Epidemiology and Impact of Anti-Pneumococcal Vaccination and COVID-19 on Resistance of Streptococcus pneumoniae Causing Invasive Disease in Piedmont, Italy
Abstract
:1. Introduction
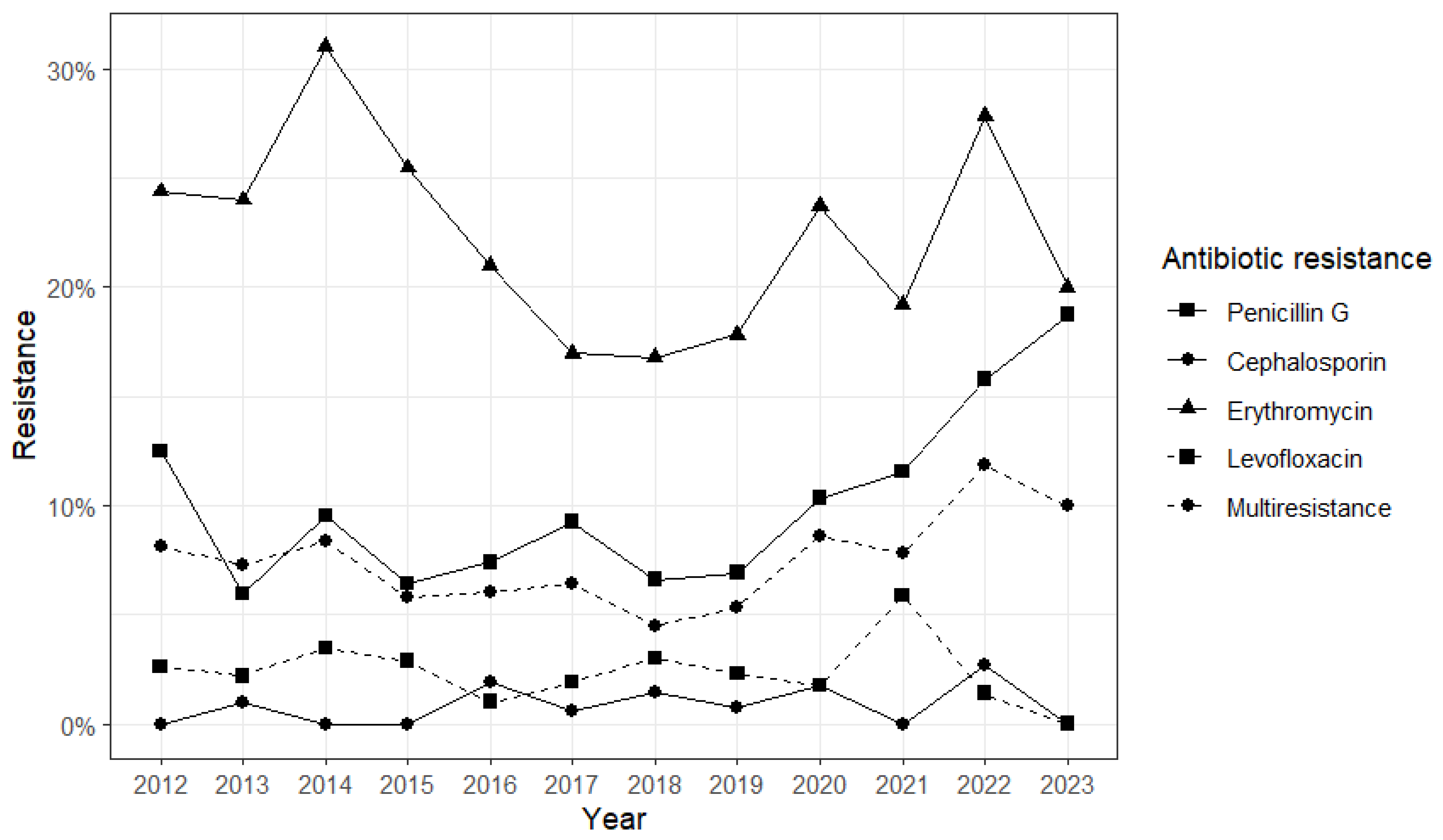
2. Results
3. Discussion
4. Materials and Methods
4.1. Clinical Data and Strain Collection
4.2. S. pneumoniae Serotyping
4.3. Antimicrobial Resistance Data and Definitions
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adegbola, R.A.; DeAntonio, R.; Hill, P.C.; Roca, A.; Usuf, E.; Hoet, B.; Greenwood, B.M. Carriage of Streptococcus pneumoniae and other respiratory bacterial pathogens in low and lower-middle income countries: A systematic review and meta-analysis. PLoS ONE 2014, 9, e103293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teixeira, R.; Kossyvaki, V.; Galvez, P.; Méndez, C. Pneumococcal Serotype Evolution and Burden in European Adults in the Last Decade: A Systematic Review. Microorganisms 2023, 11, 1376. [Google Scholar] [CrossRef] [PubMed]
- Bridy-Pappas, A.E.; Margolis, M.B.; Center, K.J.; Isaacman, D.J. Streptococcus pneumoniae: Description of the pathogen, disease epidemiology, treatment, and prevention. Pharmacotherapy 2005, 25, 1193–1212. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.R.; Link-Gelles, R.; Schaffner, W.; Lynfield, R.; Lexau, C.; Bennett, N.M.; Petit, S.; Zansky, S.M.; Harrison, L.H.; Reingold, A.; et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in th USA: Analysis of multisite, population-based surveillance. Lancet Infect. Dis. 2015, 15, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Ladhani, S.N.; Collins, S.; Djennad, A.; Sheppard, C.L.; Borrow, R.; Fry, N.K.; Andrews, N.J.; Miller, E.; Ramsay, M.E. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–2017: A prospective national observational cohort study. Lancet Infect. Dis. 2018, 18, 441–451, Erratum in Lancet Infect Dis. 2018, 18, 376. [Google Scholar] [CrossRef] [PubMed]
- van de Beek, D.; Cabellos, C.; Dzupova, O.; Esposito, S.; Klein, M.; Kloek, A.T.; Leib, S.L.; Mourvillier, B.; Ostergaard, C.; Pagliano, P.; et al. ESCMID guideline: Diagnosis and treatment of acute bacterial meningitis. Clin. Microbiol. Infect. 2016, 22 (Suppl. S3), S37–S62. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655, Erratum in Lancet 2022, 400, 1102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andrejko, K.; Ratnasiri, B.; Hausdorff, W.P.; Laxminarayan, R.; Lewnard, J.A. Antimicrobial resistance in paediatric Streptococcus pneumoniae isolates amid global implementation of pneumococcal conjugate vaccines: A systematic review and meta-regression analysis. Lancet Microbe 2021, 2, e450–e460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Plainvert, C.; Varon, E.; Viriot, D.; Kempf, M.; French Regional Pneumococcal Observatories (ORP) network. Invasive pneumococcal infections in France: Changes from 2009 to 2021 in antibiotic resistance and serotype distribution of Streptococcus pneumoniae based on data from the French Regional Pneumococcal Observatories network. Infect. Dis. Now. 2023, 53, 104632. