Evaluation of the Anti-Toxoplasma gondii Efficacy, Cytotoxicity, and GC/MS Profile of Pleopeltis crassinervata Active Subfractions
Abstract
1. Introduction
2. Results
2.1. Fractionation of the Hexane Fraction by Thin-Layer Chromatography
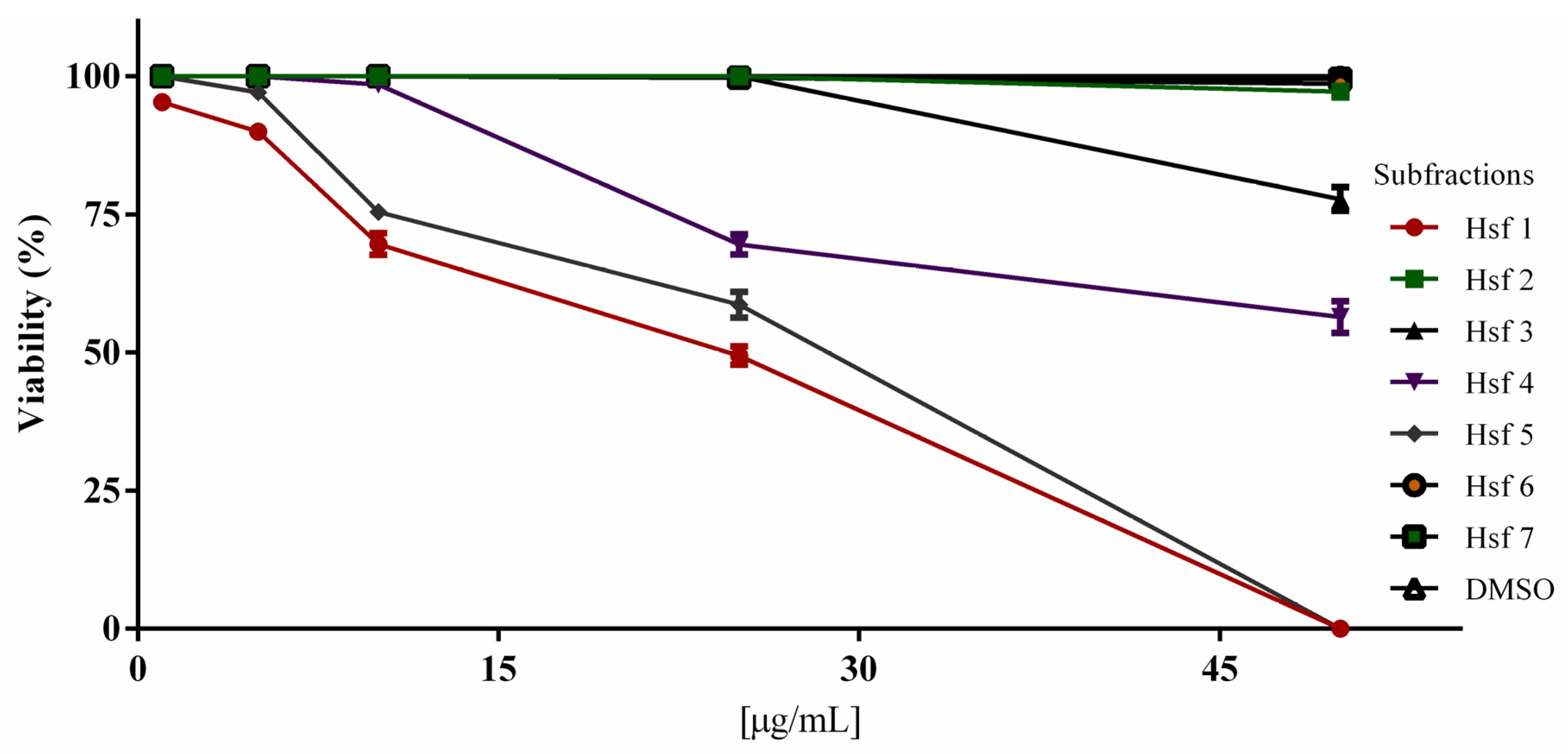
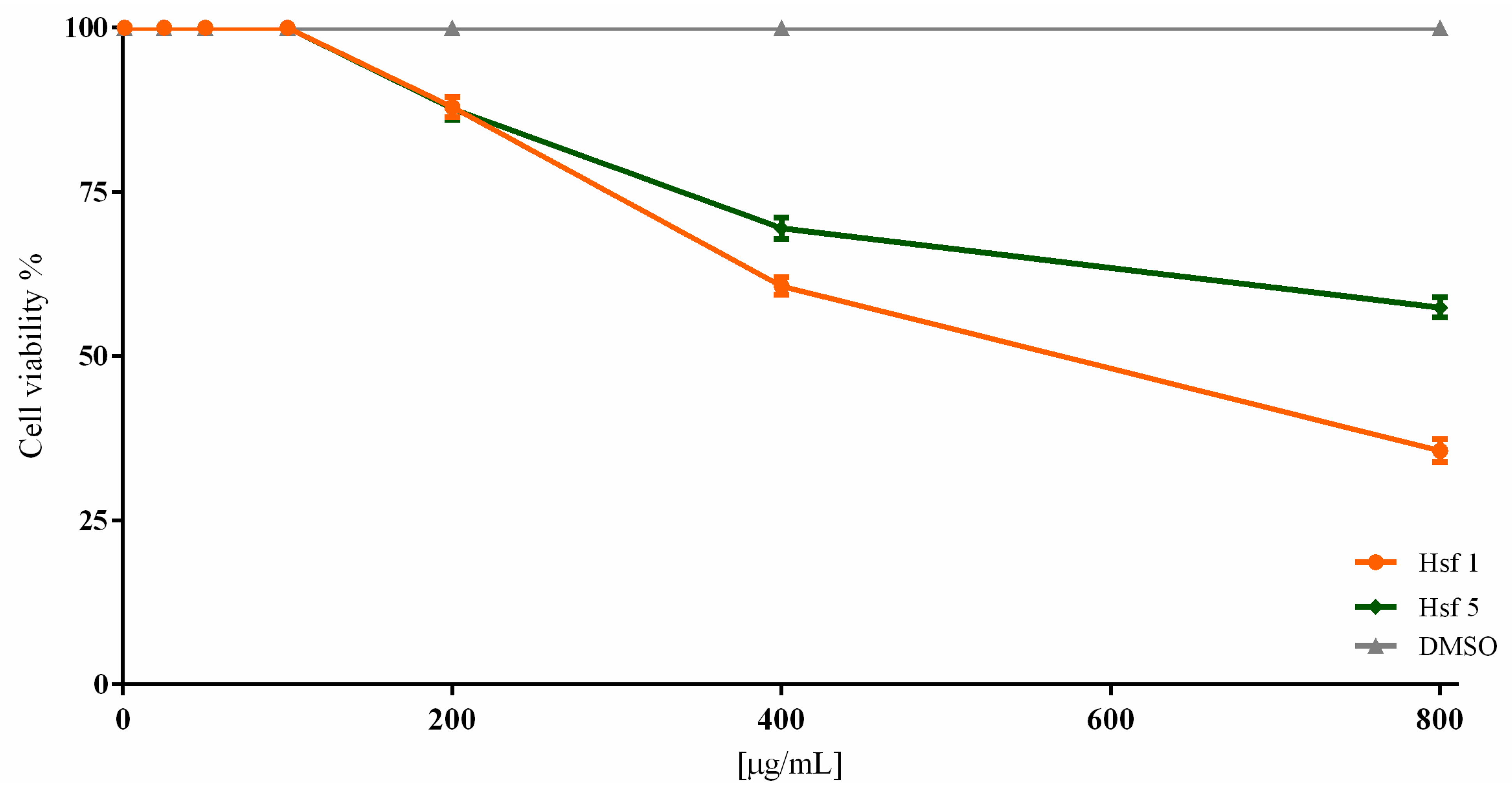
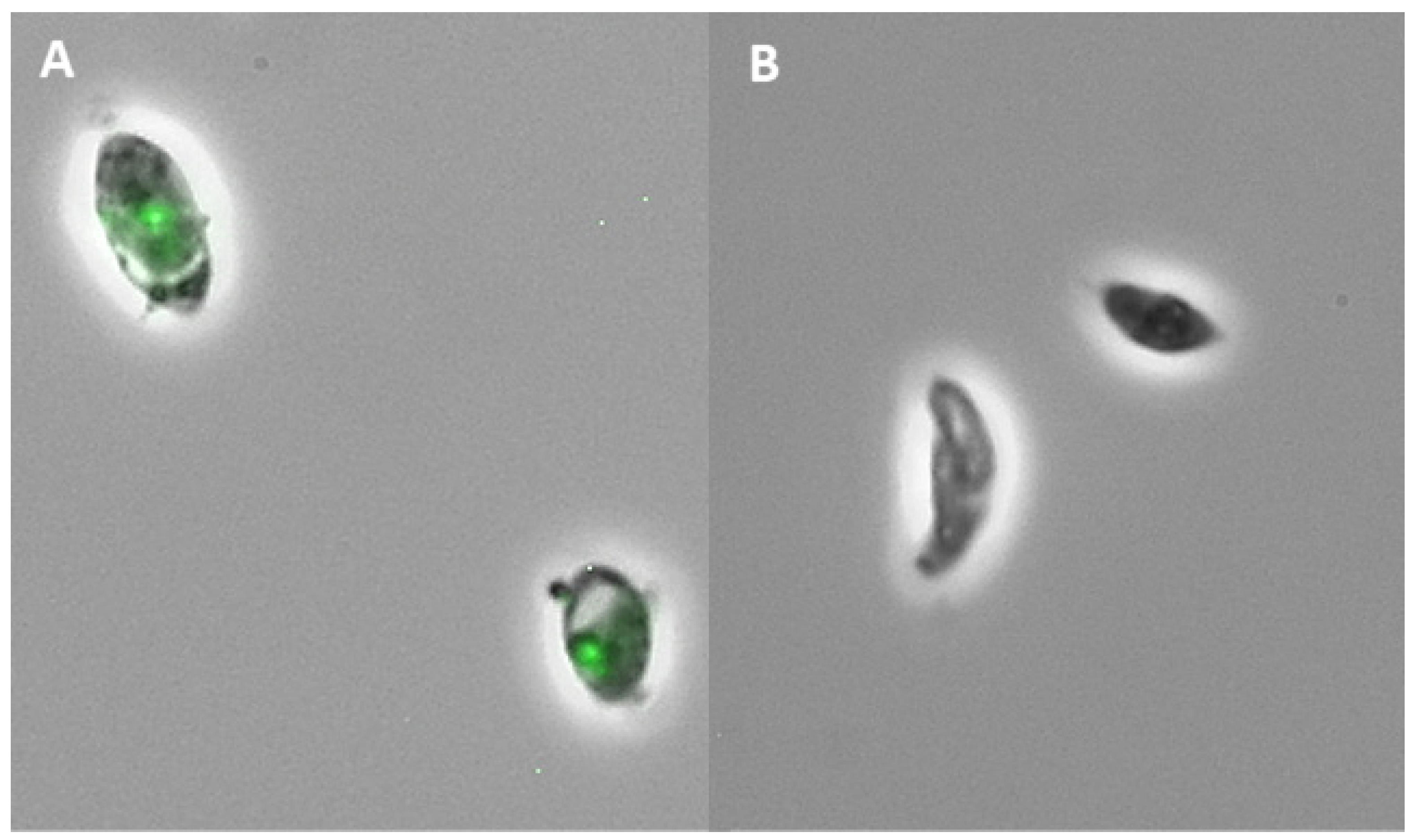
2.2. Subfraction Efficacy against T. gondii and Cytotoxicity
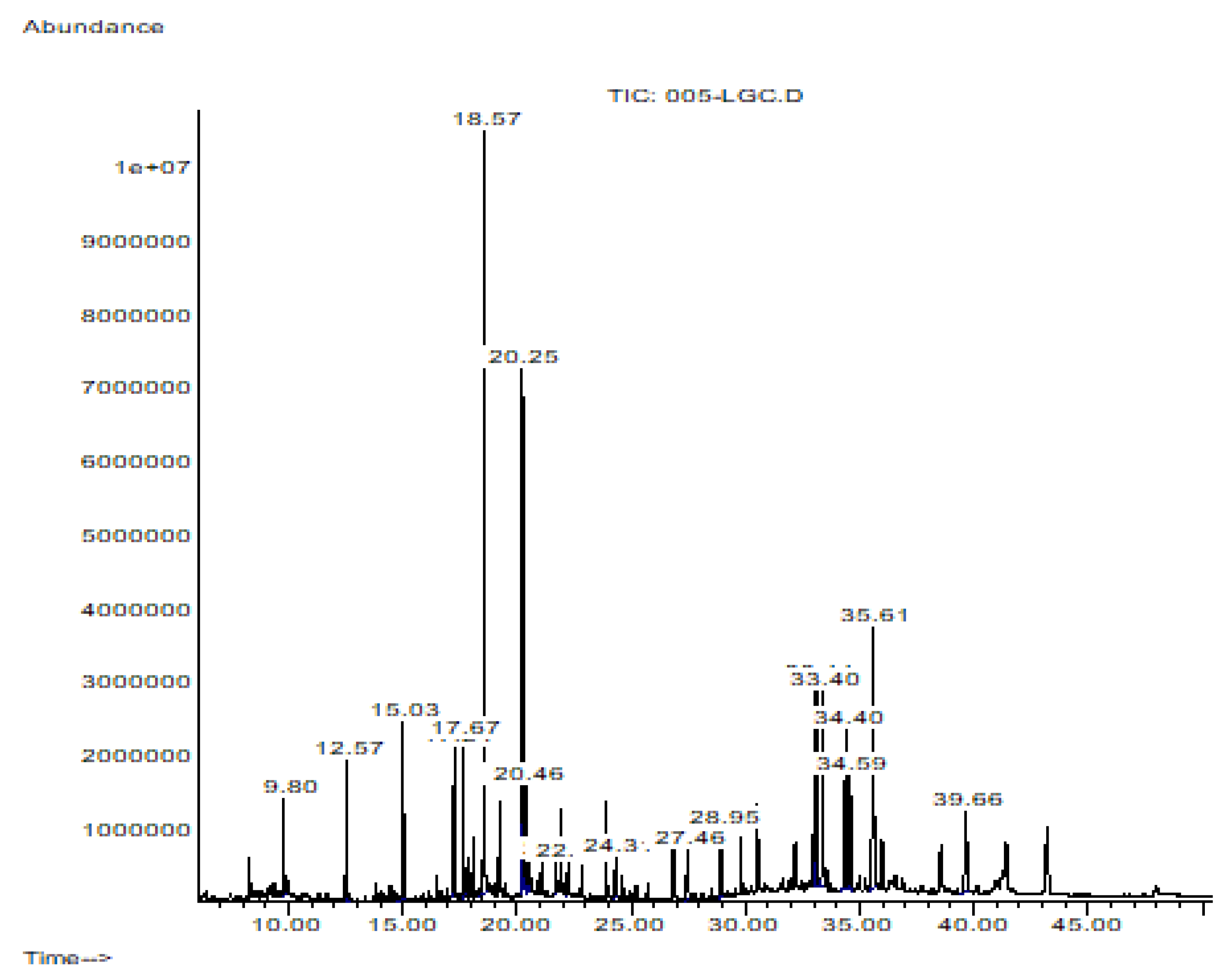
2.3. Hsf1 Compound Identification by CG-MS
3. Discussion
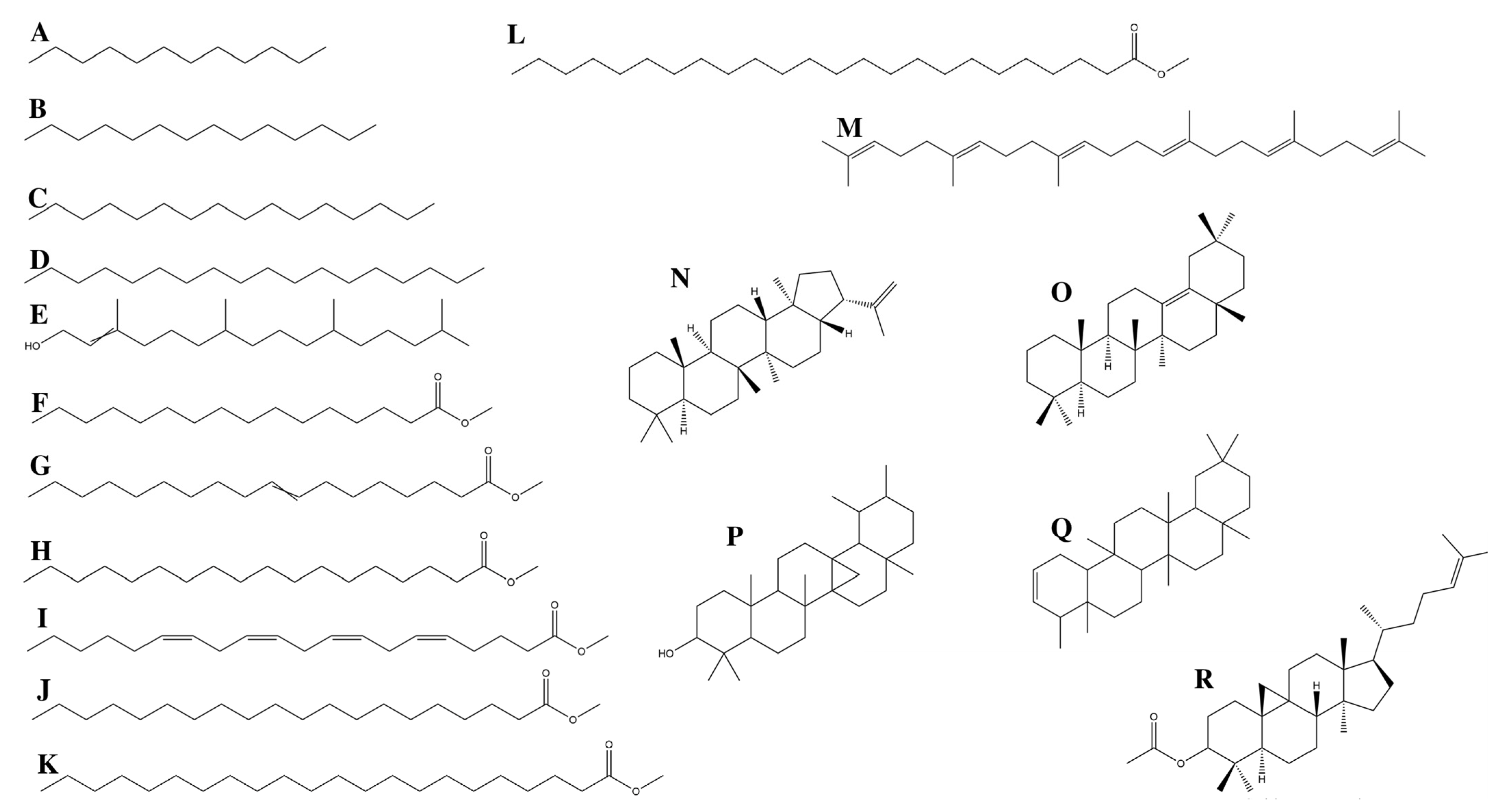
3.1. GC/MS Profiling
3.1.1. Fatty Acids
3.1.2. Terpenes
3.1.3. Alkanes
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Fractionation
4.3. Chromatographic Separation
4.4. Animals
4.5. Cell Culture
4.6. Parasites
4.7. Anti-Toxoplasma Activity Assay
4.8. Gas Chromatography Analysis
4.9. Cytotoxic Assay
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almeria, S.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H.; Shipley, A.; Dubey, J.P. Epidemiological and Public Health Significance of Toxoplasma gondii Infection in Wild Rabbits and Hares: 2010–2020. Microorganisms 2021, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.C.; Goulart, C.; Hayward, J.A.; Kupz, A.; Miller, C.M.; van Dooren, G.G. Control of human toxoplasmosis. Int. J. Parasitol. 2021, 51, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-H.; Sun, M.-M.; Elsheikha, H.M.; Fu, Y.-T.; Sugiyama, H.; Ando, K.; Sohn, W.-M.; Zhu, X.-Q.; Yao, C. Human gnathostomiasis: A neglected food-borne zoonosis. Parasites Vectors 2020, 13, 616. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Ferreira, F.; Caldart, E.T.; Pasquali, A.K.S.; Mitsuka-Breganó, R.; Freire, R.L.; Navarro, I.T. Patterns of Transmission and Sources of Infection in Outbreaks of Human Toxoplasmosis. Emerg. Infect. Dis. 2019, 25, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Hajimohammadi, B.; Ahmadian, S.; Firoozi, Z.; Askari, M.; Mohammadi, M.; Eslami, G.; Askari, V.; Loni, E.; Barzegar-Bafrouei, R.; Boozhmehrani, M.J. A Meta-Analysis of the Prevalence of Toxoplasmosis in Livestock and Poultry Worldwide. EcoHealth 2022, 19, 55–74. [Google Scholar] [CrossRef]
- Khan, K.; Khan, W. Congenital toxoplasmosis: An overview of the neurological and ocular manifestations. Parasitol. Int. 2018, 67, 715–721. [Google Scholar] [CrossRef]
