Abstract
Antimicrobial resistance is a silent pandemic exacerbated by the uncontrolled use of antibiotics. Since the discovery of penicillin, we have been largely dependent on microbe-derived small molecules to treat bacterial infections. However, the golden era of antibiotics is coming to an end, as the emergence and spread of antimicrobial resistance against these antibacterial compounds are outpacing the discovery and development of new antibiotics. The current antibiotic market suffers from various shortcomings, including the absence of profitability and investment. The most important underlying issue of traditional antibiotics arises from the inherent properties of these small molecules being mostly broad-spectrum and non-programmable. As the scientific knowledge of microbes progresses, the scientific community is starting to explore entirely novel approaches to tackling antimicrobial resistance. One of the most prominent approaches is to develop next-generation antibiotics. In this review, we discuss three innovations of next-generation antibiotics compared to traditional antibiotics as specificity, evolvability, and non-immunogenicity. We present a number of potential antimicrobial agents, including bacteriophage-based therapy, CRISPR-Cas-based antimicrobials, and microbiome-derived antimicrobial agents. These alternative antimicrobial agents possess innovative properties that may overcome the inherent shortcomings of traditional antibiotics, and some of these next-generation antibiotics are not merely far-fetched ideas but are currently in clinical development. We further discuss some related issues and challenges such as infection diagnostics and regulatory frameworks that still need to be addressed to bring these next-generation antibiotics to the antibiotic market as viable products to combat antimicrobial resistance using a diversified set of strategies.
1. Introduction
A growing number of bacterial infections such as salmonellosis, tuberculosis, pneumonia, and gonorrhea are becoming resistant to antibiotics. The World Health Organization (WHO) recently declared the spread of antimicrobial resistance (AMR) as one of the top 10 threats to global health and development, which shows that the problem of multidrug-resistant bacteria is having a negative impact on various aspects of society. Followingly, the WHO published a priority list of pathogens that urgently require new antibiotics (Table 1). In 2019, at least 1.2 million people died worldwide from multidrug-resistant bacteria [1], which was already recognized as a serious threat to the progress of modern medicine as bacterial infections can become fatal. During COVID-19, the spread of AMR has been exacerbated by infection control lapses, with significantly higher rates of hospital-acquired infections and deaths from multidrug-resistant bacteria in U.S. hospitals [2]. It is estimated that infections from multidrug-resistant bacteria could cause more than 10 million deaths per year worldwide by 2050 [1].

Table 1.
World Health Organization (WHO) priority pathogens for R&D of new antibiotics (released in 2017) and Centers for Disease Control and Prevention (CDC) antibiotic resistance threats in the United States (released in 2019).
Following the discovery of penicillin in 1928, the Golden Age of antibiotic discovery between the 1940s and the 1960s was led by a systematic survey of microbe-derived antibacterial compounds [3]. During this era, the study discovered numerous antibiotic compounds, such as neomycin and streptomycin produced by soil-dwelling actinomycetes. Most clinically relevant classes of antibiotic compounds were derived from small-molecule natural products, but the excessive use of these compounds resulted in the rapid rise of AMR. Since the 1970s, most antibiotics in clinical trials are derivatives of these antibiotic classes, with a few recent discoveries from bacteria dwelling in the newly-explored environments thanks to the advances in genome mining and pathway analysis [4,5,6]. According to a recent survey, several dozens of small-molecule antimicrobial candidates have been in clinical trials since 2000; however, only five are first-in-class with a new mechanism of action and none with Gram-negative activity [7]. The majority of the WHO list is Gram-negative bacteria (9 out of 12), as they possess an outer membrane that gives resistance to a wide range of antibiotics [8]. Particularly, new antibiotics against the carbapenem-resistant Gram-negative bacteria (e.g., Acinetobacter, Pseudomonas, Enterobacteriaceae) are critical, as carbapenems are often used to treat multidrug-resistant infections. For example, up to 7% of Enterobacteriaceae are now resistant to carbapenem due to the rapid spread of extended-spectrum β-lactamase producing strains, causing high morbidity and mortality worldwide [8,9]. We are currently in urgent need of revolutionary next-generation antibiotics that can shift the paradigm of traditional antibiotics, which are mostly broad-spectrum small molecules against which microbes quickly develop resistance.
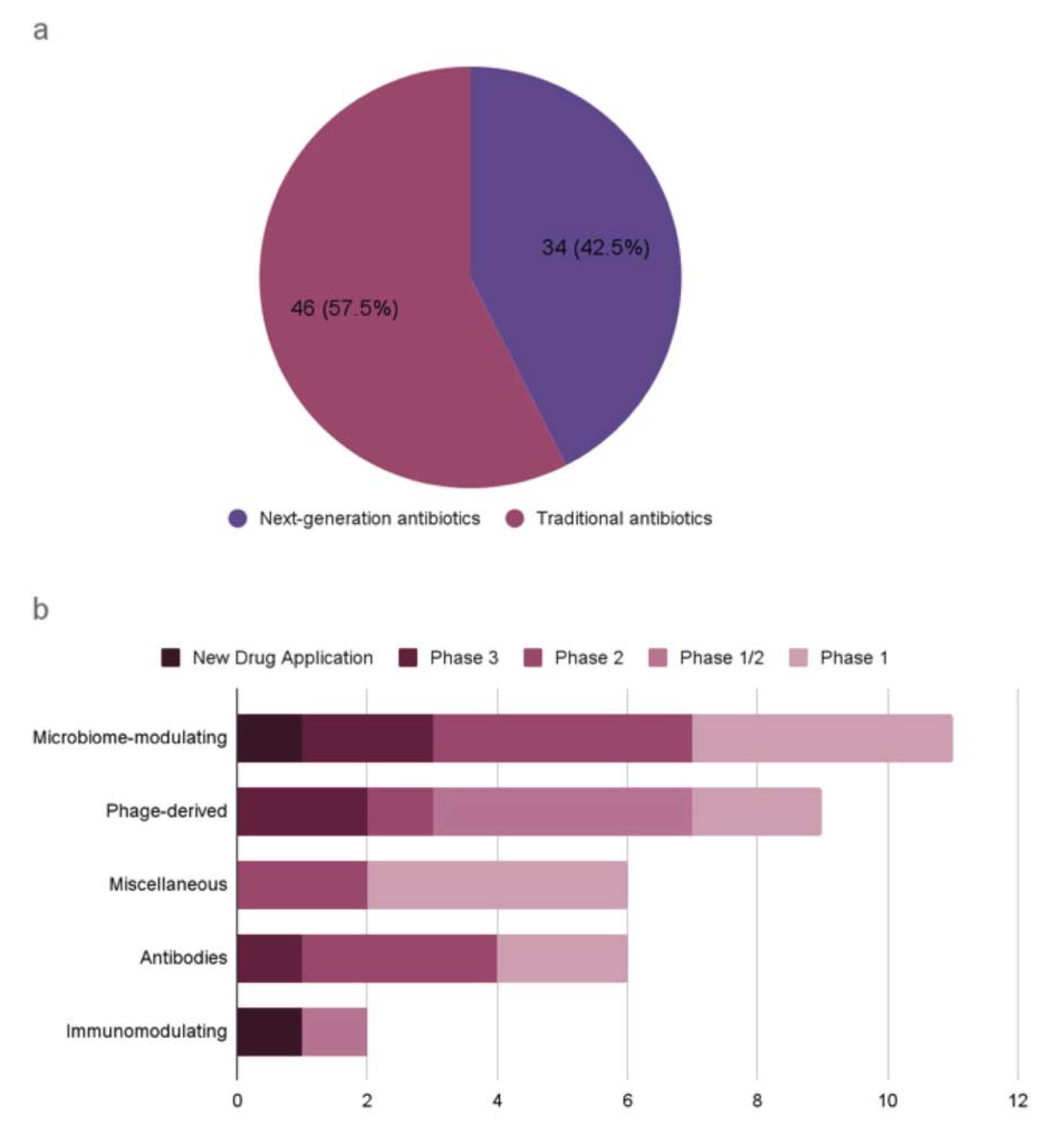
A recent WHO report on the antibacterial agents in preclinical and clinical development defines traditional antibacterials as small molecules that directly inhibit the growth of (bacteriostatic) or kill bacteria (bactericidal) by targeting essential components for bacterial survival [10]. It also defines non-traditional antibacterials as any other approaches for the treatment and prevention of bacterial infections, or preventing the development or spread of drug resistance. This report presents an analysis of antibacterial agents in clinical development worldwide, covering both traditional and non-traditional antibiotics (Figure 1). As of 2021, there are 46 traditional antibiotics and 34 non-traditional antibiotics in clinical development worldwide. For example, an antibody–drug conjugate (ADC) is being developed as alternative antimicrobials, which is an engineered human immunoglobulin G1 (IgG1) designed to cleave in phagocytic cells known as a reservoir for Staphylococcus aureus infections [11]. Other alternatives to traditional antibiotics are also in development, including phage-based therapy and proteins (Table 2). Despite the severity of AMR-related issues, too few antibiotics are currently in research and development to counteract the rapid rise in AMR.