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Johnson, K.D.; Yu, K.C.; Watts, J.A.; Gupta, V. A Multicenter Evaluation of Trends in Antimicrobial Resistance Among Streptococcus pneumoniae Isolates from Adults in the United States. Open Forum Infect. Dis. 2022, 9, ofac420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matanock, A.; Lee, G.; Gierke, R.; Kobayashi, M.; Leidner, A.; Pilishvili, T. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged ≥65 Years: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1069–1075, Erratum in MMWR Morb. Mortal. Wkly. Rep. 2020, 68, 1195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, P.J.; Hung, M.C.; Srivastav, A.; Grohskopf, L.A.; Kobayashi, M.; Harris, A.M.; Dooling, K.L.; Markowitz, L.E.; Rodriguez-Lainz, A.; Williams, W.W. Surveillance of Vaccination Coverage Among Adult Populations—United States, 2018. MMWR Surveill Summ. 2021, 70, 1–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kyaw, M.H.; Lynfield, R.; Schaffner, W.; Craig, A.S.; Hadler, J.; Reingold, A.; Thomas, A.R.; Harrison, L.H.; Bennett, N.M.; Farley, M.M.; et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N. Engl. J. Med. 2006, 354, 1455–1463, Erratum in N. Engl. J. Med. 2006, 355, 638. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Dagan, R.; Klugman, K.P.; Fritzell, B. The relationship between pneumococcal serotypes and antibiotic resistance. Vaccine 2012, 30, 2728–2737. [Google Scholar] [CrossRef] [PubMed]
- Dagan, R.; Klugman, K.P. Impact of conjugate pneumococcal vaccines on antibiotic resistance. Lancet Infect. Dis. 2008, 8, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Van Effelterre, T.; Moore, M.R.; Fierens, F.; Whitney, C.G.; White, L.; Pelton, S.I.; Hausdorff, W.P. A dynamic model of pneumococcal infection in the United States: Implications for prevention through vaccination. Vaccine 2010, 28, 3650–3660. [Google Scholar] [CrossRef] [PubMed]
- Fenoll, A.; Granizo, J.J.; Aguilar, L.; Giménez, M.J.; Aragoneses-Fenoll, L.; Hanquet, G.; Casal, J.; Tarragó, D. Temporal trends of invasive Streptococcus pneumoniae serotypes and antimicrobial resistance patterns in Spain from 1979 to 2007. J. Clin. Microbiol. 2009, 47, 1012–1020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sempere, J.; Llamosí, M.; López Ruiz, B.; Del Río, I.; Pérez-García, C.; Lago, D.; Gimeno, M.; Coronel, P.; González-Camacho, F.; Domenech, M.; et al. Effect of pneumococcal conjugate vaccines and SARS-CoV-2 on antimicrobial resistance and the emergence of Streptococcus pneumoniae serotypes with reduced susceptibility in Spain, 2004–2020: A national surveillance study. Lancet Microbe 2022, 3, e744–e752. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- PRINCIPLE Trial Collaborative Group. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): A randomised, controlled, open-label, adaptive platform trial. Lancet 2021, 397, 1063–1074. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boccia, D.; Alegiani, S.S.; Pantosti, A.; Moro, M.L.; Traversa, G. The geographic relationship between the use of antimicrobial drugs and the pattern of resistance for Streptococcus pneumoniae in Italy. Eur. J. Clin. Pharmacol. 