Improving Turnaround Times for Routine Antimicrobial Sensitivity Testing Following European Committee on Antimicrobial Susceptibility Testing Methodology in Patients with Bacteraemia
Abstract
:1. Introduction
2. Results
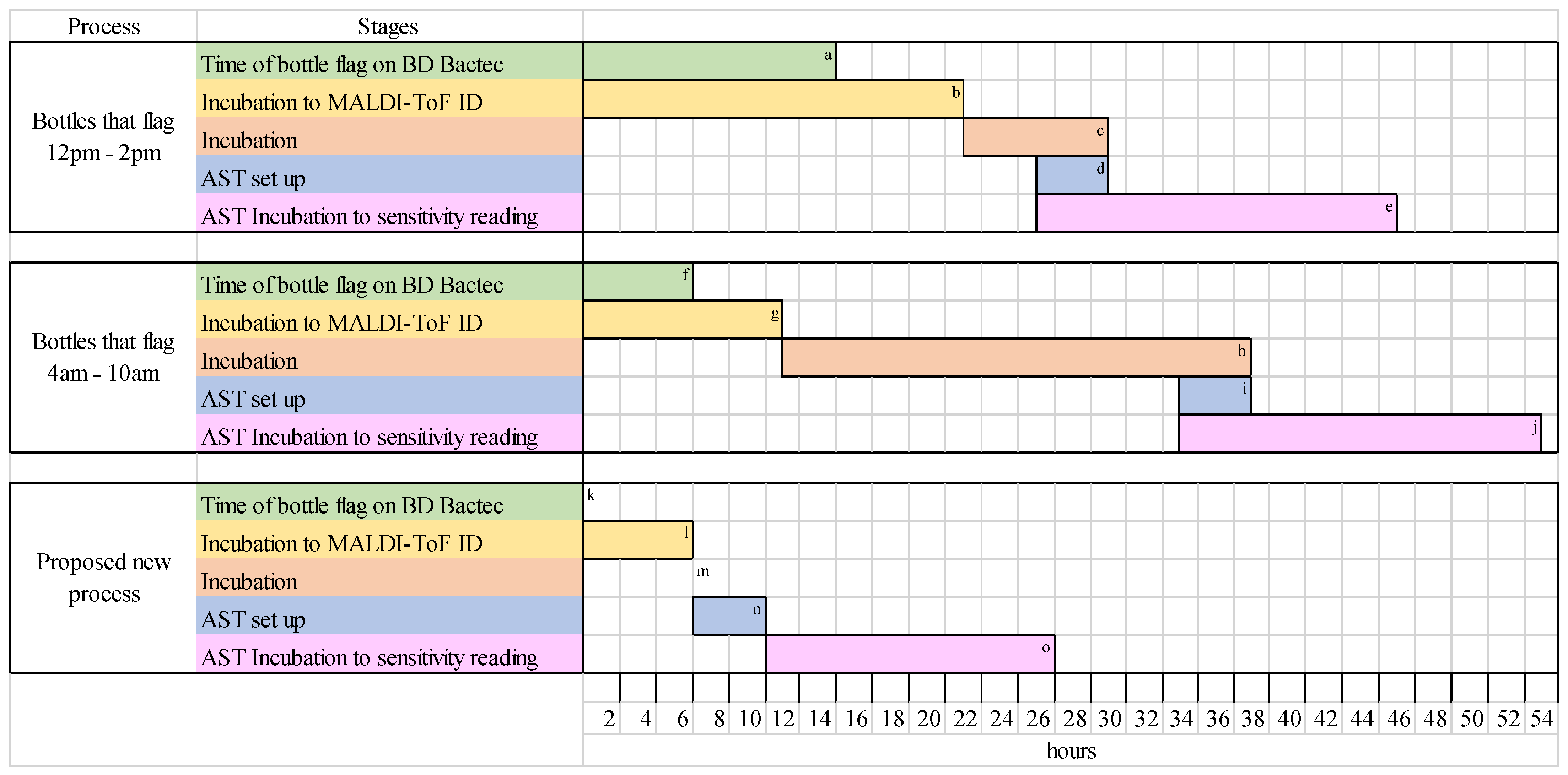
2.1. Qualitative Results
2.2. Quantitative Results
3. Discussion
4. Materials and Methods
4.1. Culture Plate Set Up and Organism Identification
4.2. Performing AST According to EUCAST
4.3. Data Collection
4.4. Data Analysis
- Qualitative results of sensitive (S), resistant (R), susceptible with increased exposure (I), and ATU from AST of 6 and 24 h cultures, for each antibiotic tested on each specimen. Qualitative results were used to determine sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), and accuracy of the 6-h culture method.