- Buonsenso, D.; Pata, D.; Turriziani Colonna, A.; Iademarco, M.; De Santis, M.; Masini, L.; Conti, G.; Molle, F.; Baldascino, A.; Acampora, A.; et al. Spyramicine and Trimethoprim-Sulfamethoxazole Combination to Prevent Mother-To-Fetus Transmission of Toxoplasma Gondii Infection in Pregnant Women: A 28-Years Single-Center Experience. Pediatr. Infect. Dis. J. 2022, 41, e223–e227. [Google Scholar] [CrossRef]
- Guglielmi, P.; Secci, D. Treatment of Toxoplasmosis: An Insight on Epigenetic Drugs; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–27. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Anacleto-Santos, J.; López-Camacho, P.; Mondragón-Flores, R.; Vega-Ávila, E.; Islas, G.B.; Mondragón-Castelán, M.; Carrasco-Ramírez, E.; Rivera-Fernández, N. Anti-toxoplasma, antioxidant and cytotoxic activities of Pleopeltis crassinervata (Fée) T. Moore hexane fraction. Saudi J. Biol. Sci. 2020, 27, 812–819. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, T.; Zhai, S.-Q.; Li, C.-H. Recent progress on anti-Toxoplasma drugs discovery: Design, synthesis and screening. Eur. J. Med. Chem. 2019, 183, 111711. [Google Scholar] [CrossRef]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug Resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar] [CrossRef] [PubMed]
- Cheraghipour, K.; Masoori, L.; Ezzatpour, B.; Roozbehani, M.; Sheikhian, A.; Malekara, V.; Niazi, M.; Mardanshah, O.; Moradpour, K.; Mahmoudvand, H. The Experimental Role of Medicinal Plants in Treatment of Toxoplasma gondii Infection: A Systematic Review. Acta Parasitol. 2021, 66, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Xi, J. Ultrahigh pressure extraction of bioactive compounds from plants—A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1097–1106. [Google Scholar] [CrossRef]
- Potterat, O.; Hamburger, M. Concepts and technologies for tracking bioactive compounds in natural product extracts: Generation of libraries, and hyphenation of analytical processes with bioassays. Nat. Prod. Rep. 2013, 30, 546. [Google Scholar] [CrossRef]
- Guo, H.-Y.; Jin, C.; Zhang, H.-M.; Jin, C.-M.; Shen, Q.-K.; Quan, Z.-S. Synthesis and Biological Evaluation of (+)-Usnic Acid Derivatives as Potential Anti-Toxoplasma gondii Agents. J. Agric. Food Chem. 2019, 67, 9630–9642. [Google Scholar] [CrossRef] [PubMed]
- Arouri, A.; Mouritsen, O.G. Membrane-perturbing effect of fatty acids and lysolipids. Prog. Lipid Res. 2013, 52, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Bunchongprasert, K.; Shao, J. Effect of fatty acid ester structure on cytotoxicity of self-emulsified nanoemulsion and transport of nanoemulsion droplets. Colloids Surf. B Biointerfaces 2020, 194, 111220. [Google Scholar] [CrossRef]