2004, 60, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Schito, A.M.; Schito, G.C.; Debbia, E.; Russo, G.; Liñares, J.; Cercenado, E.; Bouza, E. Antibacterial resistance in Streptococcus pneumoniae and Haemophilus influenzae from Italy and Spain: Data from the PROTEKT surveillance study, 1999–2000. J. Chemother. 2003, 15, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, M.; Aliberti, S.; Azzari, C.; Moriondo, M.; Nieddu, F.; Blasi, F.; Mantero, M. Serotypes and antibiotic susceptibility of Streptococcus pneumoniae isolated from hospitalised patients with community-acquired pneumonia in Italy. SAGE Open Med. 2017, 5, 2050312117720058. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monaco, M.; Camilli, R.; D’Ambrosio, F.; Del Grosso, M.; Pantosti, A. Evolution of erythromycin resistance in Streptococcus pneumoniae in Italy. J. Antimicrob. Chemother. 2005, 55, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Peradotto, M.; Bondi, A.; Lombardi, D.; Bottino, P.; Zanotto, E.; Barbui, A.M.; Cavallo, R. The impact of COVID-19 pandemic control on vaccine-preventable invasive bacterial diseases in Piedmont (Italy). Infection 2022, 50, 767–770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 1 February 2024).
- Bajema, K.L.; Gierke, R.; Farley, M.M.; Schaffner, W.; Thomas, A.; Reingold, A.L.; Harrison, L.H.; Lynfield, R.; Burzlaff, K.E.; Petit, S.; et al. Impact of Pneumococcal Conjugate Vaccines on Antibiotic-Nonsusceptible Invasive Pneumococcal Disease in the United States. J. Infect. Dis. 2022, 226, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Reyburn, R.; Maher, J.; von Mollendorf, C.; Gwee, A.; Mulholland, K.; Russell, F.; ARI Review group. The impact of the introduction of ten- or thirteen-valent pneumococcal conjugate vaccines on antimicrobial-resistant pneumococcal disease and carriage: A systematic literature review. J. Glob. Health 2023, 13, 05001. [Google Scholar] [CrossRef] [PubMed]
- Kournoutou, G.G.; Dinos, G. Azithromycin through the Lens of the COVID-19 Treatment. Antibiotics 2022, 11, 1063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://www.eucast.org/ (accessed on 1 March 2024).

| Infant (≤2 yo) (n = 74) | Child (>2 and ≤16 yo) (n = 118) | Adult (>16 and ≤64 yo) (n = 550) | Elderly (>64 yo) (n = 1334) | p-Value | |
|---|---|---|---|---|---|
| Age (years), median [IQR] | 0.644 [0.296–1.12] | 5.00 [3.42–7.22] | 52.1 [43.1–59.5] | 77.0 [70.6–83.7] | <0.001 |
| After 2010 vaccination policy | 51 (68.9%) | 67 (56.8%) | 546 (99.3%) | 1329 (99.6%) | <0.001 |
| After 2018 vaccination policy | 13 (17.6%) | 15 (12.7%) | 180 (32.7%) | 431 (32.3%) | <0.001 |
| After COVID-19 outbreak | 10 (13.5%) | 13 (11.0%) | 104 (18.9%) | 269 (20.2%) | 0.058 |
| Sepsis | 52 (70.3%) | 87 (73.7%) | 242 (44.0%) | 532 (39.9%) | <0.001 |
| Meningitis | 10 (13.5%) | 20 (16.9%) | 102 (18.5%) | 132 (9.9%) | <0.001 |
| Serotype included in the PCV13 vaccine | 33 (44.6%) | 70 (59.3%) | 182 (33.1%) | 475 (35.6%) | <0.001 |
| Serotype included in the PCV10 vaccine | 24 (32.4%) | 54 (45.8%) | 86 (15.6%) | 190 (14.2%) | <0.001 |
| Serotype | |||||