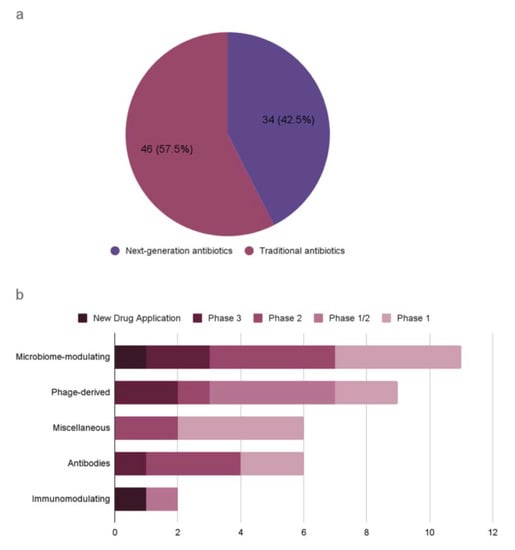
Figure 1.
Antibiotics in clinical development according to the WHO analysis (published in 2022). (a) Traditional antibiotics and next-generation antibiotics. (b) Next-generation antibiotics by antibacterial class and development phase.

Table 2.
Next-generation antibiotics in clinical development according to the WHO analysis (published in 2022). (MAA: market authorization application; NDA: new drug application).
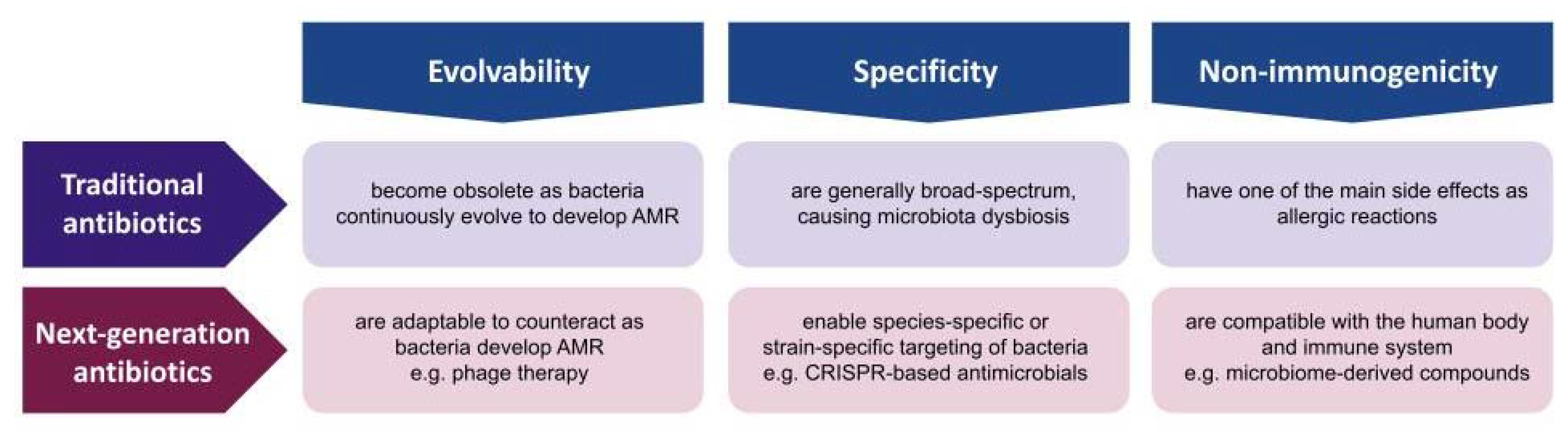
The consensus of the AMR experts is that the golden era of antibiotic discovery has passed, as the continuous and systematic study of microbe-derived small-molecule compounds led to no further discovery despite the advances in genomics, bioinformatics, combinatorial chemistry, and high-throughput screening [12]. Since the discovery of penicillin in 1928, the scientific communities possess a much broader and deeper knowledge of microbes in terms of their genome, evolution, ecosystem, and host–parasite interactions. Given the progress in microbial knowledge and technology, the solution to AMR should not be limited to microbe-derived small molecules. The advantages of small-molecule compounds are considerable, as they are easy to manufacture, store, deliver, and administer [3]. However, these compounds have inherent disadvantages of being non-evolvable, non-specific, and immunogenic; thus, it is essential to develop other types of antibiotics that may not be as convenient but have innovative properties that compensate for the challenges. In this review, we present three innovations that next-generation antibiotics should be differentiated from traditional antibiotics such as evolvability, specificity, and non-immunogenicity (Figure 2). Evolvability enables next-generation antibiotics to be updated as bacteria adapt to counteract or evade these antibacterial agents. Specificity allows these antibacterial agents to have minimal off-target effects on human microbiota. Non-immunogenicity reduces the negative impact on human cells and tissues during antimicrobial treatment. Followingly, we discuss each property in terms of traditional small-molecule antibiotics and non-traditional antimicrobial agents, and present several examples of innovation that could overcome the fundamental issues of traditional antibiotics in combating the current AMR crisis.
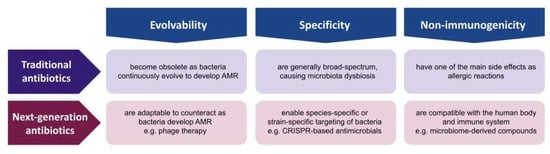
Figure 2.
Traditional antibiotics versus next-generation antibiotics. Comparison in terms of evolvability, specificity, and non-immunogenicity.
2. Evolvability
2.1. Evolution of Antimicrobial Resistance in Bacteria
Microbes are the most abundant and diverse life forms on Earth, being the most ancient root of life that stretches back 4 billion years ago [13]. It is estimated that only 1% of bacterial and archaeal species have been sequenced and cultured, and the rest of the microbial genomes remain unexplored as Microbial Dark Matter [14]. The evolutionary processes of microbes and viruses are distinctive from those of other higher organisms, as they experience high selective pressures and severe population fluctuations that may be amplified if they have within-host and between-host life cycles [15,16,17]. Most conventional antimicrobial compounds are derived from bioactive natural molecules, resulting from the interaction of diverse organisms to survive and thrive in nature [18]. Microbes are prolific producers of bioactive natural molecules, particularly soil-dwelling bacteria that make antimicrobial compounds to compete with other microbes or to use as signaling molecules with close relatives or eukaryotic hosts such as plants and insects [19]. Thus, antimicrobial resistance is ancient, and the emergence of drug resistance to these antibiotic compounds is intrinsic to the evolutionary processes of complex ecological interactions [20,21].