- De Carvalho, C.C.C.R.; Caramujo, M.J. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Bonde, C.S.; Bornancin, L.; Lu, Y.; Simonsen, H.T.; Martínez-Valladares, M.; Peña-Espinoza, M.; Mejer, H.; Williams, A.R.; Thamsborg, S.M. Bio-Guided Fractionation and Molecular Networking Reveal Fatty Acids to Be Principal Anti-Parasitic Compounds in Nordic Seaweeds. Front. Pharmacol. 2021, 12, 674520. [Google Scholar] [CrossRef]
- Taki, A.C.; Brkljača, R.; Wang, T.; Koehler, A.V.; Ma, G.; Danne, J.; Ellis, S.; Hofmann, A.; Chang, B.C.H.; Jabbar, A.; et al. Natural Compounds from the Marine Brown Alga Caulocystis cephalornithos with Potent In Vitro-Activity against the Parasitic Nematode Haemonchus contortus. Pathogens 2020, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits—A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-W.; Lee, J.; Lee, J.-H.; Park, B.-J.; Lee, E.J.; Shin, S.; Cha, G.-H.; Lee, Y.-H.; Lim, K.; Yuk, J.-M. Omega-3 Polyunsaturated Fatty Acids Prevent Toxoplasma gondii Infection by Inducing Autophagy via AMPK Activation. Nutrients 2019, 11, 2137. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Ghaly, M.F.; Fahmi, S.M. Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria. J. Basic Microbiol. 2021, 61, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, A.C.d.A.; Miranda, G.E.C.d.; Souza, M.d.F.V.d.; da Cunha, E.V.L. Analysis and characterization of methyl esters of fatty acids of some Gracilaria species. Biochem. Syst. Ecol. 2012, 44, 303–306. [Google Scholar] [CrossRef]
- Salem, A.-F.Z.; Salem, M.Z.; Gonzalez-Ronquillo, M.; Camacho, L.M.; Cipriano, M. Major chemical constituents of Leucaena leucocephala and Salix babylonica leaf extracts. J. Trop. Agric. 2011, 49, 95–98. [Google Scholar]
- Zheng, C.J.; Yoo, J.-S.; Lee, T.-G.; Cho, H.-Y.; Kim, Y.-H.; Kim, W.-G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef]
- Abubacker, M.N.; Deepalakshmi, T. In vitro Antifungal Potentials of Bioactive Compound Methyl Ester of Hexadecanoic Acid Isolated from Annona muricata Linn. (Annonaceae) Leaves. Biosci. Biotechnol. Res. Asia 2013, 10, 879–884. [Google Scholar] [CrossRef]
- El-Zawawy, L.A.; El-Said, D.; Mossallam, S.F.; Ramadan, H.S.; Younis, S.S. Triclosan and triclosan-loaded liposomal nanoparticles in the treatment of acute experimental toxoplasmosis. Exp. Parasitol. 2015, 149, 54–64. [Google Scholar] [CrossRef]
