Abstract
Introduction: Periodontitis, an infectious inflammatory condition, is a key contributor to sustained systemic inflammation, intricately linked to atherosclerotic cardiovascular disease (CVD), the leading cause of death in developed nations. Treating periodontitis with subgingival mechanical instrumentation with or without adjunctive antimicrobials reduces the microbial burden and local inflammation, while also potentially bringing systemic benefits for patients with both periodontitis and CVD. This review examines systemic effects of subgingival instrumentation with or without antimicrobial products in individuals with periodontitis and CVD, and explores intricate pathogenetic interactions between periodontitis and CVD. Material and Methods: English-language databases (PubMed MEDLINE and Cochrane Library) were searched for studies assessing the effects of nonsurgical periodontal therapies in periodontitis patients with or without CVD. Results: While the ability of periodontal therapy to reduce mortality- and morbidity-related outcomes in CVD patients with periodontitis remains uncertain, some studies indicate a decrease in inflammatory markers and blood cell counts. Subgingival mechanical instrumentation delivered over multiple short sessions carries lower risks of adverse effects, particularly systemic inflammation, compared to the full-mouth delivery, making it a preferable option for CVD patients. Conclusions: Subgingival mechanical instrumentation, ideally conducted in a quadrant-based therapeutic approach, to decontaminate periodontal pockets has the potential to reduce both local and systemic inflammation with minimal adverse effects in patients suffering from periodontitis and concurrent CVD.
1. Introduction
Periodontitis is a chronic, multifactorial infectious disease that triggers a local dysregulated immune–inflammatory response [1], leading to progressive periodontal destruction, significant oral functional impairments, systemic consequences [2], and increased medical costs [3]. Due to its enormous global burden in the general population, periodontitis has become a major public health problem. It affects around 50% of the population [4], and as of 2019, 1.1 billion severe periodontitis cases have been reported worldwide [5].
Cardiovascular disease (CVD) encompasses various pathologies such as ischemic heart disease, stroke, hypertension, rheumatic heart disease, cardiomyopathies, and atrial fibrillation, with most having an atherosclerotic origin [2]. CVD is the leading cause of mortality globally, responsible for 32% of all deaths [6]. In Europe alone, in 2017, over 3.9 million deaths were attributed to CVD [7].
Periodontitis, mostly stage III and IV phenotypes, through the considerable subgingival bacterial load, can contribute to persistent systemic inflammation, impacting the development of atherosclerotic CVD [8,9,10] and its complications [11]. Epidemiological studies have reported positive associations between periodontal disease and coronary heart disease and stroke [10]. Moreover, an increased risk of developing CVD among periodontitis patients has been identified [12,13,14,15]. Current evidence now recognizes periodontitis as an independent risk factor for atherosclerotic CVD, such as hypertension, coronary artery disease, peripheral artery disease, and their acute events [9,16]. The prerequisite pathogenetic substrate of atherosclerosis is endothelial dysfunction [17].
Numerous reviews and meta-analyses based on epidemiological studies have consistently indicated a relationship between periodontitis and CVD with an increased risk of periodontitis patients developing CVD [14,18,19,20,21] or its acute event—myocardial infarction [12,22,23,24,25]. Periodontitis and its major sequela—edentulism—increased the risk of all-cause mortality and mortality due to CVD, coronary heart disease, or cerebrovascular diseases [26]. However, so far, it has not been possible to establish the nature of the association between the two diseases [27].
Excessive and dysbiotic subgingival microbiota as well as aberrant periodontal inflammation are central elements of the pathogenic network inducing local periodontal destruction as well as systemic consequences (Figure 1A). Periodontitis triggers the initiation and progression of endothelial dysfunction, atherosclerosis, and CVD through a networking of pathogenetic mechanisms such as the release of several systemic proinflammatory molecules, unbalanced oxidative stress, the decrease of nitric oxide (NO) concentration [27], upregulation of serum cell-free DNA (cfDNA) levels, dysfunction of endothelial progenitors [28,29,30,31,32,33], or elevated levels of trimethylamine-N-oxide [33,34] induced by periodontitis-related gut dysbiosis [33,35].
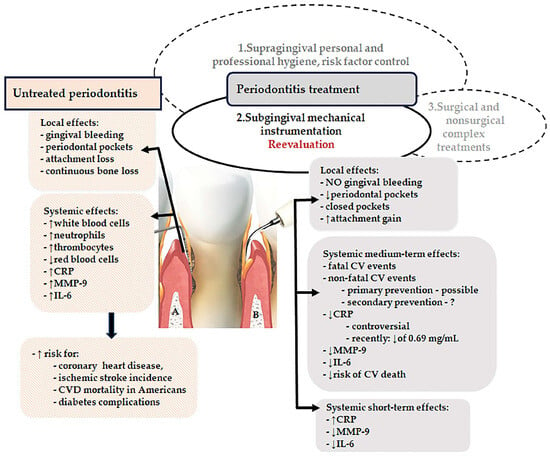
Figure 1.
Synoptic view of local and systemic effects in untreated and treated periodontitis. (A) Contaminated periodontal pocket, (B) Cleaned pocket through subgingival mechanical instrumentation (CVD, cardiovascular disease, CRP, C-reactive protein, IL-6, interleukin-6, MMP-9, metalloproteinase-9; NO, nitric oxide), ↓ decrease, ↑ increase, [Photo—Free for Canva Pro].
The implication of periodontopathogens in the development of atheromatous lesions has been suggested by their presence in atheromatous lesions [36]. Identification of periodontal bacterial DNA in coronary atheroma supports their systemic dissemination from periodontal pockets [37]. Porphyromonas gingivalis was more frequently detected in coronary atheromatous plaque samples than Aggregatibacter actinomycetemcomitans [38]. However, it is difficult to state that DNA fragments belong to living resident bacteria from the atheromas participating in vascular pathology; instead, they may merely be local bystanders [37]. Viable bacteria have been reported to be present in some thrombus samples, all of them identified as positive for bacterial DNA through qPCR assay [39].