The modes of action of most antibiotics can be categorized into five major classes: cell wall, protein synthesis, DNA synthesis, RNA synthesis, and metabolic pathway inhibitors (Table 3) [22]. Resistance to one antibiotic class can result from multiple biochemical pathways, and bacteria are capable of using a combination of resistance mechanisms to escape the effect of an antibiotic. For instance, resistance to fluoroquinolone that blocks DNA synthesis may develop from mutations in genes encoding DNA gyrase and topoisomerase IV, over-expression of efflux pumps, or protection of the protein target sites by another protein (named Qnr) [18]. Due to the difference in the cell envelope, Gram-positive bacteria and Gram-negative bacteria may differ in the predominant mechanism of resistance. For instance, β-lactam is a major class of antibiotics that inhibit cell wall synthesis, and Gram-positive bacteria mainly modify the penicillin-binding proteins, while Gram-negative bacteria produce β-lactamases, as their outer membrane can control the access of these antibiotics to the periplasmic space [18]. In overall, the biochemical routes conferring antibiotic resistance can be classified into modifying the antibiotic molecule, preventing access to the target site, changing the target site, and adjusting global cell adaptive processes. Bacteria also possess phenotypic resistance, which is non-genetically encoded and non-inheritable resistance to antibiotics through processes such as persistence, biofilms, swarming, and metabolic dormancy [23].

Table 3.
Mechanism of action and sensitivity against Gram-negative bacteria of each antibiotic group.
2.2. Phage Therapy
Bacteriophages (phages) are viruses that infect and replicate in bacteria, which are the most abundant biological agent on Earth [24]. Lytic or virulent phages infect and kill their bacteria hosts (lytic cycle), whereas lysogenic or temperate phages either integrate into their host’s genome (lysogenic cycle) or enter the lytic cycle. As phages are natural killers of bacteria in their lytic cycle, the administration of virulent phages was experimented on early in the 20th century to treat a number of bacterial infections such as cholera, dysentery, bubonic plague, conjunctivitis, and skin infections [25]. The discovery of penicillin in 1929 diminished scientific interest and investment in phage therapy, as a string of cheap and effective antibiotics were introduced to treat bacterial infections. However, phage therapy was steadily developed in places such as Georgia and Poland, which documented extensive and successful cases of phage therapy to treat multiple bacterial infections [26]. Less than a century after the discovery of penicillin, excessive use of antibiotics has resulted in the uncontrolled spread of superbugs, and the lack of new antibiotic discovery renewed therapeutic interest in the potential of phage therapy.
This renewed interest in phage therapy has driven the scientific communities to investigate and standardize various aspects of phage therapy. The minimum regulations for the therapeutic use of phages require strictly lytic phages with antimicrobial activity against the target bacteria and the removal of toxic bacterial debris [27]. Among the standardization, class phage therapy identifies and isolates naturally occurring phages, which are screened for host ranges amid pathogenic bacterial strains, and evaluated with in vitro or in vivo tests. The primary phage of interest is Caudovirales, which are the most numerous and diverse phages in the biosphere. They have a linear double-stranded genome of 15 to 500 kb, which make specific contacts to the surface receptors of their bacterial host using the tail, tail fibers, or both. Once the phage genome is injected into the host cell, they typically undergo a lytic cycle, which results in replications of hundreds of progeny virions. In the recent clinical setting, phage therapy was focused on the clinical product development against bacterial pathogens such as Staphylococcus aureus, Pseudomonas aeruginosa, and Clostridium difficile, which are difficult to treat with conventional antibiotic therapy. For instance, Phagoburn was the world-first phage therapy clinical trial using phage cocktails for the treatment of Escherichia coli and Pseudomonas aeruginosa burn wound infections, which achieved significant advancements in the regulatory framework of phage therapy [28]. Several companies have already commercialized phage products for controlling food-borne pathogens such as Escherichia coli O157:H7 and Listeria monocytogenes, thanks to the genetic homogeneity of these bacteria and the lower regulatory barriers for food production and processing [29].
Alternative antibiotics still face significant challenges; phage therapy has safety concerns of self-replicating bacteriophages in patients [30], and bacteriophage-derived agents have delivery issues to different organs given the harsh in vivo environments (e.g., low pH, cell barriers, proteases) [31]. Despite these challenges, the natural antimicrobial activities of bacteriophages are gaining attention as viable alternatives [32]; phage therapy is actively being tested in clinical trials (Table 2). Several phage-encoded endolysins, which lyse the bacterial peptidoglycan layer, are in clinical development against Gram-positive bacteria [31]. However, no phage-based antimicrobial agents have been approved yet, due to regulatory and logistical hurdles [33]. Currently, no bacteriophage-based therapeutics have passed FDA approval for clinical use, except in emergency or experimental cases [34].
2.3. Evolvability of a Bacteriophage-Based Therapy
Unlike chemical-based traditional antibiotics, bacteriophages are biological entities that are self-replicating and evolving under changing environments. This characteristic is both an advantage and disadvantage to controlling bacterial populations. This paradoxical relation also stands in natural environments where diminishing bacterial populations due to highly successful infections of lytic phages will eventually diminish the chance of their own replication too. During the host–parasite interaction, bacteria can develop resistance to phage infections, equivalent to the case of antibiotics. The difference is, however, phages also evolve to counteract the defense systems of bacteria, whose evolution can be directed and accelerated through genetic engineering to outpace the bacterial resistance and even enhance their replication and lytic activities. Previously, phages were engineered to add or improve function as natural predators of bacteria, such as an engineered enzymatic bacteriophage incorporated with a gene that degrades a polysaccharide adhesin in biofilm formation [35].
Bacteria have various defense mechanisms against these phages, such as restriction-modification systems that protect host DNA with modification and destroy foreign DNA with restriction enzymes [36], and CRISPR-Cas systems that specifically degrade previously encountered foreign genetic elements through RNA templates [37]. However, phages also have several arsenals to counteract these bacterial defense systems. For instance, recent studies revealed that phages have small proteins that have anti-CRISPR activities by inhibiting CRISPR-Cas systems via direct interference [38,39] or enzymatic activity [40,41]. Bacterial populations may develop a collective strategy to mitigate phage infection, such as a newly discovered system named cyclic oligonucleotide-based anti-phage signaling system (CBASS) that uses small signaling molecules to activate cell death released upon phage infection [42]. Such diverse bacterial defense strategies may result in unexpected results such as the depletion of phage replications during phage therapy. Furthermore, bacteria may adapt other phenotypic and genotypic changes such as decreased phage absorption due to the intense selective pressure imposed by phages [43], which may require other treatment strategies such as the use of phage cocktails and phage engineering [44].
Phages have been known to be highly specific for their hosts, which enables the targeting of pathogenic bacteria at the strain level without disturbing microbiomes in the body [45]. The inevitable off-target effects from conventional antibiotic therapy are known to cause severe disruptions in the microbiomes of the human body (see below for details). However, there is recent evidence that phages can also jump hosts, and this adaptation of phage-host specificity may lead to unexpected loss or gain in specificity [46]. The host receptor should be identified for any phage proposed for therapeutic use, to minimize off-target events and also to assemble combinations of phages that are less likely to generate resistant hosts with a single defective receptor. Furthermore, the use of lysogenic phages in phage therapy should be prohibited, as they can carry antimicrobial or virulence genes that alter the pathogenic potential of their hosts [47]. As shown above, the evolvability of bacteriophage-based therapy gives heterogeneity and plasticity in counteracting highly adaptive bacteria. On the other hand, it brings unpredictability and instability to the antimicrobial treatment, which will require constant monitoring and evaluation to minimize any potential risk.
