Chemical Characterization and Biological Properties Assessment of Euphorbia resinifera and Euphorbia officinarum Moroccan Propolis
Abstract
:1. Introduction
2. Results
2.1. Pollen Grains
2.2. Propolis Volatile Profile
2.3. Mineral Element Compounds
2.4. Total Phenol, Flavone, Flavonol, Flavanone and Dihydroflavonol Contents
2.5. Antimicrobial Activity
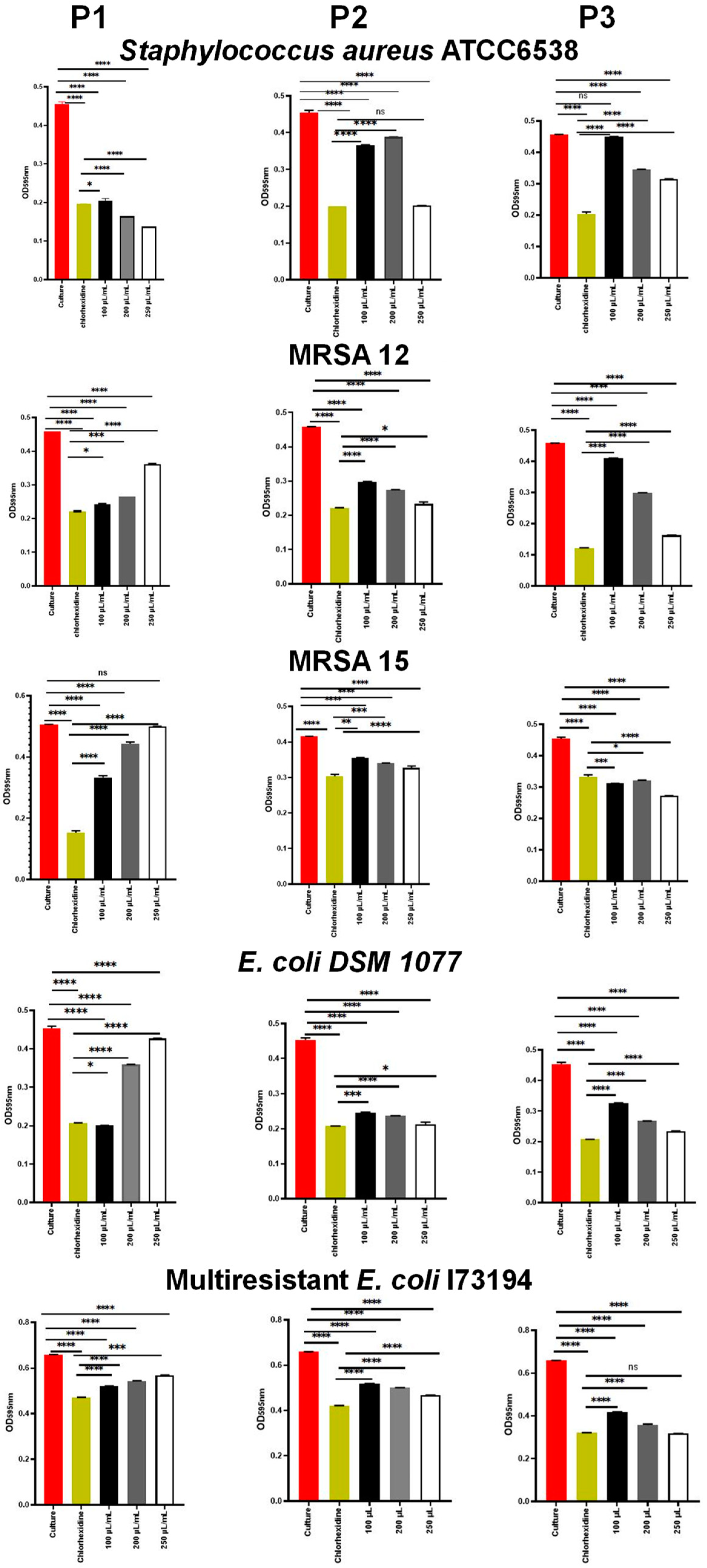
2.5.1. Antimicrobial Properties
2.5.2. Anti-Quorum-Sensing Activity
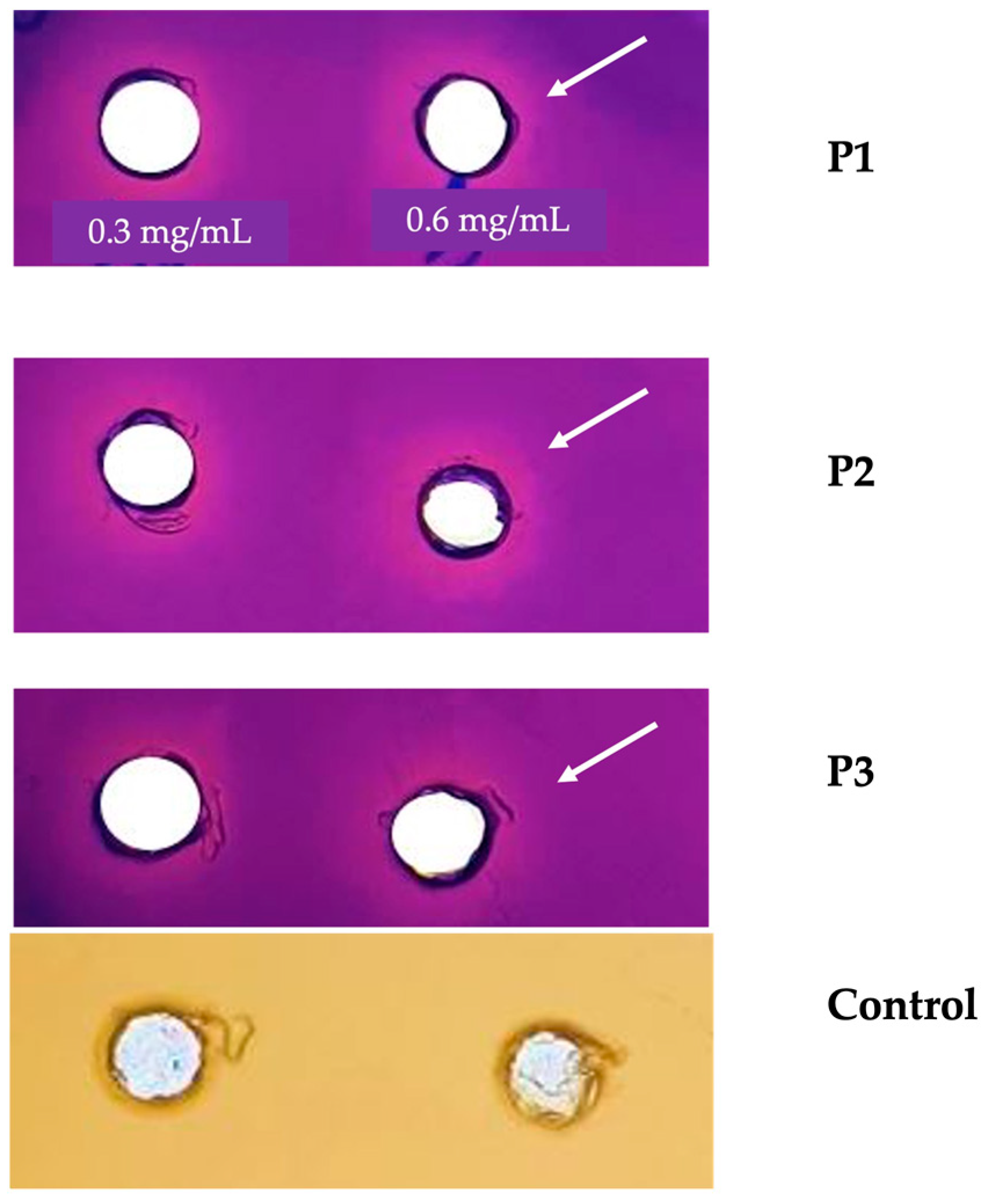
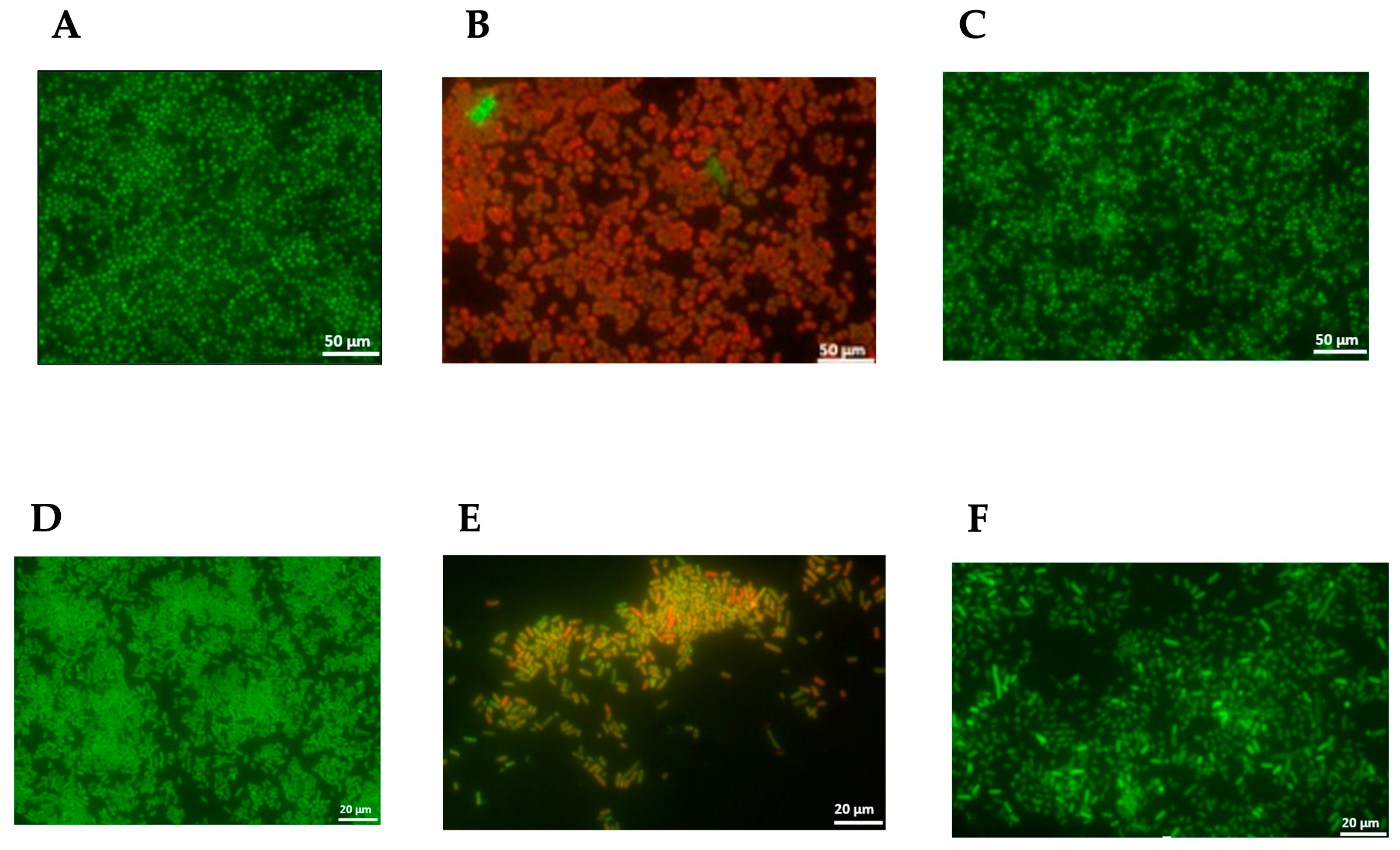
2.5.3. Anti-Adherence and Anti-Biofilm Activity
3. Discussion
3.1. Pollen Grains
3.2. Propolis Volatile Profile
3.3. Mineral Element Compounds
3.4. Total Phenol, Flavone, Flavonol, Flavanone and Dihydroflavonol Contents
3.5. Antimicrobial Activity
3.5.1. MIC and MBC Values
3.5.2. Anti-Quorum-Sensing Activity
3.5.3. Anti-Adherence and Anti-Biofilm Activity
4. Material and Methods
4.1. Propolis Collection
4.2. Evaluation of Pollen Grains
4.3. Volatile Organic Compound Extraction, Analysis and Identification
4.4. Quantification of Mineral Elements
4.5. Hydro-Alcoholic Propolis Extraction
4.6. Quantification of Total Phenol, Flavones, Flavonol, Flavanone and Dihydrofavonol Contents
4.6.1. Total Phenol Content
4.6.2. Total Flavone and Flavonol Contents
4.6.3. Total Flavanone and Dihydroflavonol Contents
4.7. Antimicrobial Activity
4.7.1. Determination of the Minimum Inhibitory Concentration
4.7.2. Evaluation of Anti-Adherence Activity
4.7.3. Determination of Anti-Biofilm Activity
4.7.4. Visualization of Biofilm Cells by Fluorescence Staining
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damodaran, T. Chapter 46—Propolis. In Nutraceuticals: Efficacy, Safety and Toxicity, 2nd ed.; Ramesh, C., Gupta, L., Srivastava, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 795–812. [Google Scholar] [CrossRef]
- Mohammadzadeh, S.; Sharriatpanahi, M.; Hamedi, M.; Amanzadeh, Y.; Sadat Ebrahimi, S.E.; Ostad, S.N. Antioxidant power of Iranian propolis extract. Food Chem. 2007, 103, 729–733. [Google Scholar] [CrossRef]
- Bankova, V.S.; de Castro, S.L.; Marcucci, M. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Becerra, T.L.; Calla-Poma, R.D.; Requena-Mendizabal, M.F.; Millones-Gómez, P. Antibacterial effect of Peruvian propolis collected during different seasons on the growth of Streptococcus mutans. Open Dentristy J. 2019, 13, 3–14. [Google Scholar] [CrossRef]
- Woźniak, M.; Mrówczyńska, L.; Waśkiewicz, A.; Rogoziński, T.; Ratajczak, I. The role of seasonality on the chemical composition, antioxidant activity and cytotoxicity of Polish propolis in human erythrocytes. Rev. Bras. Farmacogn. 2019, 29, 301–308. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial effects of flavonoids and their structure-activity relationship study: A comparative interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef]