- Quantitative results of zone diameter sizes (mm) for each antibiotic for each specimen from 6 and 24 h cultures that had AST performed on them. R2 was calculated using Microsoft Excel software. Linear regression analysis was conducted using the lm function in R (version 4.3.2; R Core Team, Vienna, Austria).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verway, M.; Brown, K.A.; Marchand-Austin, A.; Diong, C.; Lee, S.; Langford, B.; Schwartz, K.L.; MacFadden, D.R.; Patel, S.N.; Sander, B.; et al. Prevalence and Mortality Associated with Bloodstream Organisms: A Population Wide Retrospective Cohort Study. J. Clin. Microbiol. 2022, 60, e0242921. [Google Scholar] [CrossRef] [PubMed]
- Daneman, N.; Fridman, D.; Johnstone, J.; Langford, B.J.; Lee, S.M.; MacFadden, D.M.; Mponponsuo, K.; Patel, S.N.; Schwartz, K.L.; Brown, K.A. Antimicrobial resistance and mortality following E. coli bacteraemia. EClinicalMedicine 2023, 56, 101781. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Al-Hasan, M.N. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin. Microbiol. Infect. 2013, 19, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.B.; Church, D.L. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin. Microbiol. Rev. 2014, 27, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Cisterna, R.; Cabezas, V.; Gomez, E.; Busto, C.; Atutxa, I.; Ezpeleta, C. Community-acquired bacteraemia. Rev. Esp. Quimioter. 2001, 14, 369–382. [Google Scholar] [PubMed]
- Elhanan, G.; Raz, R.; Pitlik, S.D.; Sharif, R.; Konisberger, H.; Samra, Z.; Kennes, Y.; Drucker, M.; Leibovici, L. Bacteraemia in a community and university hospital. J. Antimicrob. Chemother. 1995, 36, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Whiles, B.B.; Deis, A.S.; Simpson, S.Q. Increased time to Initial Antimicrobial Administration is Associated with Progression to Septic Shock in Severe Sepsis Patients. Crit. Care Med. 2017, 45, 623–629. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation (WHO). Comprehensive Review of the WHO Global Action Plan on Antimicrobial Resistance. 2021. Available online: https://cdn.who.int/media/docs/default-source/documents/about-us/evaluation/gap-amr-final-report-v2.pdf?sfvrsn=1db7e8b0_1&download=true%22%20%5Cl%20%22:~:text=The%20GAP%20AMR%20provides%20a,national%20action%20plans%20 (accessed on 31 May 2023).
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. 2022. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf (accessed on 15 February 2022).
- Minejima, E.; Mai, N.; Bui, N.; Mert, M.; Mack, W.J.; She, R.C.; Nieberg, P.; Spellberg, B.; Wong-Beringer, A. Defining the breakpoint duration of Staphylococcus aureus bacteraemia predictive of poor outcomes. Clin. Infect. Dis. 2020, 70, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Strich, J.R.; Heil, E.L.; Masur, H. Considerations for Empiric Antimicrobial Therapy in Sepsis and Septic Shock in an Era of Antimicrobial Resistance. J. Infect. Dis. 2020, 222, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Jonasson, E.; Matuschek, E.; Kahlmeter, G. The EUCAST rapid disc diffusion method for antimicrobial susceptibility testing directly from positive blood culture bottles. J. Antimicrob. Chemother. 2020, 75, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Expected Susceptible Phenotypes. Version 1.1. 2022. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Expert_Rules/2022/Expected_Susceptible_Phenotypes_Tables_v1.1_20220325.pdf (accessed on 20 April 2022).