3. Specificity
3.1. Broad-Spectrum and Narrow-Spectrum Small-Molecule Antibiotics
Small-molecule antibiotics have variable ranges of microorganisms they can inhibit. Based on the spectrum of antimicrobial activity, they are classified as broad-spectrum antibiotics that can target a wide range of bacteria or narrow-spectrum antibiotics that can target limited species of bacteria. Extended-spectrum antibiotics can target Gram-positive bacteria but only some Gram-negative bacteria (Table 3). Generally, broad-spectrum antibiotics have higher chances of developing antimicrobial resistance, as the selective pressure for resistance is applied on both pathogenic and non-pathogenic bacteria. During this process, non-pathogenic commensal bacteria in microbiomes become a persistent reservoir for antimicrobial resistance genes that can be transferred to pathogenic bacteria [48]. Broad-spectrum antibiotics not only promote the emergence of multidrug-resistant bacteria and cause dysbiosis in the microbiome from off-target effects, but they also have more side effects such as diarrhea or rash [49]. Thus, antibiotic stewardship generally recommends identifying the specific pathogen and facilitating the use of narrow-spectrum antibiotics over broad-spectrum antibiotics whenever possible, although broad-spectrum antibiotics tend to have more clinical indications.
The development of narrow-spectrum antimicrobial agents that are genus or species-specific is one of the strategies to tackle antimicrobial resistance. Narrow-spectrum antibiotics are less likely to induce antimicrobial resistance and disrupt the human microbiome [48]. This is an important advantage as the effects of antibiotic exposure as short as seven-day have been shown to alter the gut microbiota over two years post-treatment [50]. A recent study demonstrates that repeated use of antibiotics might permanently change the size and composition of gut microbiomes [51]. Particularly, it has been observed that exposure to broad-spectrum antibiotics during early childhood disrupts the diversity and stability of the infant microbiota, which can also be disruptive to the development of the infant immune system [52]. As microbiomes play vital roles in human physiology, such as protection from pathogens and metabolite production, microbiome dysbiosis leads to disruptions to human health (see below for details). Although broad-spectrum antibiotics are essential for life-threatening infections such as sepsis or pneumonia, better identification of the causative pathogen allows switching to narrow-spectrum antibiotics that can reduce both the antimicrobial resistance and the microbiome disruption in non-life-threatening infections such as urinary tract infections and abscesses.
3.2. Specificity of CRISPR-Based Antimicrobials
CRISPR-Cas systems are microbial immune systems first discovered in bacteria, which consist of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) arrays and CRISPR-associated system (Cas) proteins [37,53]. CRISPR arrays are a remarkable component of CRISPR-Cas systems that enables RNA-mediated adaptive immunity by encoding genetic information about previous invaders such as phages or plasmids. This genetic component makes CRISPR-Cas systems programmable and specific by altering the target information, and they have been successfully adapted as genome-editing tools thanks to this characteristic [54,55]. There are two main classes and several types of CRISPR-Cas systems depending on the architecture of Cas proteins, and the diversity of these prokaryotic immune systems has been expanding as more uncultured microbes from diverse environments are being discovered [56].
A number of CRISPR-Cas systems have been recently investigated as alternative antibiotics by reprogramming them to target bacterial DNA/RNA [57,58]. In a landmark study, the prominent genome-editing tool, CRISPR-Cas9 systems, was repurposed to target multidrug-resistant bacteria [59]. They used bacteriophages and bacterial plasmids to deliver CRISPR-Cas systems encoding virulence and antimicrobial resistance templates in carbapenem-resistant Enterobacteriaceae, which significantly increased the survival rate of the worm infection model. In another pioneering study, the CRISPR-Cas9 systems delivered by a plasmid packaged in phage capsids called phagemids were reported to selectively kill virulent strains of Staphylococcus aureus [60]. These sequence-specific antimicrobials were validated also in a murine skin infection model. In another study, another type of DNA-modifying CRISPR-Cas system was used to target antibiotic-resistant bacteria by destroying their antibiotic-resistance-conferring plasmids with temperate and lytic phages as delivery vectors [61].
More recently, the potential of type VI CRISPR-Cas systems as antimicrobial tools is gaining attention because these proteins cleave targeted transcripts of invading RNA viruses. Triggered by the target RNA cleavage, type VI CRISPR-Cas systems carry out non-specific RNase activity, resulting in cleaving transcripts of the bacterial genome itself. This activity eventually leads to the dormancy of the bacterial host cell, which diminishes the phage population by disabling these phages from multiplying further into other bacterial cells. Some recent studies took advantage of this outcome to trigger bacterial cells to enter cell arrest when CRISPR-Cas systems detect the expression of antimicrobial resistance genes [62]. This strategy has advantages over other DNA-modifying CRISPR-Cas systems, as there is no need to consider the potential interference of extensive DNA repair systems in bacteria [63].
While CRISPR-Cas systems were initially applied as genome-editing tools, their specificity and programmability are also highly attractive traits as alternative antibiotics that traditional antibiotics do not possess. If we could successfully repurpose CRISPR-Cas systems as antibiotics, they can be programmed to be specific to pathogenic bacteria carrying antimicrobial resistance genes instead of disturbing the whole microbiota in the human body. Currently, no CRISPR-Cas-based therapeutics have passed FDA approval for clinical use, but there is one clinical development of non-traditional antibiotics involving CRISPR-Cas3 enhanced phages (Table 2). Since CRISPR-Cas antimicrobials are to be used against bacteria that have their own CRISPR-Cas systems, their functioning within bacteria should be investigated further to prevent unexpected events from indigenous CRISPR-Cas and other genomic systems.
3.3. Diagnostic Tests for Pathogen Identification
One of the main challenges to the utility of next-generation antibiotics is the requirement for highly specific diagnosis of bacterial pathogens. To achieve specific targeting of pathogens causing infections, rapid pathogen identification with high accuracy and sensitivity is vital. Due to the availability of broad-spectrum antibiotics, most bacterial infections have been treated without the need for diagnostic tests. Such empirical antibiotic therapy exacerbated the emergence and spread of antimicrobial resistance, which necessitates a shift towards directed antibiotic therapy along with the progress of diagnostic clinical microbiology.
Culture-based diagnosis in clinical microbiology is dependent on the growth of bacteria and has largely been unchanged for 100 years. Culture-based diagnostic processes of bacterial infections take several days, from initial cultures (~24 h) to pathogen identification and antimicrobial susceptibility testing (~24 h) [64]. This diagnostic method is also prone to false-negative results, particularly if samples are obtained during antimicrobial therapy. Other techniques such as Gram-staining microscopy and ELISA for detecting bacterial antigens or antibodies are less time-consuming, but they cannot determine antimicrobial susceptibility [65].
In the recent clinical diagnostic setting, the introduction of nucleic acid-based amplification technologies (NAATs) and MALDI-TOF mass spectrometry fingerprinting have modernized pathogen identification [64]. NAAT-based approaches include polymerase chain reaction (PCR) and next-generation sequencing (NGS), which accelerates pathogen detection to within 3–6 h [64]. PCR-based techniques have higher sensitivity than culture-based approaches, as demonstrated in cases when antimicrobial treatment is ongoing or only small sample volumes are available (e.g., bloodstream infection) [66]. However, PCR-based techniques can lead to false positives due to the presence of genetic materials after the pathogen has been neutralized, and false negatives due to the emergence of mutations or loss of the gene during antibiotic treatment. NGS has similar limitations, but it exhibits increased accuracy with the potential to detect antimicrobial resistance genes and virulence markers. MALDI-TOF MS fingerprinting uses direct colony testing on the MALDI plate to compare the generated spectrum against a reference spectrum for bacterial pathogen identification [67]. This method is rapid, accurate, and inexpensive, but only clinical samples with high numbers of bacteria, such as urine and cerebrospinal fluid, allow direct testing, and organisms with similar spectral profiles, such as E. coli and Shigella species, cannot be differentiated accurately [67]. Other approaches include fluorescent in situ hybridization (FISH) and electrochemical biosensor assays by species-specific probes for the bacterial ribosomal RNA target, and rapid antigen testing by a visible readout upon antibody-antigen binding [48].
4. Non-Immunogenicity
4.1. Effects of Antibiotics on the Immune System
According to the Centers for Disease Control and Prevention (CDC), the most common side effects of antibiotics involve the digestive system and the immune system. Due to the detrimental effect on microbiota homeostasis, antibiotics can cause nausea, diarrhea, and indigestion. The negative effects of antibiotics also include allergic reactions such as rash, coughing, wheezing, and breathing difficulties. In rare cases, antibiotics can cause a medical emergency such as anaphylaxis, which is a severe and life-threatening allergic reaction. Most emergency department visits related to antibiotic side effects are due to severe allergic reactions.