- Li, F.; Awale, S.; Tezuka, Y.; Esumi, H.; Kadota, S. Study on the constituents of mexican propolis and their cytotoxic activity against PANC-1 human pancreatic cancer cells. J. Nat. Prod. 2010, 73, 623–627. [Google Scholar] [CrossRef]
- Demir, S.; Aliyazicioglu, Y.; Turan, I.; Misir, S.; Mentese, A.; Yaman, S.O.; Akbulut, K.; Kilinc, K.; Deger, O. Antiproliferative and proapoptotic activity of Turkish propolis on human lung cancer cell line. Nutr. Cancer 2016, 68, 165–172. [Google Scholar] [CrossRef]
- Silva, J.C.; Rodrigues, S.; Feás, X.; Estevinho, L.M. Antimicrobial activity, phenolic profile and role in the inflammation of propolis. Food Chem. Toxicol. 2012, 50, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Silva, B.; Rosalen, P.L.; Alencar, S.M.; Mayer, M.P.A. Anti-inflammatory mechanisms of neovestitol from Brazilian red propolis in LPS-activated macrophages. J. Funct. Foods 2017, 36, 440–447. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Zhang, G.; Kong, L.; Peng, W.; Zhang, H. Galangin and pinocembrin from propolis ameliorate insulin resistance in HepG2 cells via regulating Akt/mTOR signaling. Evid. -Based Complement Altern. Med. 2018, 2018, 7971842. [Google Scholar] [CrossRef] [PubMed]
- Pujirahayu, N.; Bhattacharjya, D.K.; Suzuki, T.; Katayama, T. α-Glucosidase inhibitory activity of cycloartane-type triterpenes isolated from indonesian stingless bee propolis and their structure-activity relationship. Pharmaceuticals 2019, 12, 102. [Google Scholar] [CrossRef]
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Aboulghazi, A.; Ferreira-Santos, P.; Genisheva, Z.; Lyoussi, B. Effect of antioxidant-rich propolis and bee pollen extracts against D-glucose induced type 2 diabetes in rats. Food Res. Int. 2020, 138, 109802. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- El Hamzaoui, N.; Barguigua, A.; Larouz, S.; Maouloua, M. Epidemiology of burn wound bacterial infections at a Meknes hospital, Morocco. New Microbes New Infect. 2020, 38, 100764. [Google Scholar] [CrossRef]
- Rode, D.K.H.; Singh, P.K.; Drescher, K. Multicellular and unicellular responses of microbial biofilms to stress. Biol. Chem. 2020, 401, 1365–1374. [Google Scholar] [CrossRef]
- Azevedo, N.F.; Allkja, J.; Goeres, D.M. Biofilms vs. cities and humans vs. aliens—A tale of reproducibility in biofilms. Trends Microbiol. 2021, 29, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Caldara, M.; Belgiovine, C.; Secchi, E.; Rusconi, R. Environmental, microbiological, and immunological features of bacterial biofilms associated with implanted medical devices. Clin. Microbiol. Rev. 2022, 35, e0022120. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Aazza, S.; Lyoussi, B.; Bankova, V.; Popova, M.; Neto, L.; Faleiro, M.L.; Da Graça Miguel, M. Moroccan Propolis: A natural antioxidant, antibacterial, and antibiofilm against Staphylococcus aureus with no induction of resistance after continuous exposure. Evid. -Based Complement. Altern. Med. 2018, 2018, 9759240. [Google Scholar] [CrossRef]