- Flanagan, J.N.; Steck, T.R. The relationship between agar thickness and antimicrobial susceptibility testing. Indian. J. Microbiol. 2017, 57, 503–506. [Google Scholar] [CrossRef] [PubMed]
- EUCAST Reading Guide for Broth Microdilution. Version 5.0. 2024. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/MIC_testing/Reading_guide_BMD_v_5.0_2024.pdf (accessed on 22 October 2024).
- Anton-Vasquez, V.; Adjepong, S.; Suarez, C.; Planche, T. Evaluation of new rapid antimicrobial susceptibility system for Gram-negative and Gram-positive bloodstream infections: Speed and accuracy of Alfred 60AST. BMC Microbiol. 2019, 19, 268. [Google Scholar] [CrossRef] [PubMed]
- Q-Linea. ASTar—Designed to Save Lifetimes. 2021. Available online: https://www.qlinea.com/0986741_wp-uploads/2021/05/D30109.pdf (accessed on 17 April 2022).
- Charnot-Katsikas, A.; Tesic, V.; Love, N.; Hill, B.; Bethel, C.; Boonlayangoor, S.; Beavis, K.G. Use of the Accelerate Pheno System for Identification and Antimicrobial Susceptibility Testing of Pathogens in positive blood cultures and impact on time to results and workflow. J. Clin. Microbiol. 2017, 56, e01166-17. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, R.; George, S.; Burwell, R.; Rajeev, L.; Rhodes, P.A.; Singh, P.; Samuel, L. Performance of the Reveal rapid antibiotic susceptibility testing system on Gram-negative blood cultures at a large urban hospital. J. Clin. Microbiol. 2022, 60, e0009822. [Google Scholar] [CrossRef] [PubMed]
- Bruker. Instructions for Use. Available online: https://www.bruker.com/en/resources/certificates-data-sheets/ifu.html?page_1=28 (accessed on 20 March 2023).

| 24-h AST Result | |||||
|---|---|---|---|---|---|
| S | R | I | ATU | ||
| Six hour AST result | S | 631 | 1 * | ||
| R | 2 * | 213 | |||
| I | 4 | ||||
| ATU | 7 | ||||
| Organism | ATCC/NCTC Number | Resistance Mechanism |
|---|---|---|
| S. aureus | ATCC 33591 | MRSA |
| E. faecium | NCTC 12202 | VRE |
| E. coli | NCTC 13476 | IMP |
| K. pneumoniae | ATCC 700603 | ESBL |
| K. pneumoniae | NCTC 13439 | VIM-1 |
| K. pneumoniae | ATCC BAA-2814 | KPC-3 |
| K. pneumoniae | NCTC 13438 | KPC-3 |
| K. pneumoniae | NCTC 13442 | OXA-48 |
| K. pneumoniae | NCTC 13443 | NDM-1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edmondson, R.; Saeed, K.; Green, S.; O’Dwyer, M. Improving Turnaround Times for Routine Antimicrobial Sensitivity Testing Following European Committee on Antimicrobial Susceptibility Testing Methodology in Patients with Bacteraemia. Antibiotics 2024, 13, 1094. https://doi.org/10.3390/antibiotics13111094
Edmondson R, Saeed K, Green S, O’Dwyer M. Improving Turnaround Times for Routine Antimicrobial Sensitivity Testing Following European Committee on Antimicrobial Susceptibility Testing Methodology in Patients with Bacteraemia. Antibiotics. 2024; 13(11):1094. https://doi.org/10.3390/antibiotics13111094
Chicago/Turabian StyleEdmondson, Raewyn, Kordo Saeed, Steve Green, and Matthew O’Dwyer. 2024. "Improving Turnaround Times for Routine Antimicrobial Sensitivity Testing Following European Committee on Antimicrobial Susceptibility Testing Methodology in Patients with Bacteraemia" Antibiotics 13, no. 11: 1094. https://doi.org/10.3390/antibiotics13111094
APA StyleEdmondson, R., Saeed, K., Green, S., & O’Dwyer, M. (2024). Improving Turnaround Times for Routine Antimicrobial Sensitivity Testing Following European Committee on Antimicrobial Susceptibility Testing Methodology in Patients with Bacteraemia. Antibiotics, 13(11), 1094. https://doi.org/10.3390/antibiotics13111094