Infants are vulnerable to bacterial infections, especially when born preterm and/or underweight, and are often subjected to prophylactic or therapeutic antibiotic treatments [68]. It is estimated that around 40% of pregnant mothers and newborns receive antibiotics globally [69]. In fact, empiric antibiotic treatment is a common practice during pregnancy and birth, which leads to the inappropriate use of antibiotics, particularly in developing countries. In infants, the use of antibiotics has been found to cause more long-lasting negative effects on the immune system, which is not fully established and functional. Antibiotic therapy during infancy is linked to a higher risk of infections later in life, as shown in the studies that found associations between prolonged exposure to antibiotics with an increased susceptibility to diarrhea and respiratory tract infections [68].
Using the animal models, some studies demonstrated that antibiotic exposures during infancy negatively impact innate immune cells, such as dendritic cells (DCs), natural killer (NK) cells, and innate lymphoid cells. For instance, the infant mice born from the antibiotic-treated mothers after being infected with the vaccinia virus had a reduced number of splenic DCs, which are the most potent antigen-presenting cells, compared to the control mice [70]. Similarly, NK cells of the antibiotic-exposed mouse infants exhibited remarkable reductions in terms of frequency and phenotypic expression following the vaccinia virus infection. In terms of adaptive immunity, the mouse infants exposed to antibiotics in early life had their antibody-mediated responses impaired to the majority of vaccines, such as protection against tuberculosis, meningitis, and pneumococcal disease, compared to the control mouse infants [71]. Thus, these studies indicate that antibiotic exposure during early life could cause long-lasting impairments both in innate immunity and adaptive immunity.
4.2. Human Microbiome
A microbiome is a collection of cells, genes, and metabolites from the microbiota comprising bacteria, viruses, and eukaryotes within the human body. The high-throughput technological advances in sequencing and data processing have allowed the scientific community to establish a baseline of healthy microbiome compositions, to which microbiome compositions from patients with various diseases can be compared. A healthy microbiome profile is generalizable across human populations consisting of the commensal and beneficial microbiota [72]. The human microbiome is tightly involved in human health; particularly the human gut is inhabited by trillions of microbes influencing host physiology and susceptibility to diseases, including malnutrition [73], obesity [74], inflammatory bowel disease [75], neurological disorders [76] and even cancer [77]. In addition to the gut microbiome, the complex oral microbiome also plays a key role in maintaining both oral health and systemic health, and its dysbiosis has been linked to a vast array of health issues, including respiratory, cardiovascular, and cerebrovascular diseases [78].
The use of antibiotics causes dysbiosis, which is a disruption to the microbiome from an imbalance in microbiota, activities, or distributions [45], particularly during infancy and early childhood. For instance, antibiotic drugs decrease the overall diversity and increase the colonization of drug-resistant pathogens of gut microbiota in infants [79]. Several studies have linked the repeated use of antibiotics such as penicillins, macrolides, quinolones, and cephalosporins in early childhood to long-term health issues such as an increased risk of developing obesity [80] and type 2 diabetes [81]. Even in adulthood, antibiotic use has been shown to transiently or permanently affect the diversity and health of human microbiota by depleting several commensal and beneficial taxa such as lactobacilli and bifidobacteria [45]. Furthermore, antibiotics select for resistance in the gut microbiota by stimulating the expression of antibiotic resistance, stress response, and virulent phage genes [82]. There is also evidence that antibiotics can cause immunological disorders by negatively impacting the interaction between the microbiome and immune system [83] and perturbing the host proteome [84].
4.3. Non-Immunogenicity of Microbiome-Derived Antibiotics
Microbes inhabiting the same environmental niches within human microbiomes develop various strategies to gain advantages over other microbes. The human microbiota is known to produce a diverse spectrum of metabolites, such as lipids, oligosaccharides, amino acids, non-ribosomal peptides, and ribosomal peptides, specific for interacting within the human microbiota and human hosts [85]. These metabolites serve a variety of purposes, including antimicrobial, cytotoxic, immunomodulatory, and antioxidant functions. The human microbiome has revealed several natural products with antimicrobial properties across the bacterial phyla, such as the vaginal isolate lactocillin and the nasal isolate lugdunin [86,87].
The human gut is a particularly dense environment where trillions of bacteria, archaea, eukarya, and viruses coexist and coevolve, and this competition has led to various strategies to outcompete others, including the development of specialized antimicrobials. In the gut microbiome, some bacteria use direct antagonistic strategies against their neighbors, such as removing essential substrates, reducing oxidation-reduction potential, and accumulating D-amino acid [85]. More indirect strategies involve the production of metabolic compounds that limit the growth of surrounding bacteria. For example, some bacteria produce hydrogen peroxide, which is a non-specific regulatory agent with antimicrobial activities through oxidizing effects on bacterial molecular structures [88]. However, due to the non-specific activity and associated side effects such as the acidification of the environment, most of these bacterial compounds with antimicrobial activities are unsuitable for clinical applications.
Bacteria also produce antimicrobial peptides consisting of 10–50 amino acids that are target-specific. The ability of these peptides to neutralize bacteria depends on their affinities to bacterial membranes and cell walls [85]. The first category of microbiome-derived antimicrobials is non-ribosomal peptides (NRP), which are secondary metabolite peptides synthesized by multifunctional peptide synthetases. Several microbe-derived antibacterial compounds, including penicillin, vancomycin, and polymyxin, are considered non-ribosomal peptides. However, most activities of microbiome-derived NRPs are known to be cytotoxic, and only a few NRPs have been characterized from the human microbiota [89].
The second category of microbiome-derived antimicrobials is ribosomally synthesized peptides that were first discovered in 1925 and referred to as bacteriocins [85]. Generally, bacteriocins produced by Gram-positive bacteria work better against Gram-positive pathogens and Gram-negative bacteriocins against Gram-negative pathogens. Bacteriocins are heterogeneous in primary structure, molecular weight, mode of action, and heat stability, and the most current classification is based on their structure [90]. Bacteriocins have low toxicity in human cells with broad-spectrum or narrow-spectrum antimicrobial activities against bacterial cells. Another advantage of bacteriocins as antimicrobial compounds is that bacteria cannot easily develop resistance against them, as these pathogens have to alter their membrane or receptor compositions. Recently, antimicrobial peptides from the rumen microbiome exhibited therapeutic potential against seven clinical strains of Pseudomonas aeruginosa with minimal cytotoxicity against human lung cells [91]. These antimicrobial peptides increased catalytic activities at the target bacterial cell membrane and promoted the β-oxidation of fatty acids. This study illustrates the therapeutic potential of microbiome-derived peptides against bacterial infections.
Recent evidence reveals that diverse and numerous bacteriophages coexist in the human body without causing immunogenic reactions [92,93]. Some bacteriophages produce lytic enzymes that can kill bacteria, and the use of the phages derived from the human gut has been proposed as a novel therapeutic to modulate gut composition [94,95]. Phages are inherently harmless to eukaryotic cells, but they can cause immunological reactions due to the bacterial lysates and endotoxins resulting from the phage lytic cycles. Microbiome-derived bacteriophages are largely unexplored, and the uncharted repertoire of bacteriophages is a rich resource for genome mining of next-generation antibiotics [96]. Two phage-derived peptides are potential antibacterial therapeutics: lysins and tailocins. Lysins are muralytic enzymes that are used both at the early stage of infection to penetrate the DNA through the host cell envelope and at the lysis stage of infection to release the progeny virions [97,98,99]. These enzymes are effective against Gram-positive bacteria with high genus-level specificity. Tailocins are phage tail-like bacteriocins that cause lethal damage to the host cell envelope upon absorption into a bacterial surface receptor [100,101]. Tailocins are inherently devoid of genetic materials and can be engineered to target heterologous hosts that can be administered at a defined dose. Currently, the microbiome-modulating category has the highest number of non-traditional antimicrobial agents in clinical development, with one agent at the most advanced stage of new drug application (Figure 1).