- Barth, O.M.; Da Luz, C.F.P. Palynological analysis of Brazilian geopropolis sediments. Grana 2003, 42, 121–127. [Google Scholar] [CrossRef]
- Moreira, L.; Dias, L.G.; Pereira, J.A.; Estevinho, L. Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal. Food Chem. Toxicol. 2008, 46, 3482–3485. [Google Scholar] [CrossRef]
- Guzelmeric, E.; Ristivojević, P.; Trifković, J.; Dastan, T.; Yilmaz, O.; Cengiz, O.; Yesilada, E. Authentication of Turkish propolis through HPTLC fingerprints combined with multivariate analysis and palynological data and their comparative antioxidant activity. LWT-Food Sci. Technol. 2018, 87, 23–32. [Google Scholar] [CrossRef]
- d’Albore, G.R. L’origine geographique de la propolis. Apidologie 1979, 10, 241–267. [Google Scholar]
- Villanueva-Gutiérrez, R.; Moguel-Ordóñez, Y.B.; Echazarreta-González, C.M.; Arana-López, G. Monofloral honeys in the Yucatán Peninsula, Mexico. Grana 2009, 48, 214–223. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Lyoussi, B.; Miguel, M.G.; Figueiredo, A.C. Characterization of volatiles from Moroccan propolis samples. J. Essent. Oil Res. 2019, 31, 27–33. [Google Scholar] [CrossRef]
- González, M.; García, M.E.; Slanis, A.; Bonini, A.; Fiedler, S.; Fariña, L.; Dellacassa, E.; Condurso, C.; Lorenzo, D.; Russo, M.; et al. Phytochemical findings evidencing botanical origin of new propolis type from North-West Argentina. Chem. Biodivers. 2019, 16, e1800442. [Google Scholar] [CrossRef] [PubMed]
- El Menyiy, N.; Bakour, M.; El Ghouizi, A.; El Guendouz, S.; Lyoussi, B. Influence of geographic origin and plant source on physicochemical properties, mineral content, and antioxidant and antibacterial activities of Moroccan propolis. Int. J. Food Sci. 2021, 2021, 5570224. [Google Scholar] [CrossRef]
- Laaroussi, H.; Ferreira-Santos, P.; Genisheva, Z.; Bakour, M.; Ousaaid, D.; Teixeira, J.A.; Lyoussi, B. Unraveling the chemical composition, antioxidant, α-amylase and α-glucosidase inhibition of Moroccan propolis. Food Biosci. 2021, 42, 101160. [Google Scholar] [CrossRef]
- Aboulghazi, A.; Bakour, M.; Fadil, M.; Lyoussi, B. Simultaneous optimization of extraction yield, phenolic compounds and antioxidant activity of Moroccan propolis extracts: Improvement of ultrasound-assisted technique using response surface methodology. Processes 2022, 10, 297. [Google Scholar] [CrossRef]