While there were high hopes that reducing serum inflammatory markers through periodontal therapy would provide preventive benefits for CVD patients, uncertain evidence has accumulated regarding the systemic benefits provided by reducing the microbial burden through subgingival mechanical instrumentation (scaling) in patients with chronic periodontitis [40,41].
The present review aims to provide a comprehensive overview of the systemic effects induced by nonsurgical periodontal treatment, consisting of subgingival mechanical instrumentation with or without adjunctive antimicrobials, in patients suffering from both periodontitis and CVD. Additionally, this review focuses on the short-term adverse effects of mechanical instrumentation and on the complex links sustaining the pathogenetic interactions between periodontitis and CVD.
2. Results
A total number of 27 full-text papers were identified, from which 19 (7 reviews and 12 clinical trials) reporting on systemic effects of nonsurgical periodontitis treatment in patients with both CVD and periodontitis and 8 (2 reviews and 6 clinical trials) on short-term adverse effects of nonsurgical periodontitis treatment.
2.1. Medium-Term Effects of Subgingival Mechanical Instrumentation on Mortality- and Morbidity-Related Outcomes in Cardiovascular Disease Patients with Periodontitis
Several studies have documented the systemic impact of subgingival mechanical instrumentation, which is considered the gold-standard therapy for periodontitis [42], both with and without antimicrobial agents, in patients with CVD. The effects are depicted schematically in Figure 1B.
The most recent systematic review on this subject [40] analyzed the results of two RCTs from the perspective of fatal and nonfatal cardiovascular events. One parallel-arm, double-blind RCT on primary prevention of CVD in a group of periodontitis and metabolic syndrome patients (165 participants) [43] reported one death due to fatal myocardial infarction in the intervention group (subgingival mechanical instrumentation plus double antibiotic regimen) and another three nonfatal events; it could not be identified whether intervention would have reduced the incidence of all-cause death [Peto odds ratio (OR) 7.48, 95% confidence interval (CI) 0.15 to 376.98] or all CVD-related death (Peto OR 7.48, 95% CI 0.15 to 376.98) as compared with supragingival instrumentation alone plus placebo tablets (non-intervention).
Moreover, the subgingival instrumentation combined with an amoxicillin and metronidazole regimen could have increased the risk of cardiovascular events (Peto OR 7.77, 95% CI 1.07 to 56.1) when compared with supragingival instrumentation at the 12-month follow-up [43].
The second study—PAVE (2008)—was a multicenter, parallel-group, single-blind RCT focused on secondary prevention of CVD, which included 303 patients randomly treated with subgingival mechanical instrumentation and oral hygiene instruction (intervention) or oral hygiene instruction plus community follow-up (non-intervention). Unfortunately, only a small group of participants was available for one-year follow-up, which precluded any conclusion on the effects of periodontal treatment on secondary prevention of CVD in terms of all-cause death and all CVD-related death [44,45,46].
2.2. Medium-Term Effects of Subgingival Mechanical Instrumentation on Inflammatory Markers and Blood Cell Counts in Cardiovascular Disease Patients with Periodontitis
Clinical studies [33,47,48,49,50] and systematic reviews [51,52,53,54,55] have reported the systemic effects of periodontitis infection control in CVD patients in terms of changes in periodontal parameters or systemic inflammatory markers. Three months after subgingival nonsurgical periodontal therapy, a decrease in serum levels of IL-6 and CRP was observed as compared with a control group receiving only oral hygiene advice [33,47]. More recent information confirms the reduction in serum CRP levels induced by subgingival nonsurgical periodontal treatment [48,49]. Reports from a meta-analysis showed that periodontitis therapy lowered systemic inflammation via reductions in both high-sensitivity CRP (hs-CRP) [0.56 mg/L, 95% confidence interval (CI) (−0.88, −0.25), p < 0.001] and IL-6 [0.48 pg/mL, 95% CI (−0.88, −0.08), p = 0.020] levels [52]. On the other hand, another meta-analysis [40] reported an uncertain effect of subgingival mechanical instrumentation plus a double antibiotic regimen in lowering serum CRP levels (X coefficient −0.002, 95% CI −0.19 to 0.20) or fibrinogen (X coefficient 10.9, 95% CI −12.0 to 33.8). These uncertainties were accentuated by some reported biases of this meta-analysis [40] related to the methodologies of the included RCTs and some interpretations of the systematic review. Furthermore, the authors [40] failed to account for outdated measures of chronic periodontitis used in the analyzed RCTs [43,44,45,46], and the authors [40] changed its case definition during the review process, which did not correspond either to the one provided by the RCTs or to the current one [56,57].
Other systematic reviews exploring the systemic effects of subgingival infection control in periodontitis patients with CVD in terms of changes in systemic inflammatory blood marker were subject to several methodological biases, mostly related to the inadequate follow-up periods of the included studies [51,54,55] or biasing approaches during the review process (analysis of studies providing low-quality data, with different designs, or including CVD patients of different risks) [51,55,58]. The reported biases prevent comparisons between reviews and the formulation of a firm conclusion related to the analyzed subject.
A non-significant decreasing trend of CRP values at 6 months after treatment for periodontitis patients with comorbid CVD was reported [46,59]. The greatest reductions were obtained for baseline CRP levels > 3 mg/L [60]. On the other hand, infection control through subgingival mechanical instrumentation reduced serum CRP concentrations by 0.69 mg/L (95% confidence interval: −0.97 to −0.40) after 6 months in systemically healthy individuals [60], to a degree equivalent to that obtained through traditional lifestyle modifications [61] or medication [62]. This information is of extreme importance since the reduction of CRP in CVD patients to less than 2 mg/mL decreased the risk of cardiovascular death by one-third independent of other risk factors [8].
