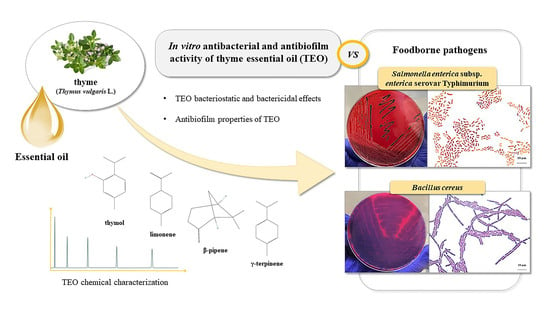
Antibacterial and Antibiofilm Efficacy of Thyme (Thymus vulgaris L.) Essential Oil against Foodborne Illness Pathogens, Salmonella enterica subsp. enterica Serovar Typhimurium and Bacillus cereus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Thyme Essential Oil
2.2. In Vitro Antibacterial Activity of Thyme Essential Oil against Salmonella enterica subsp. Enterica Serovar Typhimurium and Bacillus cereus Food Isolates
2.3. In Vitro Antibiofilm Activity of Thyme Essential Oil against Salmonella enterica subsp. Enterica Serovar Typhimurium and Bacillus cereus Food Isolates
3. Materials and Methods
3.1. Thyme Essential Oil (TEO)
3.2. Gas Chromatography-Mass Spectrometry Analysis of TEO
3.3. Meat Bacterial Isolates and Growth Conditions
3.4. In Vitro Antibacterial Assays
3.4.1. Agar Well Diffusion Method
3.4.2. Tube Dilution Method
3.5. In Vitro Antibiofilm Assays
3.5.1. Tissue Culture Plate Method (TCPM)
3.5.2. Biofilm Formation Inhibition Assay
3.6. Statistical Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. The Global Burden of Foodborne Diseases: Taking Stock and Charting the Way Forward: WHO Consultation to Develop a Strategy to Estimate the Global Burden of Foodborne Diseases; WHO: Geneva, Switzerland, 2007; pp. 25–27.
- Omer, M.K.; Álvarez-Ordoñez, A.; Prieto, M.; Skjerve, E.; Asehun, T.; Alvseike, O.A. A Systematic Review of Bacterial Foodborne Outbreaks Related to Red Meat and Meat Products. Foodborne Pathog. Dis. 2018, 15, 598–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pateiro, M.; Munekata, P.E.S.; Sant’Ana, A.S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J.M. Application of Essential Oils as Antimicrobial Agents against Spoilage and Pathogenic Microorganisms in Meat Products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, D.D.; Jo, C. Essential Oils as Potential Antimicrobial Agents in Meat and Meat Products: A Review. Trends Food Sci. Technol. 2013, 34, 96–108. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.J.; Villani, F.; Ercolini, D. Bacterial Populations and the Volatilome Associated to Meat Spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, X.; Liao, X.; Gänzle, M. Control of Pathogenic and Spoilage Bacteria in Meat and Meat Products by High Pressure: Challenges and Future Perspectives. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3476–3500. [Google Scholar] [CrossRef]
- Kalogianni, A.I.; Lazou, T.; Bossis, I.; Gelasakis, A.I. Natural Phenolic Compounds for the Control of Oxidation, Bacterial Spoilage, and Foodborne Pathogens in Meat. Foods 2020, 9, 794. [Google Scholar] [CrossRef]
- Allerberger, F.; Liesegang, A.; Grif, K.; Khaschabi, D.; Prager, R.; Danzl, J.; Höck, F.; Öttl, J.; Dierich, M.P.; Berghold, C.; et al. Occurrence of Salmonella enterica Serovar Dublin in Austria. Wien. Med. Wochenschr. 2003, 153, 148–152. [Google Scholar] [CrossRef]
- Miranda, J.M.; Mondragon, A.C.; Martinez, B.; Guarddon, M.; Rodriguez, J.A. Prevalence and Antimicrobial Resistance Patterns of Salmonella from Different Raw Foods in Mexico. J. Food Prot. 2009, 72, 966–971. [Google Scholar] [CrossRef]
- Srey, S.; Jahid, I.K.; Ha, S. Do Biofilm Formation in Food Industries: A Food Safety Concern. Food Control 2013, 31, 572–585. [Google Scholar] [CrossRef]
- Nortjé, G.L.; Vorster, S.M.; Greebe, R.P.; Steyn, P.L. Occurrence of Bacillus cereus and Yersinia enterocolitica in South African Retail Meats. Food Microbiol. 1999, 16, 213–217. [Google Scholar] [CrossRef]
- Huang, Y.; Flint, S.H.; Palmer, J.S. Bacillus cereus Spores and Toxins–The Potential Role of Biofilms. Food Microbiol. 2020, 90, 103493. [Google Scholar] [CrossRef] [PubMed]