- Matuszewska, E.; Klupczynska, A.; Maciołek, K.; Kokot, Z.J.; Matysiak, J. Multielemental analysis of bee pollen, propolis, and royal jelly collected in west-central poland. Molecules 2021, 26, 2415. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, M.I.; Revilla, I.; Betances-Salcedo, E.V.; Vivar-Quintana, A.M. Pesticide residues and heavy metals in commercially processed propolis. Microchem. J. 2018, 143, 423–429. [Google Scholar] [CrossRef]
- Inmaculada González-Martín, M.; Escuredo, O.; Revilla, I.; Vivar-Quintana, A.M.; Carmen Coello, M.; Riocerezo, C.P.; Moncada, G.W. Determination of the mineral composition and toxic element contents of propolis by near infrared spectroscopy. Sensors 2015, 15, 27854–27868. [Google Scholar] [CrossRef]
- Miguel, M.d.G.; Doughmi, O.; Aazza, S.; Antunes, D.; Lyoussi, B. Antioxidant, anti-inflammatory and acetylcholinesterase inhibitory activities of propolis from different regions of Morocco. Food Sci. Biotechnol. 2014, 23, 313–322. [Google Scholar] [CrossRef]
- Touzani, S.; Al-Waili, N.; El Menyiy, N.; Filipic, B.; Pereyra, A.; El Arabi, I.; Al-Waili, W.; Lyoussi, B. Chemical analysis and antioxidant content of various propolis samples collected from different regions and their impact on antimicrobial activities. Asian Pac. J. Trop. Med. 2018, 11, 436–442. [Google Scholar] [CrossRef]
- El-Haskoury, R.; Al-Waili, N.; Kamoun, Z.; Makni, M.; Al-Waili, A.; Lyoussi, B. Antioxidant activity and protective effect of propolis against carbon tetrachloride-induced liver and kidney injury by modulation of oxidative parameters. Vet. World 2021, 14, 3076–3083. [Google Scholar] [CrossRef]
- Almuhayawi, M.S. Propolis as a novel antibacterial agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef]
- Bouchelaghem, S. Propolis characterization and antimicrobial activities against Staphylococcus aureus and Candida albicans: A review. Saudi J. Biol. Sci. 2022, 29, 1936–1946. [Google Scholar] [CrossRef]
- Santos, F.A.; Bastos, E.M.A.; Uzeda, M.; Carvalho, M.A.R.; Farias, L.M.; Moreira, E.S.A.; Braga, F.C. Antibacterial activity of Brazilian propolis and fractions against oral anaerobic bacteria. J. Ethnopharmacol. 2002, 80, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.A.; Zullkiflee, N.; Zaini, S.N.Z.; Taha, H.; Hashim, F.; Usman, A. Phytochemicals, mineral contents, antioxidants, and antimicrobial activities of propolis produced by Brunei stingless bees Geniotrigona thoracica, Heterotrigona itama, and Tetrigona binghami. Saudi J. Biol. Sci. 2020, 27, 2902–2911. [Google Scholar] [CrossRef]
- Kartal, M.; Yildiz, S.; Kaya, S.; Kurucu, S.; Topçu, G. Antimicrobial activity of propolis samples from two different regions of Anatolia. J. Ethnopharmacol. 2003, 86, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, I.; Karpiński, T.M. Antibacterial properties of propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed]