Limited data are available regarding changes in serum calprotectin (S100A8/A9) levels among patients with periodontitis and CVD. A single study reported a notable rise in calprotectin levels in individuals with both periodontitis and coronary artery disease, which subsequently decreased 180 days after nonsurgical periodontal treatment alongside improvements in cardiac parameters [63].
While not statistically significant, a decrease in white blood cell (WBC) count was observed three months after nonsurgical periodontitis treatment in patients with CVD [64]. On the other hand, significant reductions in CRP and WBC count, along with improvements in periodontitis-related parameters, were reported one month after nonsurgical periodontal treatment in patients with both periodontitis and CVD [50]. Notably, a more pronounced reduction in WBC count was noted in patients with CVD compared to the control group [50].
2.3. Adverse Short-Term Effects of Subgingival Mechanical Instrumentation in Periodontitis Patients
Subgingival decontamination via conventional mechanical instrumentation (quadrant-based) typically occurs over multiple sessions spanning several weeks, with each session focusing on scaling a specific quadrant of the mouth.
An alternative therapeutical protocol known as full-mouth scaling (FMS) proposes treating the entire mouth within 24 h across one or two sessions, to mitigate the risk of recontamination. This approach aims to prevent the re-infection of recently cleaned periodontal pockets from untreated areas. Another option, full-mouth disinfection (FMD), combines FMS with extensive oral disinfection using chlorhexidine. In certain clinical situations, both quadrant-based and full-mouth scaling approaches may be associated with adjuvant antimicrobials applied locally or administrated systemically [42].
However, the additional local benefits of FMS and FMD compared to quadrant instrumentation are minimal, and clear recommendations for their use, particularly for FMD, are lacking [42,65,66]. While the FMS approach may seem logical to prevent recontamination, its implementation in clinical practice may present challenges, particularly in cases of moderate or severe generalized periodontitis (stage II or III periodontitis) where significant post-therapeutic bacteremia could occur.
The most recent systematic review [65] assessed the effects of full-mouth decontamination protocols in comparison to the conventional quadrant approach in periodontitis patients, highlighting an increase in body temperature as a notable short-term adverse event following full-mouth treatments [65]. This elevation in temperature may stem from repeated transient bacteremia occurring during subsequent subgingival instrumentation sessions [65].
Evidence suggests that full-mouth protocols can trigger acute systemic reactions within 24 h, manifested by a three-fold rise in CRP, a two-fold increase in IL-6, and a slight elevation in tumor necrosis factor-alpha levels in the full-mouth instrumentation group compared to the conventional quadrant instrumented group [67]. An acute-phase response one day after one-stage full-mouth instrumentation versus the quadrant approach was also reported in subjects with comorbid type 2 diabetes [68]. Consequently, there is a recommendation to carefully weigh the choice of therapeutic approach, considering the patient’s overall health status [42]. The European Federation of Periodontology and the World Heart Federation experts advise that in periodontitis, for individuals without extended edentulism, with different severities of CVD, and regardless of specific medications, nonsurgical periodontal therapy should preferably be delivered over several 30 to 45 min sessions to minimize acute systemic inflammation [16]. Moreover, individuals at risk of endocarditis ought to receive antibiotic prophylaxis in accordance with the current recommendations [16].
Other short-term effects (within 24 h) of full-mouth decontaminating approaches included tooth discoloration and alterations in taste perception among participants in the FMD group [65], resulting in challenges regarding participants’ adherence to the study during the 60-day follow-up period [69]. Furthermore, undesirable outcomes subsequent to full-mouth instrumentation in contrast to the quadrant treatment encompassed heightened analgesic usage and the occurrence of oral herpes in the full-mouth treatment groups [70]. Conversely, several randomized controlled trials revealed no discernible disparities in adverse reactions between groups subjected to full-mouth approaches versus those undergoing conventional instrumentation [71,72].
In order to more comprehensively examine the systemic implications of infection control in individuals with both periodontitis and CVD, it is recommended that future RCTs incorporate larger groups of participants, including those with associated pathologies, implement more rigorous control over confounding variables, and explicitly address outcomes related to both periodontal health and cardiovascular health [40].
3. Discussions
Until now, the evidence has been inconclusive regarding whether periodontal therapy can aid in preventing CVD in individuals diagnosed with periodontitis, or whether long-term management of periodontal inflammation could improve systemic health [40]. Furthermore, it remains unclear whether nonsurgical periodontal treatment combined with antibiotics can reduce all-cause mortality at the 12-month follow-up compared to supragingival scaling alone in patients with CVD [40].
CRP and S100A8 were chosen as relevant markers of systemic inflammation associated with both periodontitis and CVD. Increased circulating levels of CRP have been reported in periodontitis [73,74,75] as well as in CVD patients [61,76]. Subclinical inflammation, as indicated by plasma CRP levels, was associated not only with the presence of coronary atherosclerosis but also with the severity of coronary plaque burden and the clinical manifestation of ischemia [77]. Furthermore, even a slight yet persistent increase in CRP or high-sensitivity CRP (hs-CRP) has been correlated with the presence of coronary artery disease and a heightened risk of future cardiovascular events among apparently healthy individuals [78]. Considering the systemic risks associated with increased serum CRP levels [77,78] and the observed decrease in CRP concentrations following nonsurgical therapy in periodontitis patients with concurrent CVD [43,46,60], immediate infection control in these individuals is imperative.
S100A8/9, also known as calprotectin, belongs to the broader family of S100 calcium-binding proteins and has the ability to bind zinc. These proteins are predominantly found in neutrophils, monocytes, or macrophages [79] and contribute to the production of proinflammatory mediators. However, under certain specific conditions, they can also demonstrate anti-inflammatory effects [80]. Elevated serum [81] and salivary levels [82,83] of S100A8/A9 were associated with periodontitis patients. Increased concentrations of salivary S100A8 were associated with elevated metalloproteinase-9 levels, which supports the possible use of these salivary biomarkers as a rapid test kit for the early detection of periodontitis [83]. Elevated release of S100A8/A9 appears to exert deleterious effects during myocardial infarction and is linked to an unfavorable long-term prognosis [84], while the inhibition of S100A8/A9 has been effective in myocardial infarction models [80]. The reduction of serum S100A8/A9 through nonsurgical periodontal therapy [63] seems an appealing perspective in CVD individuals. However, more clinical trials should further establish the impact of periodontitis infection control on S100A8/A9 concentrations in this group of patients.