- Konstantinovic, N.; Guegan, H.; Stäjner, T.; Belaz, S.; Robert-Gangneux, F. Treatment of toxoplasmosis: Current options and future perspectives. Food Waterborne Parasitol. 2019, 15, e00036. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.J.; Romano, J.D.; Kline, J.T.; Coppens, I. Novel approaches to kill Toxoplasma gondii by exploiting the uncontrolled uptake of unsaturated fatty acids and vulnerability to lipid storage inhibition of the parasite. Antimicrob. Agents Chemother. 2018, 62, e00347-18. [Google Scholar] [CrossRef] [PubMed]
- El-Baba, C.; Baassiri, A.; Kiriako, G.; Dia, B.; Fadlallah, S.; Moodad, S.; Darwiche, N. Terpenoids’ anti-cancer effects: Focus on autophagy. Apoptosis 2021, 26, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, U.T.; Yusof, Z.N.B. Algal terpenoids: A potential source of antioxidants for cancer therapy. In Terpenes and Terpenoids-Recent Advances; IntechOpen: London, UK, 2021. [Google Scholar]
- Bisht, B.S.; Bankoti, H.; Bharti, T. A Review on Therapeutic Uses of Terpenoids. J. Drug Deliv. Ther. 2021, 11, 182–185. [Google Scholar] [CrossRef]
- Silva, F.C.O.; Ferreira, M.K.A.; Silva, A.W.; Matos, M.G.C.; Magalhães, F.; Silva, P.T.; Bandeira, P.N.; de Menezes, J.E.S.; Santos, H.S. Bioatividades de Triterpenos isolados de plantas: Uma breve revisão. Rev. Virtual Quim 2020, 12, 234–247. [Google Scholar] [CrossRef]
- Zubair, M.F.; Atolani, O.; Ibrahim, S.O.; Adebisi, O.O.; Hamid, A.A.; Sowunmi, R.A. Chemical constituents and antimicrobial properties of Phyllanthus amarus (Schum & Thonn). Bayero J. Pure Appl. Sci. 2017, 10, 238. [Google Scholar] [CrossRef]
- Ukiya, M.; Akihisa, T.; Tokuda, H.; Suzuki, H.; Mukainaka, T.; Ichiishi, E.; Yasukawa, K.; Kasahara, Y.; Nishino, H. Constituents of Compositae plants: III. Anti-tumor promoting effects and cytotoxic activity against human cancer cell lines of triterpene diols and triols from edible chrysanthemum flowers. Cancer Lett. 2002, 177, 7–12. [Google Scholar] [CrossRef]
- Hassan, A.; Rasheed, M.; Ali, M.; Ishrat, G.; Ahmed, M. Identification of Five New Triterpenoids from Ethylacetate Bark Extract of Holoptelea integrifolia (Roxb.) Planch by GC-MS. Nat. Prod. Chem. Res. 2018, 6, 338. [Google Scholar] [CrossRef]
- Darme, P.; Escotte-Binet, S.; Cordonnier, J.; Remy, S.; Hubert, J.; Sayagh, C.; Borie, N.; Villena, I.; Voutquenne-Nazabadioko, L.; Dauchez, M.; et al. Anti-Toxoplasma gondii effect of lupane-type triterpenes from the bark of black alder (Alnus glutinosa) and identification of a potential target by reverse docking. Parasite 2022, 29, 7. [Google Scholar] [CrossRef]
- Darme, P.; Spalenka, J.; Hubert, J.; Escotte-Binet, S.; Debelle, L.; Villena, I.; Sayagh, C.; Borie, N.; Martinez, A.; Bertaux, B. Investigation of antiparasitic activity of ten European tree bark extracts on Toxoplasma gondii and bioguided identification of triterpenes in Alnus glutinosa barks. Antimicrob. Agents Chemother. 2021, 66, e01098-21. [Google Scholar]