- Tamfu, A.N.; Ceylan, O.; Cârâc, G.; Talla, E.; Dinica, R.M. Antibiofilm and anti-quorum sensing potential of cycloartane-type triterpene acids from Cameroonian grassland propolis: Phenolic profile and antioxidant activity of crude extract. Molecules 2022, 27, 4872. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Bassler, B.L. Bassler Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Queiroga, M.C.; Laranjo, M.; Andrade, N.; Marques, M.; Costa, A.R.; Antunes, C.M. Antimicrobial, antibiofilm and toxicological assessment of propolis. Antibiotics 2023, 12, 347. [Google Scholar] [CrossRef]
- Santos, L.M.; Rodrigues, D.M.; Kalil, M.A.; Azevedo, V.; Meyer, R.; Umsza-Guez, M.A.; Machado, B.A.; Seyffert, N.; Portela, R.W. Activity of ethanolic and supercritical propolis extracts in Corynebacterium pseudotuberculosis and its associated biofilm. Front. Vet. Sci. 2021, 8, 700030. [Google Scholar] [CrossRef]
- Tennick, K.; Wafa, S.; Fearnley, H.; Gomez Escalada, M. Effects of propolis on the quorum sensing of selected biofilm producing bacterial species. J. Apitherapy Nat. 2018, 1, 8–19. [Google Scholar]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 5, 139–153. [Google Scholar] [CrossRef]
- Council of Europe; European Pharmacopoeia Commission; European Directorate for the Quality of medicines and Healthcare. European Pharmacopoeia, 7th ed.; Council Of Europe: Strasbourg, France, 2010. [Google Scholar]
- Boutoub, O.; El-Guendouz, S.; Manhita, A.; Dias, C.B.; Estevinho, L.M.; Paula, V.B.; Carlier, J.; Costa, M.C.; Rodrigues, B.; Raposo, S.; et al. Comparative study of the antioxidant and enzyme inhibitory activities of two types of Moroccan Euphorbia entire honey and their phenolic extracts. Foods 2021, 10, 1909. [Google Scholar] [CrossRef]
- Boutoub, O.; Aazza, S.; El-Guendouz, S.; El Ghadraoui, L.; Miguel, M. Response surface methodology (RSM) for optimization of Euphorbia resinifera and Euphorbia officinarum extracts with antioxidant and anti-diabetic activities. Pharmacogn. Mag. 2022, 18, 940–952. [Google Scholar]
- Apolónio, J.; Faleiro, M.L.; Miguel, M.G.; Neto, L. No induction of antimicrobial resistance in Staphylococcus aureus and Listeria monocytogenes during continuous exposure to eugenol and citral. FEMS Microbiol. Lett. 2014, 354, 92–101. [Google Scholar] [CrossRef]
- Walker, J.N.; Horswill, A.R. A coverslip-based technique for evaluating Staphylococcus aureus biofilm formation on human plasma. Front. Cell. Infect. Microbiol. 2012, 2, 39. [Google Scholar] [CrossRef] [PubMed]
- Sakimura, T.; Kajiyama, S.; Adachi, S.; Chiba, K.; Yonekura, A.; Tomita, M.; Koseki, H.; Miyamoto, T.; Tsurumoto, T.; Osaki, M. Biofilm-forming Staphylococcus epidermidis expressing vancomycin resistance early after adhesion to a metal surface. BioMed Res. Int. 2015, 2015, 9–11. [Google Scholar] [CrossRef] [PubMed]