Cellular circulating parameters are reliable hematological surrogates for monitoring body inflammation [85]. Periodontitis has been associated with hematological changes [86,87,88], mostly related to a higher white blood cell (WBC) count [86]. An increased WBC count is a marker of systemic inflammation in CVD [89] and a risk factor for coronary heart disease, ischemic stroke incidence, and CVD mortality in Americans [90]. Infection control through subgingival mechanical instrumentation in periodontitis patients has been linked to a decrease in WBC count, which could confirm the possible causal link between periodontitis and leukocyte count [86]. However, there are inconsistent findings regarding the decrease in WBC count following nonsurgical therapy in individuals with both periodontitis and CVD [50,64], which signals a need for further insights into this matter.
4. Material and Methods
4.1. Literature Search
A literature search was conducted in the electronic databases PubMed MEDLINE and Cochrane Library, without any restriction regarding the publication date, with the last search being performed in February 2024. The following index terms and Boolean operators were used in the established search strategy: “Cardiovascular Disease” AND “Periodontal Treatment”; “Cardiovascular Disease” AND “Periodontal Therapy”; “Inflammatory Markers” AND “Periodontal Treatment” AND “Cardiovascular Disease”; and “Inflammatory Markers” AND “Periodontal Therapy” AND “Cardiovascular Disease”; “Blood Cell Count” AND “Periodontal Treatment” AND “Cardiovascular Disease”; “Blood Cell Count” AND “Periodontal Therapy” AND “Cardiovascular Disease”.
4.2. Inclusion Criteria
We included randomized controlled trials, clinical controlled trials, and systematic reviews with or without meta-analysis evaluating the effects of nonsurgical periodontal therapies (subgingival mechanical instrumentation with or without adjunctive antimicrobials) [42] in individuals diagnosed with periodontitis, with or without CVD. The following therapeutic outcomes were considered: cardiovascular-related events (all-cause and CVD-related death, angina, myocardial infarction, stroke), blood cell counts, systemic inflammation parameters, and local and systemic adverse events induced by periodontal therapy. Non-English-language articles, experimental studies on animals, and clinical studies that assessed the effect of periodontal treatment on cardiovascular risk in patients with other systemic comorbidities were excluded from the search.
4.3. Data Collection and Synthesis
Screening of the identified papers was performed independently by two authors (I.C.M. and A.S.) by checking the titles, abstracts, and, finally, full texts of the retrieved papers regarding the predefined eligibility criteria. Screening of the reference lists of selected papers was also carried out, and the identified papers underwent the check of eligibility (I.C.M., A.P.). Publications with an unclear methodology but considered relevant and cited by relevant reviews were included in the full-text assessment to avoid missing potentially relevant information. In the event of ambiguity, consensus through discussion between the three authors participating in the literature search was achieved.
The identified clinical studies and systematic reviews were organized in three main categories regarding the influence of nonsurgical periodontal therapies on cardiovascular-related events, variations in systemic inflammatory blood markers and blood cell counts induced by the subgingival mechanical instrumentation, as well as the main adverse events associated with the subgingival mechanical instrumentation.
5. Conclusions
Periodontitis has the capacity to initiate and worsen the progression of CVD and its acute complications through intricate pathogenetic mechanisms, largely involving the release of proinflammatory molecules.
In patients with both periodontitis and concurrent CVD, subgingival mechanical instrumentation can mitigate systemic inflammation, as evidenced by reduced levels of proinflammatory markers such as C-reactive protein, calprotectin, and white blood cell counts. Decreasing the systemic inflammatory burden lowers the risk of developing CVD and experiencing acute events.
To minimize acute systemic inflammatory effects, it is advisable to implement quadrant-based instrumentation protocols at staggered intervals rather than using full-mouth subgingival mechanical instrumentation in individuals with periodontitis and CVD.
Author Contributions
Conceptualization, A.C., A.R., I.C.M. and A.S.; methodology, C.S.C., M.N., A.R. and A.P.; validation, M.N., A.R. and A.S.; resources, C.S.C.; writing—original draft preparation, C.S.C., A.C. and I.C.M.; writing—review and editing, A.R., A.P. and A.S.; visualization, M.N. and A.S.; supervision, A.R.; funding acquisition, C.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This review is part of an internal research project funded by the Iuliu Hațieganu University of Medicine and Pharmacy Cluj-Napoca, România (grant number 4504/01.10.2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Meyle, J.; Chapple, I. Molecular Aspects of the Pathogenesis of Periodontitis. Periodontol. 2000 2015, 69, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Sanz, M.; Shapira, L.; Brotons, C.; Chapple, I.; Frese, T.; Graziani, F.; Hobbs, F.D.R.; Huck, O.; Hummers, E.; et al. Association between Periodontal Diseases and Cardiovascular Diseases, Diabetes and Respiratory Diseases: Consensus Report of the Joint Workshop by the European Federation of Periodontology (EFP) and the European Arm of the World Organization of Family Doctors (WONCA Europe). J. Clin. Periodontol. 2023, 50, 819–841. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Iwasaki, M.; Yoshihara, A.; Miyazaki, H. Association between Periodontitis and Medical Expenditure in Older Adults: A 33-month Follow-up Study. Geriatr. Gerontol. Int. 2016, 16, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A.; Genco, R.J. Periodontitis in US Adults. J. Am. Dent. Assoc. 2018, 149, 576–588.e6. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, Regional, and National Burden of Severe Periodontitis, 1990–2019: An Analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [CrossRef] [PubMed]
- Cardiovascular Diseases (CVDs). Who.int. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 8 April 2024).