- Steenackers, H.; Hermans, K.; Vanderleyden, J.; De Keersmaecker, S.C.J. Salmonella Biofilms: An Overview on Occurrence, Structure, Regulation and Eradication. Food Res. Int. 2012, 45, 502–531. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial Biofilms: From the Natural Environment to Infectious Diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ordóñez, A.; Coughlan, L.M.; Briandet, R.; Cotter, P.D. Biofilms in Food Processing Environments: Challenges and Opportunities. Annu. Rev. Food Sci. Technol. 2019, 10, 173–195. [Google Scholar] [CrossRef]
- Sindelar, J.J.; Milkowski, A.L. Sodium Nitrite in Processed Meat and Poultry Meats: A Review of Curing and Examining the Risk/Benefit of Its Use. Am. Meat Sci. Assoc. 2011, 3, 1–14. [Google Scholar]
- El-Wahab, H.M.F.A.; Moram, G.S.E.D. Toxic Effects of Some Synthetic Food Colorants and/or Flavor Additives on Male Rats. Toxicol. Ind. Health 2013, 29, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Sweis, I.E.; Cressey, B.C. Potential role of the common food additive manufactured citric acid in eliciting significant inflammatory reactions contributing to serious disease states: A series of four case reports. Toxicol. Rep. 2018, 5, 808–812. [Google Scholar] [CrossRef]
- Rath, D. A critical review on food adulteration and its risk on health. IJNRD 2022, 7, 353–356. [Google Scholar]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential Oils as Antimicrobials in Food Systems—A Review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Rehman, A.; Mahdi, A.A.; Al-Ansi, W.; Wei, M.; Yanyu, Z.; Yao, W. Application of essential oils as preservatives in food systems: Challenges and future prospectives—A review. Phytochem. Rev. 2022, 21, 1209–1246. [Google Scholar] [CrossRef]
- Zang, E.; Jiang, L.; Cui, H.; Li, X.; Yan, Y.; Liu, Q.; Li, M. Only Plant-based Food Additives: An Overview on Application, Safety, and Key Challenges in the Food Industry. Food Rev. Int. 2022, 1–32. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Abdolmaleki, K.; Javanmardi, F.; Hadidi, M.; Mousavi Khaneghah, A. Recent advances in plant-based compounds for mitigation of mycotoxin contamination in food products: Current status, challenges and perspectives. Int. J. Food Sci. 2022, 57, 2159–2170. [Google Scholar] [CrossRef]
- Balasubramaniam, V.G.; Ramakrishnan, S.R.; Antony, U. Opportunities and Challenges of Plant Extracts in Food Industry. In Plant Extracts: Applications in the Food Industry, 1st ed.; Academic Press; Elsevier: London, UK, 2021; pp. 295–315. ISBN 9780128224939. [Google Scholar]
- Ballester-Costa, C.; Sendra, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical Composition and In Vitro Antibacterial Properties of Essential Oils of Four Thymus Species from Organic Growth. Ind. Crops Prod. 2013, 50, 304–311. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernandez-Lopez, J. In Vitro Antioxidant and Antifungal Properties of Essential Oils Obtained from Aromatic Herbs Endemic to the Southeast of Spain. J. Food Prot. 2013, 76, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Ed-Dra, A.; Nalbone, L.; Filali, F.R.; Trabelsi, N.; El Majdoub, Y.O.; Bouchrif, B.; Giarratana, F.; Giuffrida, A. Comprehensive Evaluation on the Use of Thymus vulgaris Essential Oil as Natural Additive against Different Serotypes of Salmonella enterica. Sustainability 2021, 13, 4594. [Google Scholar] [CrossRef]
- Sadekuzzaman, M.; Mizan, M.F.R.; Kim, H.S.; Yang, S.; Ha, S. Do Activity of Thyme and Tea Tree Essential Oils against Selected Foodborne Pathogens in Biofilms on Abiotic Surfaces. LWT 2018, 89, 134–139. [Google Scholar] [CrossRef]