| Sample | Propolis Type | Pollen Species | (%) | Region/Year | Coordinates |
|---|---|---|---|---|---|
| P1 | Euphorbia resinifera | Euphorbia resinifera | 58.6 ± 1.1 | Beni Mellal-Khénifra | 32°22′06″ N, |
| Genista hirsuta | 9.0 ± 0.4 | (2019) | 6°22′09″ W | ||
| Asparagus albus | 8.0 ± 1.4 | ||||
| Populus nigra | 7.1 ± 1.0 | ||||
| Kleinia anteuphorbium | 5.9 ± 0.4 | ||||
| Caesalpinia spinosa | 4.0 ± 0.7 | ||||
| Pinus pinaster | 3.9 ± 0.3 | ||||
| Eucalyptus cinerea | 1.6 ± 0.4 | ||||
| Convolvulus arvensis | 1.6 ± 0.4 | ||||
| P2 | Euphorbia officinarum | Euphorbia officinarum | 46.6 ± 0.5 | Souss-Massa-Tiznit | 29°43′ N, |
| Hypericum elodes | 13.9 ± 1.5 | (2019) | 8°58′ W | ||
| Quercus rotindifolia | 10.3 ± 1.0 | ||||
| Populus nigra | 7.1 ± 1.0 | ||||
| Eucalyptus cinerea | 6.7 ± 0.5 | ||||
| Pinus pinaster | 6.4 ± 0.2 | ||||
| Kleinia anteuphorbium | 3.4 ± 0.8 | ||||
| Ilex aquifolium | 3.3 ± 0.3 | ||||
| Asparagus albus | 1.9 ± 0.6 | ||||
| P3 | Euphorbia officinarum | Euphorbia officinarum | 44.8 ± 0.5 | Guelmim-Oued noun | 28°27′ N, |
| Smilax aspera | 21.0 ± 1.4 | (2019) | 10°07′ W | ||
| Cistus crepis | 14.6 ± 1.0 | ||||
| Caesalpinia spinosa | 9.9 ± 0.8 | ||||
| Campanula rotundifolia | 5.7 ± 0.3 | ||||
| Hypericum elodes | 3.9 ± 0.6 | ||||
| Populus nigra | 3.8 ± 0.8 | ||||
| Convolvulus arvensis | 3.0 ± 0.17 | ||||
| Ilex aquifolium | 2.9 ± 0.2 | ||||
| Quercus rotindifolia | 1.8 ± 0.3 |
| Components | RI | P1 | P2 |
|---|---|---|---|
| 3-Methyl-2-butenol | 726 | t | 0.3 |
| Hexanal | 739 | t | |
| n-Octane | 800 | t | |
| Hexanol | 883 | t | |
| Heptanal | 897 | t | |
| n-Nonane | 900 | 0.3 | 0.6 |
| Tricyclene | 921 | 0.4 | 0.2 |
| α-Thujene | 924 | 0.7 | 0.3 |
| α-Pinene | 930 | 15.9 | 14.7 |
| Camphene | 938 | 1.4 | 1.0 |
| Thuja-2,4(10)-diene * | 940 | 1.5 | 1.0 |
| Sabinene | 958 | 3.0 | 2.1 |
| β-Pinene | 963 | 6.8 | 6.5 |
| n-Octanal | 973 | 1.0 | 1.2 |
| 1,2,4-Trimethyl benzene | 975 | t | |
| β-Myrcene | 975 | 0.5 | t |
| Hexyl acetate | 995 | 0.5 | 0.8 |
| α-Terpinene | 1002 | 0.7 | 0.3 |
| p-Cymene | 1003 | 1.3 | 1.3 |
| 1,8-Cineole | 1005 | 0.2 | 1.2 |
| β-Phellandrene | 1005 | t | t |
| Limonene | 1009 | 0.7 | 1.3 |
| 2-Methyl butyric acid butyl ester | 1017 | t | |
| trans-β-Ocimene | 1027 | t | t |
| γ-Terpinene | 1035 | 1.6 | 1.2 |
| 2,5-Dimethyl styrene | 1059 | 0.4 | 0.4 |
| Terpinolene | 1064 | 0.4 | 0.4 |
| n-Nonanal | 1073 | 2.4 | 3.8 |
| α-Campholenal | 1092 | 1.9 | 2.0 |
| n-Undecane | 1100 | t | t |
| trans-Pinocarveol | 1106 | 1.9 | 1.4 |
| cis-Verbenol | 1114 | t | t |
| trans-Verbenol | 1114 | 0.5 | 1.1 |
| Pinocarvone | 1121 | 1.2 | 0.9 |
| Terpinen-4-ol | 1148 | 0.9 | 0.4 |
| Myrtenal | 1153 | 1.3 | 1.0 |
| Verbenone | 1164 | 0.4 | 0.3 |
| Myrtenol | 1168 | 1.9 | 2.3 |