- Endo, M.; Shigetomi, K.; Mitsuhashi, S.; Igarashi, M.; Ubukata, M. Isolation, structure determination and structure–activity relationship of anti-toxoplasma triterpenoids from Quercus crispula Blume outer bark. J. Wood Sci. 2019, 65, 3. [Google Scholar] [CrossRef]
- De Pablos, L.M.; González, G.; Rodrigues, R.; García Granados, A.; Parra, A.; Osuna, A. Action of a Pentacyclic Triterpenoid, Maslinic Acid, against Toxoplasma gondii. J. Nat. Prod. 2010, 73, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Mahizan, N.A.; Yang, S.K.; Moo, C.L.; Song, A.A.; Chong, C.M.; Chong, C.W.; Abushelaibi, A.; Lim, S.E.; Lai, K.S. Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [PubMed]
- De-Almeida, S.C.X.; da-Silva, Â.; Sousa, N.R.T.; Amorim, I.H.F.; Leite, B.G.; Neves, K.R.T.; Costa, J.G.M.; Felipe, C.F.B.; de-Barros Viana, G.S. Antinociceptive and anti-inflammatory activities of a triterpene-rich fraction from Himatanthus drasticus. Braz. J. Med. Biol. Res. 2019, 52, e7798. [Google Scholar] [CrossRef]
- Pereira, A.C.A.; Silva, R.J.; Franco, P.S.; de Oliveira Gomes, A.; Souza, G.; Milian, I.C.B.; Ribeiro, M.; Rosini, A.M.; Guirelli, P.M.; Ramos, E.L.P. Cyclooxygenase (COX)-2 inhibitors reduce Toxoplasma gondii infection and upregulate the pro-inflammatory immune response in Calomys callosus rodents and human monocyte cell line. Front. Microbiol. 2019, 10, 225. [Google Scholar] [CrossRef]
- de Souza, G.; Silva, R.J.; Milián, I.C.B.; Rosini, A.M.; de Araújo, T.E.; Teixeira, S.C.; Oliveira, M.C.; Franco, P.S.; da Silva, C.V.; Mineo, J.R.; et al. Cyclooxygenase (COX)-2 modulates Toxoplasma gondii infection, immune response and lipid droplets formation in human trophoblast cells and villous explants. Sci. Rep. 2021, 11, 12709. [Google Scholar] [CrossRef]
- Wu, R.-Z.; Zhou, H.-Y.; Song, J.-F.; Xia, Q.-H.; Hu, W.; Mou, X.-D.; Li, X. Chemotherapeutics for Toxoplasma gondii: Molecular Biotargets, Binding Modes, and Structure–Activity Relationship Investigations. J. Med. Chem. 2021, 64, 17627–17655. [Google Scholar] [CrossRef]
- Smith, T.J. Squalene: Potential chemopreventive agent. Expert Opin. Investig. Drugs 2000, 9, 1841–1848. [Google Scholar] [CrossRef]
- Adedoyin, B.J.; Okeniyi, S.O.; Garba, S.; Salihu, L. Cytotoxicity, antioxidant and antimicrobial activities of essential oil extracted from Euphorbia heterophylla plant. Topclass J. Herb. Med. 2013, 2, 84–89. [Google Scholar]