- Thielmann, J.; Muranyi, P.; Kazman, P. Screening Essential Oils for Their Antimicrobial Activities against the Foodborne Pathogenic Bacteria Escherichia coli and Staphylococcus aureus. Heliyon 2019, 5, e01860. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Barkauskaite, S.; Duffy, B.; Jaiswal, A.K.; Swarna, J. Characterization and Antimicrobial Activity of Biodegradable Active Packaging Enriched with Clove and Thyme Essential Oil for Food Packaging Application. Foods 2020, 9, 1117. [Google Scholar] [CrossRef]
- Rafiq, R.; Hayek, S.A.; Anyanwu, U.; Hardy, B.I.; Giddings, V.L.; Ibrahim, S.A.; Tahergorabi, R.; Kang, H.W. Antibacterial and Antioxidant Activities of Essential Oils from Artemisia herba-alba Asso., Pelargonium Capitatum × Radens and Laurus nobilis L. Foods 2016, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Barber, X.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. Effect of Chitosan Edible Films Added with Thymus Moroderi and Thymus Piperella Essential Oil on Shelf-Life of Cooked Cured Ham. J. Food Sci. Technol. 2015, 52, 6493–6501. [Google Scholar] [CrossRef] [Green Version]
- Trevisan, D.A.C.; Campanerut-Sá, P.A.Z.; da Silva, A.F.; Batista, A.F.P.; Seixas, F.A.V.; Peralta, R.M.; de Sá-Nakanishi, A.B.; de Abreu Filho, B.A.; Junior, M.M.; Mikcha, J.M.G. Action of Carvacrol in Salmonella typhimurium Biofilm: A Proteomic Study. J. Appl. Biomed. 2020, 18, 106–114. [Google Scholar] [CrossRef]
- Basavegowda, N.; Patra, J.K.; Baek, K.H. Essential Oils and Mono/Bi/Tri-Metallic Nanocomposites as Alternative Sources of Antimicrobial Agents to Combat Multidrug-Resistant Pathogenic Microorganisms: An Overview. Molecules 2020, 25, 1058. [Google Scholar] [CrossRef] [Green Version]
- Guillín, Y.; Cáceres, M.; Torres, R.; Stashenko, E.; Ortiz, C. Effect of Essential Oils on the Inhibition of Biofilm and Quorum Sensing in Salmonella enteritidis 13076 and Salmonella typhimurium 14028. Antibiotics 2021, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Vunnava, A. Review on Thymus vulgaris Traditional Uses and Pharmacological Properties. Med. Aromat. Plants 2014, 3, 1000167. [Google Scholar]
- Benameur, Q.; Gervasi, T.; Pellizzeri, V.; Pľuchtová, M.; Tali-Maama, H.; Assaous, F.; Guettou, B.; Rahal, K.; Gruľová, D.; Dugo, G.; et al. Antibacterial Activity of Thymus vulgaris Essential Oil Alone and in Combination with Cefotaxime against BlaESBL Producing Multidrug Resistant Enterobacteriaceae Isolates. Nat. Prod. Res. 2019, 33, 2647–2654. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.K.; Musa, F.H.; Mehdi, B.Y.; Al-Rawe, A.M. Impacts of the Alcoholic Extract and Essential Oil of Thymus vulgaris L. against the Causative Agent of Acne Formation (Staphylococcus aureus). Syst. Rev. Pharm. 2020, 11, 495–498. [Google Scholar] [CrossRef]
- Lemos, M.F.; Lemos, M.F.; Pacheco, H.P.; Guimarães, A.C.; Fronza, M.; Endringer, D.C.; Scherer, R. Seasonal variation affects the composition and antibacterial and antioxidant activities of Thymus vulgaris. Ind. Crops. Prod. 2017, 95, 543–548. [Google Scholar] [CrossRef]
- Kabdal, T.; Kumar, R.; Prakash, O.; Nagarkoti, K.; Rawat, D.S.; Srivastava, R.M.; Dubey, S.K. Seasonal variation in the essential oil composition and biological activities of Thymus linearis Benth. Collected from the Kumaun region of Uttarakhand, India. Biochem. Syst. Ecol. 2022, 103, 104449. [Google Scholar] [CrossRef]
- Kim, M.; Sowndhararajan, K.; Kim, S. The Chemical Composition and Biological Activities of Essential Oil from Korean Native Thyme Bak-Ri-Hyang (Thymus quinquecostatus Celak.). Molecules 2022, 27, 4251. [Google Scholar] [CrossRef]