| Hexyl butanoate (=Hexyl butyrate) | 1173 | t | t |
| n-Decanal | 1180 | 4.3 | 7.8 |
| trans-Carveol | 1189 | 0.2 | 0.1 |
| n-Dodecane (C12) | 1200 | t | |
| Cuminaldehyde | 1200 | t | |
| Carvone | 1210 | t | |
| Hexyl 2-methyl butyrate | 1220 | 0.6 | 1.1 |
| 2-trans-Decenal | 1236 | 0.7 | 0.4 |
| Nonanoic acid | 1263 | t | |
| Bornyl acetate (=Borneol acetate) | 1265 | 3.3 | 3.0 |
| n-Undecanal | 1288 | 0.2 | 0.2 |
| trans-Theaspirane | 1300 | ||
| n-Tridecane | 1300 | t | 0.2 |
| α-Terpenyl acetate | 1334 | 1.3 | 1.2 |
| trans-2-Undecenal | 1334 | 0.3 | 0.2 |
| α-Cubebene | 1345 | 0.3 | 0.2 |
| α-Copaene | 1375 | t | 0.2 |
| Hexyl hexanoate | 1375 | 0.3 | t |
| β-Bourbonene | 1379 | 2.3 | 2.0 |
| n-Dodecanal | 1397 | 0.7 | 1.0 |
| n-Tetradecane | 1400 | t | 0.1 |
| β-Caryophyllene | 1414 | 0.8 | 0.6 |
| β-Copaene | 1426 | 0.2 | 0.1 |
| Aromadendrene | 1428 | 0.2 | |
| α-Humulene | 1447 | 0.4 | 0.2 |
| allo-Aromadendrene | 1456 | 0.7 | 1.5 |
| Germacrene D | 1474 | 0.4 | 0.2 |
| Valencene | 1484 | 0.3 | |
| α-Muurolene | 1494 | 0.2 | |
| trans,trans-α-Farnesene | 1500 | 0.9 | 1.1 |
| trans-Calamenene | 1505 | t | t |
| δ-Cadinene | 1505 | 0.6 | 0.4 |
| α-Calacorene | 1525 | 0.2 | 0.2 |
| Elemol | 1530 | t | 0.1 |
| Spathulenol | 1551 | 6.4 | 3.0 |
| β-Caryophyllene oxide | 1561 | 0.3 | 0.5 |
| Cedrol | 1574 | 1.5 | 1.0 |
| γ-Eudesmol | 1609 | 0.2 | |
| T-Cadinol | 1616 | 0.3 | |
| δ-Cadinol | 1621 | 0.4 | |
| β-Eudesmol | 1622 | t | 0.4 |
| α-Eudesmol | 1634 | 0.3 | 0.7 |
| Cadalene | 1640 | 0.5 | |
| α-Bisabolol | 1656 | 2.0 | 0.7 |
| n-Heptadecane | 1700 | 0.3 | 0.6 |
| n-Octadecane | 1800 | t | |
| n-Nonadecane | 1900 | 0.7 | 1.7 |
| Hexadecanoic acid (=Palmitic acid) | 1908 | 0.1 | |
| n-Eicosane | 2000 | t | t |
| Abietatriene | 2045 | 0.1 | 0.2 |
| n-Heneicosane | 2100 | 0.7 | 1.7 |
| n-Docosane | 2200 | 0.2 | |
| n-Tricosane | 2300 | 1.0 | 2.0 |
| n-Tetracosane | 2400 | t | t |
| n-Pentacosane | 2500 | 0.7 | 1.4 |
| n-Hexacosane | 2600 | t | 0.1 |
| n-Octacosane | 2800 | t | 0.1 |
| n-Heptacosane | 2700 | 0.8 | t |
| n-Nonacosane | 2900 | 0.4 | 0.8 |
| n-Triacontane | 3000 | t | 0.1 |
| n-Hentriacontane | 3100 | 0.4 | 1.0 |
| % Identification | 84.9 | 87.6 | |
| Grouped components | |||
| Monoterpene hydrocarbons | 35.3 | 30.7 | |
| Oxygen-containing monoterpenes | 15.0 | 14.9 | |
| Sesquiterpene hydrocarbons | 7.0 | 7.7 | |
| Oxygen-containing sesquiterpenes | 11.2 | 6.6 | |
| Diterpene hydrocarbons | 0.1 | 0.2 | |
| Oxygen-containing diterpenes | t | t | |
| Phenylpropanoids | t | t | |
| Fatty acids | t | 0.1 | |
| Alkanes | 5.0 | 10.0 | |
| Others | 11.3 | 17.4 |
| Element Content (mg/g) | |||
|---|---|---|---|
| Sample | P1 | P2 | P3 |
| Ca | 16.61 ± 0.20 a | 1.35 ± 0.16 a | 1.13 ± 0.2 a |
| Co | <LOD1 | <LOD1 | <LOD1 |
| Cr | <LOD2 | <LOD2 | <LOD2 |
