk filters and its components were identified by GCMS. | Antibacterial activity | [140] |
| Ismail et al., 2020 | Pimenta dioica and racemosa | Leaves and berries of Pimenta dioica, were separately hydro distilled to obtain VO. The VO composition was assessed by GCMS. | Antibacterial and antibiofilm activities | [141] |
| Gaber et al., 2020 | Bee products | Honey was obtained from hives and was diluted as 50% w/v solution in Muller-Hinton broth. Propolis was gathered by collecting the hive scrapings and placed in water and heated in oven for 2 h. Wax was collected from surface, while propolis was at the bottom of container. Bee venom was collected using electric shock method. | Antibacterial activity | [144] |
| Salem et al., 2021 | Star anise | Star anise powder was extracted with methyl ter-butyl ether: water 3:1 v/v followed by methanol: water 3:1 v/v and the aqueous methanol extract was used for the study, and its components were identified by LCMS | Antibacterial and antibiofilm activities | [142] |
| El-Shiekh et al., 2021 | Caralluma quadrangula | The plant was extracted with methanol and fractionated with methylene chloride and n-butanol. From the n-butanol fraction russeliosides A-D, pregnane glycosides, were isolated. In addition to a flavonoid glycoside Rus. E | Antibacterial and antibiofilm activities | [143] |
| Mahmoud et al., 2021 | Syzygium aromaticum | Seeds were extracted, separately, with hot water, ethanol and ethyl acetate and were analyzed using GCMS | Antibacterial activity | [146] |
| Sherif et al., 2021 and Abdelaziz et al., 2021 | Cinnamic and gallic acids | Cinnamic and gallic acid were purchased commercially | Antibacterial and antibiofilm activities | [147,148] |
| Abd El-Wahed et al., 2021 | beehive air volatiles | Beehive air is a representative sample consisting of propolis: honey: wax: bee bread: royal jelly: larvae drones: larvae queen: venom (10:10:10:10:10:10:1:1:1:0.2), respectively. The constituents were analyzed by solid phase microextraction SPME-GCMS | Antibacterial activity | [145] |
| Amer et al., 2021 | Nigella sativa and Lawsonia inermis | The powdered plants were extracted with ethanol using Soxhlet apparatus. | Antibacterial activity | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elwakil, W.H.; Rizk, S.S.; El-Halawany, A.M.; Rateb, M.E.; Attia, A.S. Multidrug-Resistant Acinetobacter baumannii Infections in the United Kingdom versus Egypt: Trends and Potential Natural Products Solutions. Antibiotics 2023, 12, 77. https://doi.org/10.3390/antibiotics12010077
Elwakil WH, Rizk SS, El-Halawany AM, Rateb ME, Attia AS. Multidrug-Resistant Acinetobacter baumannii Infections in the United Kingdom versus Egypt: Trends and Potential Natural Products Solutions. Antibiotics. 2023; 12(1):77. https://doi.org/10.3390/antibiotics12010077
Chicago/Turabian StyleElwakil, Wafaa H., Soha S. Rizk, Ali M. El-Halawany, Mostafa E. Rateb, and Ahmed S. Attia. 2023. "Multidrug-Resistant Acinetobacter baumannii Infections in the United Kingdom versus Egypt: Trends and Potential Natural Products Solutions" Antibiotics 12, no. 1: 77. https://doi.org/10.3390/antibiotics12010077
APA StyleElwakil, W. H., Rizk, S. S., El-Halawany, A. M., Rateb, M. E., & Attia, A. S. (2023). Multidrug-Resistant Acinetobacter baumannii Infections in the United Kingdom versus Egypt: Trends and Potential Natural Products Solutions. Antibiotics, 12(1), 77. https://doi.org/10.3390/antibiotics12010077