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Danesh, J.; Wheeler, J.G.; Hirschfield, G.M.; Eda, S.; Eiriksdottir, G.; Rumley, A.; Lowe, G.D.O.; Pepys, M.B.; Gudnason, V. C-Reactive Protein and Other Circulating Markers of Inflammation in the Prediction of Coronary Heart Disease. N. Engl. J. Med. 2004, 350, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, T.; Sharma, P.; Walter, C.; Weston, P.; Beck, J. The Epidemiological Evidence behind the Association between Periodontitis and Incident Atherosclerotic Cardiovascular Disease. J. Clin. Periodontol. 2013, 40 (Suppl. 14), S70–S84. [Google Scholar] [CrossRef]
- Libby, P.; Loscalzo, J.; Ridker, P.M.; Farkouh, M.E.; Hsue, P.Y.; Fuster, V.; Hasan, A.A.; Amar, S. Inflammation, Immunity, and Infection in Atherothrombosis. J. Am. Coll. Cardiol. 2018, 72, 2071–2081. [Google Scholar] [CrossRef]
- Xu, S.; Song, M.; Xiong, Y.; Liu, X.; He, Y.; Qin, Z. The Association between Periodontal Disease and the Risk of Myocardial Infarction: A Pooled Analysis of Observational Studies. BMC Cardiovasc. Disord. 2017, 17, 50. [Google Scholar] [CrossRef]
- Humphrey, L.L.; Fu, R.; Buckley, D.I.; Freeman, M.; Helfand, M. Periodontal Disease and Coronary Heart Disease Incidence: A Systematic Review and Meta-Analysis. J. Gen. Intern. Med. 2008, 23, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Leng, W.-D.; Zeng, X.-T.; Kwong, J.S.W.; Hua, X.-P. Periodontal Disease and Risk of Coronary Heart Disease: An Updated Meta-Analysis of Prospective Cohort Studies. Int. J. Cardiol. 2015, 201, 469–472. [Google Scholar] [CrossRef]
- Zeng, X.-T.; Leng, W.-D.; Lam, Y.-Y.; Yan, B.P.; Wei, X.-M.; Weng, H.; Kwong, J.S.W. Periodontal Disease and Carotid Atherosclerosis: A Meta-Analysis of 17,330 Participants. Int. J. Cardiol. 2016, 203, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Del Castillo, A.M.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and Cardiovascular Diseases. Consensus Report. Glob. Heart 2020, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Baselet, B.; Sonveaux, P.; Baatout, S.; Aerts, A. Pathological Effects of Ionizing Radiation: Endothelial Activation and Dysfunction. Cell. Mol. Life Sci. 2019, 76, 699–728. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A.; Bush, R.B.; Paju, S. Associations between Periodontal Disease and Risk for Nosocomial Bacterial Pneumonia and Chronic Obstructive Pulmonary Disease. A Systematic Review. Ann. Periodontol. 2003, 8, 54–69. [Google Scholar] [CrossRef]
- Janket, S.-J.; Baird, A.E.; Chuang, S.-K.; Jones, J.A. Meta-Analysis of Periodontal Disease and Risk of Coronary Heart Disease and Stroke. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 559–569. [Google Scholar] [CrossRef]
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The Prevalence and Incidence of Coronary Heart Disease Is Significantly Increased in Periodontitis: A Meta-Analysis. Am. Heart J. 2007, 154, 830–837. [Google Scholar] [CrossRef]
- Larvin, H.; Kang, J.; Aggarwal, V.R.; Pavitt, S.; Wu, J. Risk of Incident Cardiovascular Disease in People with Periodontal Disease: A Systematic Review and Meta-analysis. Clin. Exp. Dent. Res. 2021, 7, 109–122. [Google Scholar] [CrossRef]
- Rydén, L.; Buhlin, K.; Ekstrand, E.; de Faire, U.; Gustafsson, A.; Holmer, J.; Kjellström, B.; Lindahl, B.; Norhammar, A.; Nygren, Å.; et al. Periodontitis Increases the Risk of a First Myocardial Infarction: A Report from the PAROKRANK Study. Circulation 2016, 133, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Filho, I.S.; Coelho, J.M.F.; Miranda, S.S.; Cruz, S.S.; Trindade, S.C.; Cerqueira, E.M.M.; Passos-Soares, J.S.; da Costa, M.C.N.; Vianna, M.I.P.; Figueiredo, A.C.M.G.; et al. Severe and Moderate Periodontitis Are Associated with Acute Myocardial Infarction. J. Periodontol. 2020, 91, 1444–1452. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, B.; Huo, N.; Cai, C.; Liu, H.; Xu, J. Association between Myocardial Infarction and Periodontis: A Meta -Analysis of Case-Control Studies. Front. Physiol. 2016, 7, 519. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, N.; Ahlqvist, J.; Näslund, U.; Buhlin, K.; Gustafsson, A.; Kjellström, B.; Klinge, B.; Rydén, L.; Levring Jäghagen, E. Associations among Periodontitis, Calcified Carotid Artery Atheromas, and Risk of Myocardial Infarction. J. Dent. Res. 2020, 99, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Romandini, M.; Baima, G.; Antonoglou, G.; Bueno, J.; Figuero, E.; Sanz, M. Periodontitis, Edentulism, and Risk of Mortality: A Systematic Review with Meta-Analyses. J. Dent. Res. 2021, 100, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Naderi, S.; Merchant, A.T. The Association between Periodontitis and Cardiovascular Disease: An Update. Curr. Atheroscler. Rep. 2020, 22, 1–5. [Google Scholar] [CrossRef]