| Cu | <LOD3 | <LOD3 | <LOD3 |
| Fe | 1.20 ± 0.02 d | 0.75 ± 0.12 b | 0.19 ± 0.009 c |
| K | 1.46 ± 0.03 c | 0.65 ± 0.005 c | 0.43 ± 0.01 b |
| Mg | 2.39 ± 0.02 b | 0.53 ± 0.05 d | 0.19 ± 0.02 c |
| Mn | 0.04 ± 0.01 f | 0.017 ± 0.01 g | 0.0047 ± 0.0005 g |
| Na | 0.49 ± 0.01 e | 0.09 ± 0.001 e | 0.08 ± 0.01 e |
| Ni | 0.0037 ± 0.0006 h | 0.0026 ± 0.0001 h | 0.0042 ± 0.0004 g |
| Zn | 0.033 ± 0.001 g | 0.04 ± 0.03 f | 0.005 ± 0.001 f |
| Sample | Phenol (mg GAE/g Propolis) | Flavonol/Flavones (mg QE/g Propolis) | Dihydroflavonol (mg Naringenin Eq/g Propolis) |
|---|---|---|---|
| P1 | 21.7 ± 1.2 b | 0.4 ± 0.0 b | 6.1 ± 0.1 b |
| P2 | 39.7 ± 1.0 a | 0.7 ± 0.1 a | 7.6 ± 0.2 a |
| P3 | 1.3 ± 0.1 c | 0.1 ± 0.0 c | 3.1 ± 0.1 c |
| Bacteria | MIC (µL/mL) ‡ | MBC (µL/mL) ‡ | |
|---|---|---|---|
| Euphorbia resinifera (P1) | S. aureus ATCC 6538 | 50 a | 150 a |
| MRSA12 | 100 b | 300 c | |
| MRSA15 | 120 c | 250 b | |
| E. coli DSM 1077 | 150 d | 250 b | |
| Euphorbia officinarum (P2) | S. aureus ATCC 6538 (C48) | 150 a | 200 a |
| MRSA12 | 100 b | 200 a | |
| MRSA15 | 250 d | 300 b | |
| E. coli DSM 1077 | 200 c | 350 c | |
| Euphorbia officinarum (P3) | S. aureus ATCC 6538 (C48) | 250 a | 300 a |
| MRSA12 | 200 b | 400 b | |
| MRSA15 | 300 c | 450 c | |
| E. coli DSM 1077 | 350 d | 400 b |
| Step | Temperature (°C) | Slope Time (min:s) | Step Time (min:s) | Pressure (psi) | Magnetic Stirring |
|---|---|---|---|---|---|
| 1 | Room temperature | - | 00:15 | <50 | Mean |
| 2 | 90 | 02:00 | 02:00 | 250 | Mean |
| 3 | 190 | 06:00 | 05:00 | 300 | Mean |
| 4 | 230 | 10:00 | 05:00 | 300 | Mean |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutoub, O.; El-Guendouz, S.; Matos, I.; El Ghadraoui, L.; Costa, M.C.; Carlier, J.D.; Faleiro, M.L.; Figueiredo, A.C.; Estevinho, L.M.; Miguel, M.G. Chemical Characterization and Biological Properties Assessment of Euphorbia resinifera and Euphorbia officinarum Moroccan Propolis. Antibiotics 2024, 13, 230. https://doi.org/10.3390/antibiotics13030230
Boutoub O, El-Guendouz S, Matos I, El Ghadraoui L, Costa MC, Carlier JD, Faleiro ML, Figueiredo AC, Estevinho LM, Miguel MG. Chemical Characterization and Biological Properties Assessment of Euphorbia resinifera and Euphorbia officinarum Moroccan Propolis. Antibiotics. 2024; 13(3):230. https://doi.org/10.3390/antibiotics13030230
Chicago/Turabian StyleBoutoub, Oumaima, Soukaina El-Guendouz, Isabel Matos, Lahsen El Ghadraoui, Maria Clara Costa, Jorge Dias Carlier, Maria Leonor Faleiro, Ana Cristina Figueiredo, Letícia M. Estevinho, and Maria Graça Miguel. 2024. "Chemical Characterization and Biological Properties Assessment of Euphorbia resinifera and Euphorbia officinarum Moroccan Propolis" Antibiotics 13, no. 3: 230. https://doi.org/10.3390/antibiotics13030230