- Popović, M.; Jukić Špika, M.; Veršić Bratinčević, M.; Ninčević, T.; Matešković, A.; Mandušić, M.; Rošin, J.; Nazlić, M.; Dunkić, V.; Vitanović, E. Essential Oil Volatile Fingerprint Differentiates Croatian cv. Oblica from Other Olea europaea L. Cultivars. Molecules 2021, 26, 3533. [Google Scholar] [CrossRef]
- Senthilkumar, A.; Thangamani, A.; Karthishwaran, K.; Cheruth, A.J. Essential oil from the seeds of Moringa peregrina: Chemical composition and antioxidant potential. S. Afr. J. Bot. 2020, 129, 100–105. [Google Scholar] [CrossRef]
- Naman, C.B.; Gomes, C.M.; Gupta, G. Chapter 9—Phytodrugs and Immunomodulators for the Therapy of Leishmaniasis. In Natural Products and Drug Discovery; Mandal, S.C., Mandal, V., Konishi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 213–275. [Google Scholar]
- Tsukamoto, S.; Kato, H.; Hirota, H.; Fusetani, N. Antibacterial and antifungal sulfated alkane and alkenes from the hepatopancreas of the ascidian Halocynthia roretzi. J. Nat. Prod. 1994, 57, 1606–1609. [Google Scholar] [CrossRef] [PubMed]
- Kizhakkekalam, V.K.; Chakraborty, K. Seaweed-associated heterotrophic bacteria: New paradigm of prospective anti-infective and anticancer agents. Arch. Microbiol. 2021, 203, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Ibáñez-Escribano, A.; Meneses-Marcel, A.; Marrero-Ponce, Y.; Nogal-Ruiz, J.J.; Arán, V.J.; Gómez-Barrio, A.; Escario, J.A. A sequential procedure for rapid and accurate identification of putative trichomonacidal agents. J. Microbiol. Methods 2014, 105, 162–167. [Google Scholar] [CrossRef]
- Rivera Fernández, N.; Mondragón Castelán, M.; González Pozos, S.; Ramírez Flores, C.J.; Mondragón González, R.; Gómez De León, C.T.; Castro Elizalde, K.N.; Marrero Ponce, Y.; Arán, V.J.; Martins Alho, M.A.; et al. A new type of quinoxalinone derivatives affects viability, invasion, and intracellular growth of Toxoplasma gondii tachyzoites in vitro. Parasitol. Res. 2016, 115, 2081–2096. [Google Scholar] [CrossRef]
- Risopatrón, J.; Merino, O.; Cheuquemán, C.; Figueroa, E.; Sánchez, R.; Farías, J.G.; Valdebenito, I. Effect of the age of broodstock males on sperm function during cold storage in the trout (Oncorhynchus mykiss). Andrologia 2018, 50, e12857. [Google Scholar] [CrossRef]
- Spalenka, J.; Escotte-Binet, S.; Bakiri, A.; Hubert, J.; Renault, J.-H.; Velard, F.; Duchateau, S.; Aubert, D.; Huguenin, A.; Villena, I. Discovery of New Inhibitors of Toxoplasma gondii via the Pathogen Box. Antimicrob. Agents Chemother. 2018, 62, e01640-17. [Google Scholar] [CrossRef]