- Isola, G.; Santonocito, S.; Lupi, S.M.; Polizzi, A.; Sclafani, R.; Patini, R.; Marchetti, E. Periodontal Health and Disease in the Context of Systemic Diseases. Mediat. Inflamm. 2023, 2023, 9720947. [Google Scholar] [CrossRef]
- Chopra, H.; Hung, M.K.; Kwong, D.L.; Zhang, C.F.; Pow, E.H.N. Insights into Endothelial Progenitor Cells: Origin, Classification, Potentials, and Prospects. Stem Cells Int. 2018, 2018, 9847015. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhang, S.; Ouyang, X.; Wang, Y.; Jiang, Y.; An, N. Crosstalk between Akt and NF-κB Pathway Mediates Inhibitory Effect of Gas6 on Monocytes-endothelial Cells Interactions Stimulated by P. Gingivalis-LPS. J. Cell. Mol. Med. 2020, 24, 7979–7990. [Google Scholar] [CrossRef]
- Deng, H.; Gong, Y.; Chen, Y.; Zhang, G.; Chen, H.; Cheng, T.; Jin, L.; Wang, Y. Porphyromonas gingivalis Lipopolysaccharide Affects the Angiogenic Function of Endothelial Progenitor Cells via Akt/FoxO1 Signaling. J. Periodontal. Res. 2022, 57, 859–868. [Google Scholar] [CrossRef]
- Vázquez-Reza, M.; Custodia, A.; López-Dequidt, I.; Aramburu-Núñez, M.; Romaus-Sanjurjo, D.; Ouro, A.; Botelho, J.; Machado, V.; Iglesias-Rey, R.; Pías-Peleteiro, J.M.; et al. Periodontal Inflammation Is Associated with Increased Circulating Levels of Endothelial Progenitor Cells: A Retrospective Cohort Study in a High Vascular Risk Population. Ther. Adv. Chronic Dis. 2023, 14, 20406223231178276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, S.; Ren, J.; Zou, H.; Liu, Y.; Chen, Y.; Qiu, Y.; Zhuang, W.; Tao, J.; Yang, J. Association of Enhanced Circulating Trimethylamine N-oxide with Vascular Endothelial Dysfunction in Periodontitis Patients. J. Periodontol. 2022, 93, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.-H.; Chen, C.-Y.; Chen, I.-C.; Huang, H.-L.; Lu, Y.-W.; Kuo, C.-S.; Chang, C.-C.; Huang, P.-H.; Chen, J.-W.; Lin, S.-J. Trimethylamine N-Oxide, Circulating Endothelial Progenitor Cells, and Endothelial Function in Patients with Stable Angina. Sci. Rep. 2019, 9, 4249. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, S.; Nagao-Kitamoto, H.; Jiao, Y.; Gillilland, M.G., III; Hayashi, A.; Imai, J.; Sugihara, K.; Miyoshi, M.; Brazil, J.C.; Kuffa, P.; et al. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell 2020, 182, 447–462.e14. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Nemoto, H.; Nomura, R.; Inaba, H.; Yoshioka, H.; Taniguchi, K.; Amano, A.; Ooshima, T. Detection of Oral Bacteria in Cardiovascular Specimens. Oral Microbiol. Immunol. 2009, 24, 64–68. [Google Scholar] [CrossRef]
- Joshi, C.; Bapat, R.; Anderson, W.; Dawson, D.; Hijazi, K.; Cherukara, G. Detection of Periodontal Microorganisms in Coronary Atheromatous Plaque Specimens of Myocardial Infarction Patients: A Systematic Review and Meta-Analysis. Trends Cardiovasc. Med. 2021, 31, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Gaetti-Jardim, E.; Marcelino, S.L.; Feitosa, A.C.R.; Romito, G.A.; Avila-Campos, M.J. Quantitative Detection of Periodontopathic Bacteria in Atherosclerotic Plaques from Coronary Arteries. J. Med. Microbiol. 2009, 58, 1568–1575. [Google Scholar] [CrossRef]
- Pessi, T.; Karhunen, V.; Karjalainen, P.P.; Ylitalo, A.; Airaksinen, J.K.; Niemi, M.; Pietila, M.; Lounatmaa, K.; Haapaniemi, T.; Lehtimäki, T.; et al. Bacterial Signatures in Thrombus Aspirates of Patients with Myocardial Infarction. Circulation 2013, 127, 1219–1228. [Google Scholar] [CrossRef]
- Ye, Z.; Cao, Y.; Miao, C.; Liu, W.; Dong, L.; Lv, Z.; Iheozor-Ejiofor, Z.; Li, C. Periodontal Therapy for Primary or Secondary Prevention of Cardiovascular Disease in People with Periodontitis. Cochrane Libr. 2022, 10, CD009197. [Google Scholar] [CrossRef]
- Liu, W.; Cao, Y.; Dong, L.; Zhu, Y.; Wu, Y.; Lv, Z.; Iheozor-Ejiofor, Z.; Li, C. Periodontal Therapy for Primary or Secondary Prevention of Cardiovascular Disease in People with Periodontitis. Cochrane Libr. 2019, 12, CD009197. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of Stage I–III Periodontitis—The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [CrossRef]
- López, N.J.; Quintero, A.; Casanova, P.A.; Ibieta, C.I.; Baelum, V.; López, R. Effects of Periodontal Therapy on Systemic Markers of Inflammation in Patients with Metabolic Syndrome: A Controlled Clinical Trial. J. Periodontol. 2012, 83, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.D.; Couper, D.J.; Falkner, K.L.; Graham, S.P.; Grossi, S.G.; Gunsolley, J.C.; Madden, T.; Maupome, G.; Offenbacher, S.; Stewart, D.D.; et al. The Periodontitis and Vascular Events (PAVE) Pilot Study: Adverse Events. J. Periodontol. 2008, 79, 90–96. [Google Scholar] [CrossRef]
- Couper, D.J.; Beck, J.D.; Falkner, K.L.; Graham, S.P.; Grossi, S.G.; Gunsolley, J.C.; Madden, T.; Maupome, G.; Offenbacher, S.; Stewart, D.D.; et al. The Periodontitis and Vascular Events (PAVE) Pilot Study: Recruitment, Retention, and Community Care Controls. J. Periodontol. 2008, 79, 80–89. [Google Scholar] [CrossRef]