5. Conclusions
The research and development of next-generation antibiotics are affected by profitability challenges, as the market size of a drug is proportional to the prevalence of the disease. However, the evolvability and specificity of next-generation antibiotics may compensate for the lower prevalence from specific pathogen targeting by lengthening the viability of antimicrobials with reduced rates of resistance. If the innovations of next-generation antibiotics can overcome the challenges of traditional antibiotics and are more effective in reducing mortality, morbidity, and length of hospitalization, the case for a higher price may also be made in high-income countries.
To prepare for the imminent post-antibiotic era, a shift in medical culture and education from empirical antibiotic therapy to directed antibiotic therapy is necessary. Furthermore, a shift in the pharmaceutical industry to invest in innovative next-generation antibiotics rather than broad-spectrum small-molecule antibiotics is essential. Next-generation antibiotics still have technological limitations and regulatory hurdles to overcome. However, they represent another scientific asset that will progress modern medicine by expanding the repertoire of antibiotics from being exclusively chemotherapeutic small molecules to a diversified range of tools and agents to control antimicrobial resistance that humanity will continue to face. In closing, further research to explore novel microbes and microbial communities is essential to inspire nature-derived antimicrobial agents to be repurposed as next-generation antibiotics.
Funding
This research was funded by Ghent University Global Campus (GUGC), Incheon, Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author thanks the members of the Center for Biotech Data Science at GUGC for their constant encouragement, support, and motivation.
Conflicts of Interest
The author declares no conflict of interest.
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Lastinger, L.M.; Alvarez, C.R.; Kofman, A.; Konnor, R.Y.; Kuhar, D.T.; Nkwata, A.; Patel, P.R.; Pattabiraman, V.; Xu, S.Y.; Dudeck, M.A. Continued increases in the incidence of healthcare-associated infection (HAI) during the second year of the coronavirus disease 2019 (COVID-19) pandemic. Infect. Control. Hosp. Epidemiology 2022, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Vila-Farres, X.; Inoyama, D.; Ternei, M.; Cohen, L.J.; Gordon, E.A.; Reddy, B.V.B.; Charlop-Powers, Z.; Zebroski, H.A.; Gallardo-Macias, R.; et al. Discovery of MRSA active antibiotics using primary sequence from the human microbiome. Nat. Chem. Biol. 2016, 12, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Munnoch, J.T.; Devine, R.; Holmes, N.A.; Seipke, R.F.; Wilkinson, K.A.; Wilkinson, B.; Hutchings, M.I. Formicamycins, antibacterial polyketides produced by Streptomyces formicae isolated from African Tetraponera plant-ants. Chem. Sci. 2017, 8, 3218–3227. [Google Scholar] [CrossRef]
- Saha, S.; Zhang, W.; Zhang, G.; Zhu, Y.; Chen, Y.; Liu, W.; Yuan, C.; Zhang, Q.; Zhang, H.; Zhang, L.; et al. Activation and characterization of a cryptic gene cluster reveals a cyclization cascade for polycyclic tetramate macrolactams. Chem. Sci. 2017, 8, 1607–1612. [Google Scholar] [CrossRef]
- Butler, M.S.; Paterson, D.L. Antibiotics in the clinical pipeline in October 2019. J. Antibiot. 2020, 73, 329–364. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Lepore, C.; Silver, L.; Theuretzbacher, U.; Thomas, J.; Visi, D. The small-molecule antibiotics pipeline: 2014–2018. Nat. Rev. Drug Discov. 2019, 18, 739–740. [Google Scholar] [CrossRef]
- World Health Organization. Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; World Health Organization: Geneva, Switzerland, 2021.
- Rymut, S.M.; Deng, R.; Owen, R.; Saad, O.; Berhanu, A.; Lim, J.; Carrasco-Triguero, M.; Couch, J.; Peck, M. 1305. Comparison of Pharmacokinetics of DSTA4637S, a novel THIOMABTM Antibody-Antibiotic Conjugate, in Patients with Staphylococcus aureus Bacteremia Receiving Standard-of-Care Antibiotics with Pharmacokinetics in Healthy Volunteers. Open Forum. Infect. Dis. 2020, 7, S666–S667. [Google Scholar] [CrossRef]
- Donadio, S.; Maffioli, S.; Monciardini, P.; Sosio, M.; Jabes, D. Antibiotic discovery in the twenty-first century: Current trends and future perspectives. J. Antibiot. 2010, 63, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Woese, C.R.; Fox, G.E. Phylogenetic structure of the prokaryotic domain: The primary kingdoms. Proc. Natl. Acad. Sci. USA 1977, 74, 5088–5090. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.-Y.; Liu, L.; Hua, Z.-S.; Fang, B.-Z.; Zhou, E.-M.; Salam, N.; Hedlund, B.; Li, W.-J. Microbial dark matter coming to light: Challenges and opportunities. Natl. Sci. Rev. 2021, 8, nwaa280. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Wolf, Y.I. Evolution of microbes and viruses: A paradigm shift in evolutionary biology? Front. Cell Infect. Microbiol. 2012, 2, 119. [Google Scholar] [CrossRef]
- Shim, H. Feature Learning of Virus Genome Evolution With the Nucleotide Skip-Gram Neural Network. Evol. Bioinform. 2019, 15, 1176934318821072. [Google Scholar] [CrossRef]
- Shim, H.; Laurent, S.; Matuszewski, S.; Foll, M.; Jensen, J.D. Detecting and Quantifying Changing Selection Intensities from Time-Sampled Polymorphism Data. G3 2016, 6, 893–904. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 464–473. [Google Scholar] [CrossRef]
- Traxler, M.F.; Kolter, R. Natural products in soil microbe interactions and evolution. Nat. Prod. Rep. 2015, 32, 956–970. [Google Scholar] [CrossRef]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef]
- Thomas, C.M.; Nielsen, K.M. Mechanisms of, and Barriers to, Horizontal Gene Transfer between Bacteria. Nat. Rev. Microbiol. 2005, 3, 711–721. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482. [Google Scholar] [CrossRef] [PubMed]
- Olivares, J.; Ebernardini, A.; Egarcia-Leon, G.; Ecorona, F.; Esanchez, M.B.; Martinez, J.L. The intrinsic resistome of bacterial pathogens. Front. Microbiol. 2013, 4, 103. [Google Scholar] [CrossRef] [PubMed]
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, F.L.G.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019, 32, e00066-18. [Google Scholar] [CrossRef]
- Chanishvili, N. Phage Therapy—History from Twort and d’Herelle through soviet experience to current approaches. Adv. Virus Res. 2012, 83, 3–40. [Google Scholar]
- Young, R.; Gill, J.J. MICROBIOLOGY. Phage therapy redux--What is to be done? Science 2015, 350, 1163–1164. [Google Scholar] [CrossRef]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.-A.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage Applications for Food Production and Processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef]