| 3 | 3 (4.1%) | 8 (6.8%) | 69 (12.5%) | 219 (16.4%) | <0.001 |
| 8 | 10 (13.5%) | 7 (5.9%) | 98 (17.8%) | 199 (14.9%) | |
| 12F | 0 (0%) | 10 (8.5%) | 44 (8.0%) | 95 (7.1%) | |
| Other | 50 (67.6%) | 86 (72.9%) | 267 (48.5%) | 668 (50.1%) | |
| Not typed | 11 (14.9%) | 7 (5.9%) | 72 (13.1%) | 153 (11.5%) | |
| Penicillin G resistance | 14 (21.9%) | 8 (8.6%) | 29 (6.5%) | 97 (8.9%) | <0.001 |
| Cephalosporin resistance | 1 (1.7%) | 0 (0%) | 1 (0.2%) | 10 (0.9%) | 0.303 |
| Erythromycin resistance | 25 (40.3%) | 15 (16.0%) | 81 (18.6%) | 236 (21.6%) | <0.001 |
| Levofloxacin resistance | 0 (0%) | 1 (1.1%) | 4 (0.9%) | 25 (2.4%) | 0.181 |
| Multiresistance | 12 (20.3%) | 3 (3.3%) | 14 (3.3%) | 74 (6.9%) | <0.001 |
| Serotype | 3 (n = 299) | 8 (n = 314) | 12F (n = 149) | Other (n = 1071) | Overall (n = 2076) |
|---|---|---|---|---|---|
| Resistant to: | |||||
| Penicillin G | 7 (3.0%) | 4 (1.6%) | 1 (0.8%) | 114 (13.1%) | 148 (8.8%) |
| Cephalosporin | 0 (0%) | 1 (0.4%) | 0 (0%) | 9 (1.0%) | 12 (0.7%) |
| Erythromycin | 42 (18.0%) | 10 (4.0%) | 4 (3.2%) | 258 (29.2%) | 357 (21.2%) |
| Levofloxacin | 4 (1.7%) | 0 (0%) | 1 (0.8%) | 18 (2.1%) | 30 (1.8%) |
| Multiresistant | 4 (1.7%) | 2 (0.8%) | 0 (0%) | 81 (9.6%) | 103 (6.3%) |
| Penicillin G Resistance | Erythromycin Resistance | Multiresistance | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | ORs | 95%CI | p-Value | ORs | 95%CI | p-Value | ORs | 95%CI | p-Value |
| 2010 vaccination policy: After | 3.87 | 1.29–12.8 | 0.019 | 0.86 | 0.36–2.1 | 0.734 | 3.78 | 1.00–16.9 | 0.060 |
| Age category (ref. Child): Infant | 3.64 | 1.37–10.4 | 0.012 | 5.08 | 2.27–12.0 | <0.001 | 11.5 | 3.15–56.9 | 0.001 |
| Serotype included in the PCV 13 vaccine: Yes | 5.98 | 1.98–20.3 | 0.002 | 4.30 | 1.70–11.8 | 0.003 | 10.6 | 2.61–56.7 | 0.002 |
| Observations | 157 | 156 | 150 | 150 | 150 | ||||
| R2 Tjur | 0.134 | 0.177 | 0.227 | 0.227 | 0.227 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondi, A.; Koumantakis, E.; Curtoni, A.; Barbui, A.M.; Peradotto, M.; Lombardi, D.; Casale, R.; Alizzi, S.; Zanotto, E.; Charrier, L.; et al. Epidemiology and Impact of Anti-Pneumococcal Vaccination and COVID-19 on Resistance of Streptococcus pneumoniae Causing Invasive Disease in Piedmont, Italy. Antibiotics 2024, 13, 740. https://doi.org/10.3390/antibiotics13080740
Bondi A, Koumantakis E, Curtoni A, Barbui AM, Peradotto M, Lombardi D, Casale R, Alizzi S, Zanotto E, Charrier L, et al. Epidemiology and Impact of Anti-Pneumococcal Vaccination and COVID-19 on Resistance of Streptococcus pneumoniae Causing Invasive Disease in Piedmont, Italy. Antibiotics. 2024; 13(8):740. https://doi.org/10.3390/antibiotics13080740
Chicago/Turabian StyleBondi, Alessandro, Emanuele Koumantakis, Antonio Curtoni, Anna Maria Barbui, Marco Peradotto, Daniela Lombardi, Roberto Casale, Silvia Alizzi, Elisa Zanotto, Lorena Charrier, and et al. 2024. "Epidemiology and Impact of Anti-Pneumococcal Vaccination and COVID-19 on Resistance of Streptococcus pneumoniae Causing Invasive Disease in Piedmont, Italy" Antibiotics 13, no. 8: 740. https://doi.org/10.3390/antibiotics13080740