- Offenbacher, S.; Beck, J.D.; Moss, K.; Mendoza, L.; Paquette, D.W.; Barrow, D.A.; Couper, D.J.; Stewart, D.D.; Falkner, K.L.; Graham, S.P.; et al. Results from the Periodontitis and Vascular Events (PAVE) Study: A Pilot Multicentered, Randomized, Controlled Trial to Study Effects of Periodontal Therapy in a Secondary Prevention Model of Cardiovascular Disease. J. Periodontol. 2009, 80, 190–201. [Google Scholar] [CrossRef] [PubMed]
- D’Aiuto, F.; Ready, D.; Tonetti, M.S. Periodontal Disease and C-reactive Protein-associated Cardiovascular Risk. J. Periodontal. Res. 2004, 39, 236–241. [Google Scholar] [CrossRef]
- Souza, D.; Okawa, A.B.; Silva, R.T.; Araújo, C.O. Short-Term Changes on C Reactive Protein (CRP) Levels after Non-Surgical Periodontal Treatment in Systemically Healthy Individuals. Clin. Oral Investig. 2017, 21, 477–484. [Google Scholar] [CrossRef]
- Subha, D.S.; Pradeep, T. Periodontal Therapy with 0.25% Lemongrass Oil Mouthwash in Reducing Risk of Cardiovascular Diseases: A 3-Arm Prospective Parallel Experimental Study. Ethiop. J. Health Sci. 2017, 27, 531–540. [Google Scholar] [CrossRef]
- Hussain Bokhari, S.A.; Khan, A.A.; Tatakis, D.N.; Azhar, M.; Hanif, M.; Izhar, M. Non-surgical Periodontal Therapy Lowers Serum Inflammatory Markers: A Pilot Study. J. Periodontol. 2009, 80, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, E.; Malekzadeh, T.; Dongari-Bagtzoglou, A. Effect of Periodontal Treatment on Serum C-reactive Protein Levels: A Systematic Review and Meta-analysis. J. Periodontol. 2006, 77, 1635–1642. [Google Scholar] [CrossRef]
- Orlandi, M.; Muñoz Aguilera, E.; Marletta, D.; Petrie, A.; Suvan, J.; D’Aiuto, F. Impact of the Treatment of Periodontitis on Systemic Health and Quality of Life: A Systematic Review. J. Clin. Periodontol. 2022, 49 (Suppl. 24), 314–327. [Google Scholar] [CrossRef]
- Disidoro, O.; Perrotti, V.; Hui, W.L.; Piattelli, A.; Iaculli, F.; Quaranta, A. The Impact of Non-Surgical Therapy of Periodontal Disease on Surrogate Markers for Cardiovascular Disease: A Literature Review. Am. J. Dent. 2019, 32, 191–200. [Google Scholar]
- Lam, O.L.T.; Zhang, W.; Samaranayake, L.P.; Li, L.S.W.; McGrath, C. A Systematic Review of the Effectiveness of Oral Health Promotion Activities among Patients with Cardiovascular Disease. Int. J. Cardiol. 2011, 151, 261–267. [Google Scholar] [CrossRef]
- Roca-Millan, E.; Gonzalez-Navarro, B.; Sabater-Recolons, M.M.; Mari-Roig, A.; Jane-Salas, E.; Lopez-Lopez, J. Periodontal Treatment on Patients with Cardiovascular Disease: Systematic Review and Meta-Analysis. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e681–e690. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and Grading of Periodontitis: Framework and Proposal of a New Classification and Case Definition. J. Periodontol. 2018, 89 (Suppl. 1), S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus Report of Workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S173–S182. [Google Scholar] [CrossRef]
- Pitiphat, W.; Matangkasombut, O.; Merchant, A. Letter to the Editor: Re: Effect of Periodontal Treatment on Serum C-reactive Protein Levels. J. Periodontol. 2007, 78, 1184–1185. [Google Scholar] [CrossRef] [PubMed]
- Hada, D.S.; Garg, S.; Ramteke, G.B.; Ratre, M.S. Effect of Non-surgical Periodontal Treatment on Clinical and Biochemical Risk Markers of Cardiovascular Disease: A Randomized Trial. J. Periodontol. 2015, 86, 1201–1211. [Google Scholar] [CrossRef]
- Luthra, S.; Orlandi, M.; Hussain, S.B.; Leira, Y.; Botelho, J.; Machado, V.; Mendes, J.J.; Marletta, D.; Harden, S.; D’Aiuto, F. Treatment of Periodontitis and C-reactive Protein: A Systematic Review and Meta-analysis of Randomized Clinical Trials. J. Clin. Periodontol. 2023, 50, 45–60. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Mahendra, J.; Muralidharan, J.; Srinivasan, S.; Mahendra, L.; Cherian, S.M.; Fathima, L.; Prakash, P.; Namasivayam, A.; Dave, P.H.; Bedi, M.; et al. Calprotectin and Periostin Levels in Periodontitis Patients with Coronary Artery Disease. Oral Dis. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Duan, X.Q.; Hu, R.; Ouyang, X.Y. Effect of Non-Surgical Periodontal Therapy on Serum Levels of TNF-a, IL-6 and C-Reactive Protein in Periodontitis Subjects with Stable Coronary Heart Disease. Chin. J. Dent. Res. 2013, 16, 145–151. [Google Scholar]
- Jervøe-Storm, P.-M.; Eberhard, J.; Needleman, I.; Worthington, H.V.; Jepsen, S. Full-Mouth Treatment Modalities (within 24 Hours) for Periodontitis in Adults. Cochrane Libr. 2022, 6, CD004622. [Google Scholar] [CrossRef]