- Peng, H.; Borg, R.E.; Dow, L.P.; Pruitt, B.L.; Chen, I.A. Controlled phage therapy by photothermal ablation of specific bacterial species using gold nanorods targeted by chimeric phages. Proc. Natl. Acad. Sci. USA 2020, 117, 1951–1961. [Google Scholar] [CrossRef]
- Murray, E.; Draper, L.A.; Ross, R.P.; Hill, C. The Advantages and Challenges of Using Endolysins in a Clinical Setting. Viruses 2021, 13, 680. [Google Scholar] [CrossRef]
- Harada, L.K.; Silva, E.C.; Campos, W.F.; Del Fiol, F.S.; Vila, M.; Dąbrowska, K.; Krylov, V.N.; Balcão, V.M. Biotechnological applications of bacteriophages: State of the art. Microbiol. Res. 2018, 212–213, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, K.; Gerstmans, H.; Saafan, A.; Dishisha, T.; Briers, Y. The Preclinical and Clinical Progress of Bacteriophages and Their Lytic Enzymes: The Parts are Easier than the Whole. Viruses 2019, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten, K.M.C.; Campbell, J.L.; et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob. Agents Chemother. 2022, 66, e0207121. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.K.; Collins, J.J. Dispersing biofilms with engineered enzymatic bacteriophage. Proc. Natl. Acad. Sci. USA 2007, 104, 11197–11202. [Google Scholar] [CrossRef]
- Luria, S.E.; Human, M.L. A nonhereditary, host-induced variation of bacterial viruses. J. Bacteriol. 1952, 64, 557–569. [Google Scholar] [CrossRef]
- Mojica, F.J.M.; Díez-Villaseñor, C.; García-Martínez, J.; Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 2005, 60, 174–182. [Google Scholar] [CrossRef]
- Bondy-Denomy, J.; Pawluk, A.; Maxwell, K.L.; Davidson, A.R. Bacteriophage genes that inactivate the CRISPR/Cas bacterial immune system. Nature 2013, 493, 429–432. [Google Scholar] [CrossRef]
- Park, H.-M.; Park, Y.; Vankerschaver, J.; Van Messem, A.; De Neve, W.; Shim, H. Rethinking Protein Drug Design with Highly Accurate Structure Prediction of Anti-CRISPR Proteins. Pharmaceuticals 2022, 15, 310. [Google Scholar] [CrossRef]
- Dong, L.; Guan, X.; Li, N.; Zhang, F.; Zhu, Y.; Ren, K.; Yu, L.; Zhou, F.; Han, Z.; Gao, N.; et al. An anti-CRISPR protein disables type V Cas12a by acetylation. Nat. Struct. Mol. Biol. 2019, 26, 308–314. [Google Scholar] [CrossRef]
- Knott, G.J.; Thornton, B.W.; Lobba, M.J.; Liu, J.-J.; Al-Shayeb, B.; Watters, K.E.; Doudna, J.A. Broad-spectrum enzymatic inhibition of CRISPR-Cas12a. Nat. Struct. Mol. Biol. 2019, 26, 315–321. [Google Scholar] [CrossRef]
- Cohen, D.; Melamed, S.; Millman, A.; Shulman, G.; Oppenheimer-Shaanan, Y.; Kacen, A.; Doron, S.; Amitai, G.; Sorek, R. Cyclic GMP-AMP signalling protects bacteria against viral infection. Nature 2019, 574, 691–695. [Google Scholar] [CrossRef]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef]
- Lu, T.K.; Koeris, M.S. The next generation of bacteriophage therapy. Curr. Opin. Microbiol. 2011, 14, 524–531. [Google Scholar] [CrossRef]
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef]
- De Sordi, L.; Khanna, V.; Debarbieux, L. The Gut Microbiota Facilitates Drifts in the Genetic Diversity and Infectivity of Bacterial Viruses. Cell Host Microbe 2017, 22, 801–808.e3. [Google Scholar] [CrossRef]
- Citorik, R.J.; Mimee, M.; Lu, T.K. Bacteriophage-based synthetic biology for the study of infectious diseases. Curr. Opin. Microbiol. 2014, 19, 59–69. [Google Scholar] [CrossRef]
- Melander, R.J.; Zurawski, D.V.; Melander, C. Narrow-spectrum antibacterial agents. MedChemComm 2018, 9, 12–21. [Google Scholar] [CrossRef]
- Gerber, J.; Ross, R.; Bryan, M.; Localio, A.R.; Szymczak, J.; Fiks, A.; Barkman, D.; Odeniyi, F.; Conaboy, K.; Bell, L.; et al. Comparing Broad- and Narrow-Spectrum Antibiotics for Children with Ear, Sinus, and Throat Infections; Patient-Centered Outcomes Research Institute (PCORI): Washington, DC, USA, 2022. [Google Scholar]
- Jernberg, C.; Löfmark, S.; Edlund, C.; Jansson, J.K. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007, 1, 56–66. [Google Scholar] [CrossRef]
- Raymann, K.; Shaffer, Z.; Moran, N.A. Antibiotic exposure perturbs the gut microbiota and elevates mortality in honeybees. PLoS Biol. 2017, 15, e2001861. [Google Scholar] [CrossRef]
- Schulfer, A.; Blaser, M.J. Risks of Antibiotic Exposures Early in Life on the Developing Microbiome. PLoS Pathog. 2015, 11, e1004903. [Google Scholar] [CrossRef]
- Makarova, K.S.; Grishin, N.V.; Shabalina, S.A.; Wolf, Y.I.; Koonin, E.V. A putative RNA-interference-based immune system in prokaryotes: Computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biol. Direct. 2006, 1, 7. [Google Scholar] [CrossRef]
- Deltcheva, E.; Chylinski, K.; Sharma, C.M.; Gonzales, K.; Chao, Y.; Pirzada, Z.A.; Eckert, M.R.; Vogel, J.; Charpentier, E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 2011, 471, 602–607. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR–Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2019, 18, 67–83. [Google Scholar] [CrossRef]
- Bikard, D.; Barrangou, R. Using CRISPR-Cas systems as antimicrobials. Curr. Opin. Microbiol. 2017, 37, 155–160. [Google Scholar] [CrossRef]
- Park, H.-M.; Park, Y.; Berani, U.; Bang, E.; Vankerschaver, J.; Van Messem, A.; De Neve, W.; Shim, H. In silico optimization of RNA-protein interactions for CRISPR-Cas13-based antimicrobials. Biol. Direct. 2022, 17, 27. [Google Scholar] [CrossRef]
- Citorik, R.J.; Mimee, M.; Lu, T.K. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat. Biotechnol. 2014, 32, 1141–1145. [Google Scholar] [CrossRef]
- Bikard, D.; Euler, C.W.; Jiang, W.; Nussenzweig, P.M.; Goldberg, G.W.; Duportet, X.; Fischetti, V.; Marraffini, L. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat Biotechnol. 2014, 32, 1146–1150. [Google Scholar] [CrossRef]
- Yosef, I.; Manor, M.; Kiro, R.; Qimron, U. Temperate and lytic bacteriophages programmed to sensitize and kill antibiotic-resistant bacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 7267–7272. [Google Scholar] [CrossRef]
- Kiga, K.; Tan, X.-E.; Ibarra-Chávez, R.; Watanabe, S.; Aiba, Y.; Sato’O, Y.; Li, F.-Y.; Sasahara, T.; Cui, B.; Kawauchi, M.; et al. Development of CRISPR-Cas13a-based antimicrobials capable of sequence-specific killing of target bacteria. Nat. Commun. 2020, 11, 2934. [Google Scholar] [CrossRef]
- Shim, H. Investigating the Genomic Background of CRISPR-Cas Genomes for CRISPR-Based Antimicrobials. Evol. Bioinform. 2022, 18, 11769343221103887. [Google Scholar] [CrossRef] [PubMed]
- Maurer, F.P.; Christner, M.; Hentschke, M.; Rohde, H. Advances in Rapid Identification and Susceptibility Testing of Bacteria in the Clinical Microbiology Laboratory: Implications for Patient Care and Antimicrobial Stewardship Programs. Infect. Dis. Rep. 2017, 9, 6839. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, A.M.; Gilbert, D.N.; Ginocchio, C.C.; Hanson, K.E.; May, L.; Quinn, T.C.; Tenover, F.C.; Alland, D.; Blaschke, A.J.; Bonomo, R.A.; et al. Better Tests, Better Care: Improved Diagnostics for Infectious Diseases. Clin. Infect. Dis. 2013, 57 (Suppl. S3), S139–S170. [Google Scholar] [CrossRef] [PubMed]