- Srikantha, K.V.; Chethan Kumar, V.K.; Nagaratna, S.J.; Sunil Kumar, K.N.; Suchitra, N. Gas chromatography–Mass spectrometric analysis of Haritakyadi eye drops: A poly herbal compound for ophthalmia neonatorum. J. Ayurvedic Herb. Med. 2019, 5, 103–105. [Google Scholar]
- Escribano, A.I.; Marcel, A.M.; Tugores, Y.M.; Ruiz, J.J.N.; Redó, V.J.A.; García-Trevijano, J.A.E.; Barrio, A.G. Validation of a modified fluorimetric assay for the screening of trichomonacidal drugs. Memórias Inst. Oswaldo Cruz 2012, 107, 637–643. [Google Scholar] [CrossRef]
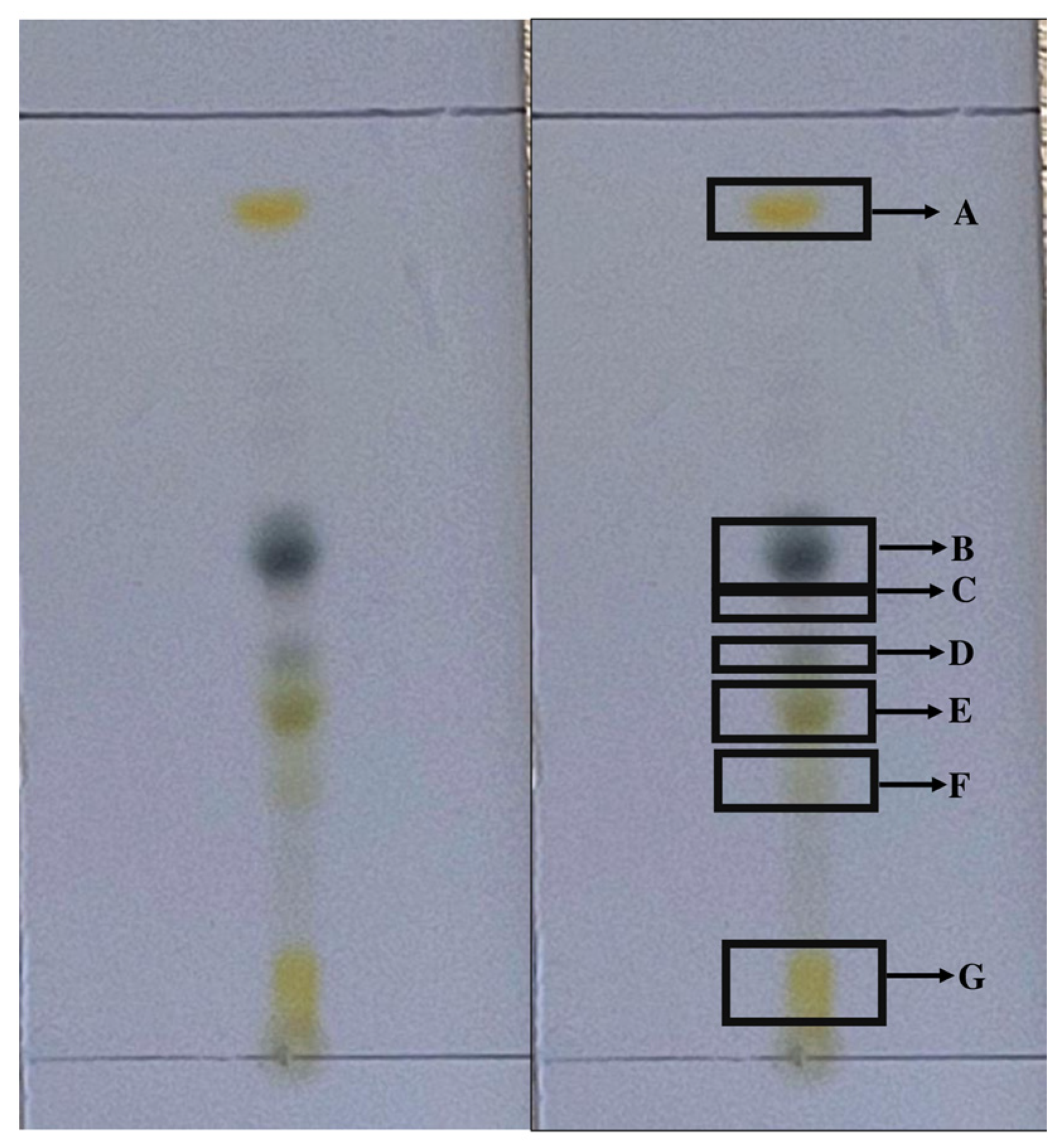
| Subfraction | Hsf1 | Hsf2 | Hsf3 | Hsf4 | Hsf5 | Hsf6 | Residual |
|---|---|---|---|---|---|---|---|
| Yield mg/500 mg Hexane fraction | 167.3 | 42.5 | 11.4 | 71.4 | 27.8 | 42.8 | 130 |
| Yield % | 33.3 | 8.5 | 2.28 | 14.28 | 5.56 | 8.84 | 26 |
| Batch | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Hexane fraction (g) | 1. 5409 | 1.3013 | 1.5087 | 1.7367 | 1.4950 | 1.8501 |
| Active subfraction (g) | 0.8511 | 0.4132 | 0.7375 | 0.6338 | 0.5968 | 0.9885 |
| Hsf1 yield (%) | 55.23 | 31.75 | 48.88 | 36.49 | 39.91 | 53.42 |
| Sample | 1 µg/mL | 5 µg/mL | 10 µg/mL | 25 µg/mL | 50 µg/mL | µg/mL |
|---|---|---|---|---|---|---|
| E (%) | E (%) | E (%) | E (%) | E (%) | IC50 | |
| Hsf1 | 95.35 ± 1.52 | 90 ± 2.18 | 69.67 ± 3.38 | 49.45 ± 2.83 | 0 | 23.69 |
| Hsf2 | 100 | 100 | 100 | 100 | 96.12 ± 1.83 | NC |
| Hsf3 | 100 | 100 | 100 | 100 | 77.8 ± 3.67 | NC |
| Hsf4 | 100 | 100 | 98.6 ± 1.71 | 69.56 ± 3.16 | 56.45 ± 4.99 | 53.18 |
| Hsf5 | 100 | 97.12 ± 1.16 | 75.45 ± 2.36 | 58.57 ± 4.04 | 0 | 28.69 |
| Hsf6 | 100 | 100 | 100 | 99.7 ± 1.20 | 98.67 ± 0.38 | NC |
| Hsf7 | 100 | 100 | 100 | 99.7 ± 0.38 | 99.55 ± 0.50 | NC |
| Elution Order | Compound | Retention Time | Formula | Area % | Synonyms |
|---|---|---|---|---|---|
| 1 | Dodecane | 9.803 | C12H26 | 1.795 | Dihexyl |
| 2 | Tetradecane | 12.568 | C14H30 | 2.656 | N- Tetradecane |
| 3 | Hexadecane | 15.032 | C16H34 | 4.492 | etane |
| 4 | Octadecane | 17.245 | C18H38 | 2.395 | n-Octadecane |
| 5 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 17.666 | C20H40O | 2.450 | Phytol |
| 6 | Hexadecanoic acid, methyl ester | 18.566 | C17H34O2 | 18.054 | Methyl Palmitate |
| 7 | 8-Octadecenoic acid, methyl ester | 20.247 | C19H36O2 | 12.535 | Methyl 8-octadecenoate |
| 8 | Octadecanoic acid, methyl ester | 20.464 | C19H38O2 | 1.910 | Methyl Estereate |
| 9 | 5,8,11,14-Eicpsatetraenoic acid, methyl ester, (all-Z)- | 21.679 | C21H34O2 | 0.606 | Arachidonic acid methyl ester |