- Hudaib, M.; Aburjai, T. Volatile Components of Thymus vulgaris L. from Wild-Growing and Cultivated Plants in Jordan. Flavour Fragr. J. 2007, 22, 322–327. [Google Scholar] [CrossRef]
- Do, T.K.T.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Authenticity of essential oils. TrAC Trends Anal. Chem. 2015, 66, 146–157. [Google Scholar] [CrossRef]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial Activity and Chemical Composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis Essential Oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Hashemi, M.; Ghahfarokhi, F.T. Essential Oil and Chemical Compositions of Wild and Cultivated Thymus Daenensis Celak and Thymus vulgaris L. Ind. Crops Prod. 2013, 48, 43–48. [Google Scholar] [CrossRef]
- Shabnum, S.; Wagay, M.G. Essential Oil Composition of Thymus vulgaris L. and Their Uses. J. Res. Dev. 2011, 11, 83–94. [Google Scholar]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Jordán, M.J.; Martínez, R.M.; Goodner, K.L.; Baldwin, E.A.; Sotomayor, J.A. Seasonal Variation of Thymus Hyemalis Lange and Spanish Thymus vulgaris L. Essential Oils Composition. Ind. Crops Prod. 2006, 24, 253–263. [Google Scholar] [CrossRef]
- Quynh, C.T.T.; Trang, V.T. Volatile Composition, Antioxidant Property and Antimicrobial Activities against Food-Borne Bacteria of Vietnamese Thyme (Thymus vulgaris L.) Essential Oil. Vietnam J. Sci. Technol. 2019, 57, 127. [Google Scholar] [CrossRef] [Green Version]
- Boskovic, M.; Djordjevic, J.; Ivanovic, J.; Janjic, J.; Zdravkovic, N.; Glisic, M.; Glamoclija, N.; Baltic, B.; Djordjevic, V.; Baltic, M. Inhibition of Salmonella by Thyme Essential Oil and Its Effect on Microbiological and Sensory Properties of Minced Pork Meat Packaged under Vacuum and Modified Atmosphere. Int. J. Food Microbiol. 2017, 258, 58–67. [Google Scholar] [CrossRef]
- Gonzalez, K.; Johnson, A.; Gonsalves, V.; Santos, A. Antimicrobial Properties of Thyme Essential Oil on Salmonella typhimurium. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Valizadeh, S.; Mahmodi, R.; Fakheri, T.; Katiraie, F.; Rahmani, V. Investigating the phytochemical, antibacterial and antifungal effects of Thymus vulgaris and Cuminum cyminum essential oils. Med. Lab. J. 2016, 10, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Penalver, P.; Huerta, B.; Borge, C.; Astorga, R.; Romero, R.; Perea, A. Antimicrobial activity of five essential oils against origin strains of the Enterobacteriaceae family. APMIS 2005, 113, 1–6. [Google Scholar]
- Turgis, M.; Vu, K.D.; Dupont, C.; Lacroix, M. Combined antimicrobial effect of essential oils and bacteriocins against foodborne pathogens and food spoilage bacteria. Int. Food Res. J. 2012, 48, 696–702. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.; Correa-Pacheco, Z.; Bautista-Baños, S.; y Gómez, Y.G. Release study and inhibitory activity of thyme essential oil-loaded chitosan nanoparticles and nanocapsules against foodborne bacteria. Int. J. Biol. Macromol. 2017, 103, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Liu, L.; Wu, X.; Sun, Y.; Liu, Z. Effect of Thyme Essential Oil against Bacillus cereus Planktonic Growth and Biofilm Formation. Appl. Microbiol. Biotechnol. 2018, 102, 10209–10218. [Google Scholar] [CrossRef]
- Čabarkapa, I.; Čolović, R.; Đuragić, O.; Popović, S.; Kokić, B.; Milanov, D.; Pezo, L. Anti-Biofilm Activities of Essential Oils Rich in Carvacrol and Thymol against Salmonella enteritidis. Biofouling 2019, 35, 361–375. [Google Scholar] [CrossRef]