- Opota, O.; Jaton, K.; Greub, G. Microbial diagnosis of bloodstream infection: Towards molecular diagnosis directly from blood. Clin. Microbiol. Infect. 2015, 21, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. MALDI-TOF MS for the Diagnosis of Infectious Diseases. Clin. Chem. 2015, 61, 100–111. [Google Scholar] [CrossRef]
- Shekhar, S.; Petersen, F.C. The Dark Side of Antibiotics: Adverse Effects on the Infant Immune Defense Against Infection. Front. Pediatr. 2020, 8, 544460. [Google Scholar] [CrossRef]
- Stokholm, J.; Schjørring, S.; Pedersen, L.; Bischoff, A.L.; Følsgaard, N.; Carson, C.G.; Chawes, B.L.K.; Bønnelykke, K.; Mølgaard, A.; Krogfelt, K.A.; et al. Prevalence and Predictors of Antibiotic Administration during Pregnancy and Birth. PLoS ONE 2013, 8, e82932. [Google Scholar] [CrossRef]
- Gonzalez-Perez, G.; Hicks, A.L.; Tekieli, T.M.; Radens, C.M.; Williams, B.L.; Lamousé-Smith, E.S.N. Maternal Antibiotic Treatment Impacts Development of the Neonatal Intestinal Microbiome and Antiviral Immunity. J. Immunol. 2016, 196, 3768–3779. [Google Scholar] [CrossRef]
- Lynn, M.A.; Tumes, D.J.; Choo, J.M.; Sribnaia, A.; Blake, S.J.; Leong, L.E.X.; Young, G.P.; Marshall, H.S.; Wesselingh, S.L.; Rogers, G.B.; et al. Early-Life Antibiotic-Driven Dysbiosis Leads to Dysregulated Vaccine Immune Responses in Mice. Cell Host Microbe 2018, 23, 653–660.e5. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Gonzalez, A.; Stombaugh, J.; Lozupone, C.; Turnbaugh, P.J.; Gordon, J.I.; Knight, R. The mind-body-microbial continuum. Dialog- Clin. Neurosci. 2011, 13, 55–62. [Google Scholar] [CrossRef]
- Rajer, M.; Segelov, E. (Eds.) Current Cancer Treatment; In-Tech: Hong Kong, China, 2020. [Google Scholar] [CrossRef]
- Stone, V.; Xu, P. Targeted antimicrobial therapy in the microbiome era. Mol. Oral Microbiol. 2017, 32, 446–454. [Google Scholar] [CrossRef]
- Moore, A.M.; Ahmadi, S.; Patel, S.; Gibson, M.K.; Bin Wang, B.; Ndao, I.M.; Deych, E.; Shannon, W.; Tarr, P.I.; Warner, B.B.; et al. Gut resistome development in healthy twin pairs in the first year of life. Microbiome 2015, 3, 27. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Mikkelsen, K.H.; Knop, F.K.; Frost, M.; Hallas, J.; Pottegård, A. Use of Antibiotics and Risk of Type 2 Diabetes: A Population-Based Case-Control Study. J. Clin. Endocrinol. Metab. 2015, 100, 3633–3640. [Google Scholar] [CrossRef]
- Maurice, C.F.; Haiser, H.J.; Turnbaugh, P.J. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell 2013, 152, 39–50. [Google Scholar] [CrossRef]
- Mårild, K.; Ye, W.; Lebwohl, B.; Green, P.H.R.; Blaser, M.J.; Card, T.; Ludvigsson, J. Antibiotic exposure and the development of coeliac disease: A nationwide case-control study. BMC Gastroenterol. 2013, 13, 109. [Google Scholar] [CrossRef]
- Morgun, A.; Dzutsev, A.; Dong, X.; Greer, R.L.; Sexton, D.J.; Ravel, J.; Schuster, M.; Hsiao, W.; Matzinger, P.; Shulzhenko, N. Uncovering effects of antibiotics on the host and microbiota using transkingdom gene networks. Gut 2015, 64, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gutierrez, E.; Mayer, M.J.; Cotter, P.D.; Narbad, A. Gut microbiota as a source of novel antimicrobials. Gut Microbes 2019, 10, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.S.; Cimermancic, P.; Schulze, C.J.; Wieland Brown, L.C.; Martin, J.; Mitreva, M.; Clardy, J.; Linington, R.G.; Fischbach, M.A. A Systematic Analysis of Biosynthetic Gene Clusters in the Human Microbiome Reveals a Common Family of Antibiotics. Cell 2014, 158, 1402–1414. [Google Scholar] [CrossRef]
- Zipperer, A.; Konnerth, M.C.; Laux, C.; Berscheid, A.; Janek, D.; Weidenmaier, C.; Burian, M.; Schilling, N.A.; Slavetinsky, C.; Marschal, M.; et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 2016, 535, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Pridmore, R.D.; Pittet, A.-C.; Praplan, F.; Cavadini, C. Hydrogen peroxide production by Lactobacillus johnsonii NCC 533 and its role in anti-Salmonella activity. FEMS Microbiol. Lett. 2008, 283, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.S.; Fischbach, M.A. HUMAN MICROBIOTA. Small molecules from the human microbiota. Science 2015, 349, 1254766. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Mulkern, A.J.; Oyama, L.B.; Cookson, A.R.; Creevey, C.J.; Wilkinson, T.J.; Olleik, H.; Maresca, M.; da Silva, G.C.; Fontes, P.P.; Bazzolli, D.M.S.; et al. Microbiome-derived antimicrobial peptides offer therapeutic solutions for the treatment of Pseudomonas aeruginosa infections. NPJ Biofilms Microbiomes 2022, 8, 70. [Google Scholar] [CrossRef]
- Manrique, P.; Bolduc, B.; Walk, S.T.; van der Oost, J.; de Vos, W.M.; Young, M.J. Healthy human gut phageome. Proc. Natl. Acad. Sci. USA 2016, 113, 10400–10405. [Google Scholar] [CrossRef]
- Townsend, E.M.; Kelly, L.; Muscatt, G.; Box, J.D.; Hargraves, N.; Lilley, D.; Jameson, E. The Human Gut Phageome: Origins and Roles in the Human Gut Microbiome. Front. Cell. Infect. Microbiol. 2021, 11, 643214. [Google Scholar] [CrossRef]
- Scarpellini, E.; Ianiro, G.; Attili, F.; Bassanelli, C.; De Santis, A.; Gasbarrini, A. The human gut microbiota and virome: Potential therapeutic implications. Dig. Liver Dis. 2015, 47, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.; Semenkovich, N.P.; Whiteson, K.; Rohwer, F.; Gordon, J.I. Going viral: Next-generation sequencing applied to phage populations in the human gut. Nat. Rev. Genet. 2012, 10, 607–617. [Google Scholar] [CrossRef]
- Shim, H.; Shivram, H.; Lei, S.; Doudna, J.A.; Banfield, J.F. Diverse ATPase Proteins in Mobilomes Constitute a Large Potential Sink for Prokaryotic Host ATP. Front. Microbiol. 2021, 12, 691847. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, V.A. Bacteriophage lysins as effective antibacterials. Curr. Opin. Microbiol. 2008, 11, 393–400. [Google Scholar] [CrossRef]
- Nie, T.; Meng, F.; Lu, F.; Bie, X.; Zhao, H.; Sun, J.; Lu, Z.; Lu, Y. An endolysin Salmcide-p1 from bacteriophage fmb-p1 against gram-negative bacteria. J. Appl. Microbiol. 2022, 133, 1597–1609. [Google Scholar] [CrossRef]
- Oechslin, F.; Zhu, X.; Dion, M.B.; Shi, R.; Moineau, S. Phage endolysins are adapted to specific hosts and are evolutionarily dynamic. PLoS Biol. 2022, 20, e3001740. [Google Scholar] [CrossRef]
- Ghequire, M.G.; De Mot, R. The Tailocin Tale: Peeling off Phage Tails. Trends Microbiol. 2015, 23, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Mitra, A.K.; Hurst, M.R.H. Investigating the Process of Sheath Maturation in Antifeeding Prophage: A Phage Tail-Like Protein Translocation Structure. J. Bacteriol. 2021, 203, e0010421. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).