| 10 | Eicosanoid acid, methyl ester | 22.224 | C21H42O2 | 0.677 | Methyl arachisate |
| 11 | Docosanoic acid, methyl ester | 24.313 | C23H46O2 | 1.335 | Behenic acid, methyl ester |
| 12 | Tetracosanoic acid, methyl ester | 27.459 | C25H50O2 | 2.327 | Methyl lignocerate |
| 13 | Squalene | 28.951 | C30H50 | 2.215 | Spinacene |
| 14 | A-Neogammacer-22(29)-ene | 33.109 | C30H50 | 7.219 | Diploptene |
| 15 | Olean-13(18)-ene | 33.404 | C30H50 | 16.194 | - |
| 16 | 6a,14a-methanopicene, perhydro-1,2,4a,6b,9,9,12a-heptamethyl-10-hydroxy | 34.586 | C30H5O | 4.723 | - |
| 17 | 2,2,4a,8a,9,12b,14a-Octamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,12,12a,12b,13,14,14a,14b-eicosahydropicene | 35.611 | C30H50 | 12.996 | - |
| 18 | 9,19-Cyclolanost-24-en-3-ol, acetate | 39.664 | C32H5O2 | 5.422 | Cycloartenol acetate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anacleto-Santos, J.; Calzada, F.; López-Camacho, P.Y.; López-Pérez, T.d.J.; Carrasco-Ramírez, E.; Casarrubias-Tabarez, B.; Fortoul, T.I.; Rojas-Lemus, M.; López-Valdés, N.; Rivera-Fernández, N. Evaluation of the Anti-Toxoplasma gondii Efficacy, Cytotoxicity, and GC/MS Profile of Pleopeltis crassinervata Active Subfractions. Antibiotics 2023, 12, 889. https://doi.org/10.3390/antibiotics12050889
Anacleto-Santos J, Calzada F, López-Camacho PY, López-Pérez TdJ, Carrasco-Ramírez E, Casarrubias-Tabarez B, Fortoul TI, Rojas-Lemus M, López-Valdés N, Rivera-Fernández N. Evaluation of the Anti-Toxoplasma gondii Efficacy, Cytotoxicity, and GC/MS Profile of Pleopeltis crassinervata Active Subfractions. Antibiotics. 2023; 12(5):889. https://doi.org/10.3390/antibiotics12050889
Chicago/Turabian StyleAnacleto-Santos, Jhony, Fernando Calzada, Perla Yolanda López-Camacho, Teresa de Jesús López-Pérez, Elba Carrasco-Ramírez, Brenda Casarrubias-Tabarez, Teresa I. Fortoul, Marcela Rojas-Lemus, Nelly López-Valdés, and Norma Rivera-Fernández. 2023. "Evaluation of the Anti-Toxoplasma gondii Efficacy, Cytotoxicity, and GC/MS Profile of Pleopeltis crassinervata Active Subfractions" Antibiotics 12, no. 5: 889. https://doi.org/10.3390/antibiotics12050889
APA StyleAnacleto-Santos, J., Calzada, F., López-Camacho, P. Y., López-Pérez, T. d. J., Carrasco-Ramírez, E., Casarrubias-Tabarez, B., Fortoul, T. I., Rojas-Lemus, M., López-Valdés, N., & Rivera-Fernández, N. (2023). Evaluation of the Anti-Toxoplasma gondii Efficacy, Cytotoxicity, and GC/MS Profile of Pleopeltis crassinervata Active Subfractions. Antibiotics, 12(5), 889. https://doi.org/10.3390/antibiotics12050889