- Grigore-Gurgu, L.; Ionela Bucur, F.; Borda, D.; Alexa, E.-A.; Neagu, C.; Ioana Nicolau, A. Biofilms Formed by Pathogens in Food and Food Processing Environments. In Bacterial Biofilms; IntechOpen: London, UK, 2020; pp. 1–32. [Google Scholar]
- Soni, K.A.; Oladunjoye, A.; Nannapaneni, R.; Schilling, M.W.; Silva, J.L.; Mikel, B.; Bailey, R.H. Inhibition and Inactivation of Salmonella typhimurium Biofilms from Polystyrene and Stainless Steel Surfaces by Essential Oils and Phenolic Constituent Carvacrol. J. Food Prot. 2013, 76, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Alsaraf, S.; Hadi, Z.; Al-Lawati, W.M.; Al Lawati, A.A.; Khan, S.A. Chemical Composition, in Vitro Antibacterial and Antioxidant Potential of Omani Thyme Essential Oil along with in Silico Studies of Its Major Constituent. J. King Saud Univ.-Sci. 2020, 32, 1021–1028. [Google Scholar] [CrossRef]
- Perez, C.; Pauli, M.; Bazerque, P. An Antibiotic Assay by the Agar Well Diffusion Method. Acta Biol. Med. Exp. 1990, 15, 113–115. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2022; ISBN 978-1-68440-134-5. [Google Scholar]
- Sateriale, D.; Imperatore, R.; Colicchio, R.; Pagliuca, C.; Varricchio, E.; Volpe, M.G.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Phytocompounds vs. Dental Plaque Bacteria: In Vitro Effects of Myrtle and Pomegranate Polyphenolic Extracts Against Single-Species and Multispecies Oral Biofilms. Front. Microbiol. 2020, 11, 592265. [Google Scholar] [CrossRef]
- Bakkiyaraj, D.; Nandhini, J.R.; Malathy, B.; Pandian, S.K. The Anti-Biofilm Potential of Pomegranate (Punica granatum L.) Extract against Human Bacterial and Fungal Pathogens. Biofouling 2013, 29, 929–937. [Google Scholar] [CrossRef]





| N° Peak | RI | Relative Peak Area % | Identified Compound | Structure |
|---|---|---|---|---|
| 1 | 973 | 1.232 ± 0.01 | sabinene |  |
| 2 | 978 | 7.177 ± 0.04 | β-pinene |  |
| 3 | 996 | 0.518 ± 0.00 | α-phellandrene |  |
| 4 | 1021 | 1.680 ± 0.50 | o-cymene |  |
| 5 | 1035 | 39.391 ± 0.20 | limonene |  |
| 6 | 1064 | 4.405 ± 0.02 | γ-terpinene |  |
| 7 | 1302 | 44.435 ± 0.22 | thymol |  |
| Antibacterial Agent | S. Typhimurium ST1 | B. cereus BC3 | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| TEO | 20 µL mL−1 | 100 µL mL−1 | 10 µL mL−1 | 80 µL mL−1 |
| GNT | 30 μg mL−1 | 500 μg mL−1 | - | - |
| AMX | - | - | 50 μg mL−1 | 200 μg mL−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sateriale, D.; Forgione, G.; De Cristofaro, G.A.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Antibacterial and Antibiofilm Efficacy of Thyme (Thymus vulgaris L.) Essential Oil against Foodborne Illness Pathogens, Salmonella enterica subsp. enterica Serovar Typhimurium and Bacillus cereus. Antibiotics 2023, 12, 485. https://doi.org/10.3390/antibiotics12030485
Sateriale D, Forgione G, De Cristofaro GA, Pagliuca C, Colicchio R, Salvatore P, Paolucci M, Pagliarulo C. Antibacterial and Antibiofilm Efficacy of Thyme (Thymus vulgaris L.) Essential Oil against Foodborne Illness Pathogens, Salmonella enterica subsp. enterica Serovar Typhimurium and Bacillus cereus. Antibiotics. 2023; 12(3):485. https://doi.org/10.3390/antibiotics12030485
Chicago/Turabian StyleSateriale, Daniela, Giuseppina Forgione, Giuseppa Anna De Cristofaro, Chiara Pagliuca, Roberta Colicchio, Paola Salvatore, Marina Paolucci, and Caterina Pagliarulo. 2023. "Antibacterial and Antibiofilm Efficacy of Thyme (Thymus vulgaris L.) Essential Oil against Foodborne Illness Pathogens, Salmonella enterica subsp. enterica Serovar Typhimurium and Bacillus cereus" Antibiotics 12, no. 3: 485. https://doi.org/10.3390/antibiotics12030485