- Eberhard, J.; Jepsen, S.; Jervøe-Storm, P.-M.; Needleman, I.; Worthington, H.V. Full-Mouth Treatment Modalities (within 24 Hours) for Chronic Periodontitis in Adults. Cochrane Libr. 2015, 4, CD004622. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Cei, S.; Orlandi, M.; Gennai, S.; Gabriele, M.; Filice, N.; Nisi, M.; D’Aiuto, F. Acute-phase Response Following Full-mouth versus Quadrant Non-surgical Periodontal Treatment: A Randomized Clinical Trial. J. Clin. Periodontol. 2015, 42, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Gennai, S.; Marruganti, C.; Peric, M.; Ghiadoni, L.; Marhl, U.; Petrini, M. Acute-phase Response Following One-stage Full-mouth versus Quadrant Non-surgical Periodontal Treatment in Subjects with Comorbid Type 2 Diabetes: A Randomized Clinical Trial. J. Clin. Periodontol. 2023, 50, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.C.; Cortelli, J.R.; Cortelli, S.C.; Miranda Cota, L.O.; Machado Costa, L.C.; Moreira Castro, M.V.; Oliveira Azevedo, A.M.; Costa, F.O. Clinical and Microbiologic Evaluation of Scaling and Root Planing per Quadrant and One-stage Full-mouth Disinfection Associated with Azithromycin or Chlorhexidine: A Clinical Randomized Controlled Trial. J. Periodontol. 2015, 86, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Apatzidou, D.A.; Kinane, D.F. Quadrant Root Planing versus Same-day Full-mouth Root Planing: I. Clinical Findings. J. Clin. Periodontol. 2004, 31, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Del Peloso Ribeiro, É.; Bittencourt, S.; Sallum, E.A.; Nociti, F.H., Jr.; Gonçalves, R.B.; Casati, M.Z. Periodontal Debridement as a Therapeutic Approach for Severe Chronic Periodontitis: A Clinical, Microbiological and Immunological Study. J. Clin. Periodontol. 2008, 35, 789–798. [Google Scholar] [CrossRef]
- Koshy, G.; Kawashima, Y.; Kiji, M.; Nitta, H.; Umeda, M.; Nagasawa, T.; Ishikawa, I. Effects of Single-visit Full-mouth Ultrasonic Debridement versus Quadrant-wise Ultrasonic Debridement. J. Clin. Periodontol. 2005, 32, 734–743. [Google Scholar] [CrossRef]
- Slade, G.D.; Offenbacher, S.; Beck, J.D.; Heiss, G.; Pankow, J.S. Acute-Phase Inflammatory Response to Periodontal Disease in the US Population. J. Dent. Res. 2000, 79, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.G.; Craandijk, J.; Hoek, F.J.; Dillen, P.M.E.W.; Van Der Velden, U. Elevation of Systemic Markers Related to Cardiovascular Diseases in the Peripheral Blood of Periodontitis Patients. J. Periodontol. 2000, 71, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Torrungruang, K.; Vathesatogkit, P.; Mahanonda, R.; Thienpramuk, L. Periodontitis and Hypertension Are Linked through Systemic Inflammation: A 5-year Longitudinal Study. J. Clin. Periodontol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, N.; Chai, J.T.; Fisher, E.A.; Choudhury, R.P. Inflammatory Processes in Cardiovascular Disease: A Route to Targeted Therapies. Nat. Rev. Cardiol. 2017, 14, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Rammos, A.; Bechlioulis, A.; Kekiopoulou, A.; Kekiopoulos, P.; Katsouras, C.S.; Sioka, C. Myocardial Perfusion Imaging and C-Reactive Protein in Myocardial Ischemia: A Retrospective Single-Center Study. Life 2024, 14, 261. [Google Scholar] [CrossRef] [PubMed]
- Lapić, I.; Padoan, A.; Bozzato, D.; Plebani, M. Erythrocyte Sedimentation Rate and C-Reactive Protein in Acute Inflammation. Am. J. Clin. Pathol. 2020, 153, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Perera, C.; McNeil, H.P.; Geczy, C.L. S100 Calgranulins in Inflammatory Arthritis. Immunol. Cell Biol. 2010, 88, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Marriott, E.; Singanayagam, A.; El-Awaisi, J. Inflammation as the Nexus: Exploring the Link between Acute Myocardial Infarction and Chronic Obstructive Pulmonary Disease. Front. Cardiovasc. Med. 2024, 11, 1362564. [Google Scholar] [CrossRef] [PubMed]
- Haririan, H.; Andrukhov, O.; Pablik, E.; Neuhofer, M.; Moritz, A.; Rausch-Fan, X. Comparative Analysis of Calcium-binding Myeloid-related Protein-8/14 in Saliva and Serum of Patients with Periodontitis and Healthy Individuals. J. Periodontol. 2016, 87, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Punj, V.; Kim, J.-M.; Lee, S.; Ulmer, T.S.; Lu, W.; Rice, J.C.; An, W. MMP-9 Facilitates Selective Proteolysis of the Histone H3 Tail at Genes Necessary for Proficient Osteoclastogenesis. Genes Dev. 2016, 30, 208–219. [Google Scholar] [CrossRef]
- Kim, H.-D.; Kim, S.; Jeon, S.; Kim, S.-J.; Cho, H.-J.; Choi, Y.-N. Diagnostic and Prognostic Ability of Salivary MMP-9 and S100A8 for Periodontitis. J. Clin. Periodontol. 2020, 47, 1191–1200. [Google Scholar] [CrossRef]
- Honore, P.M.; Perriens, E.; Blackman, S. Potential Confounders in Linking Elevated S100A8/A9 to Left Ventricular Dysfunction in Septic Shock Patients. Crit. Care 2023, 27, 480. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; de Salis, I.; Hamilton, W.; Salisbury, C. ‘I’m Fishing Really—Inflammatory Marker Testing in Primary Care: A Qualitative Study. Br. J. Gen. Pract. 2016, 66, e200–e206. [Google Scholar] [CrossRef] [PubMed]
- Irwandi, R.A.; Kuswandani, S.O.; Harden, S.; Marletta, D.; D’Aiuto, F. Circulating Inflammatory Cell Profiling and Periodontitis: A Systematic Review and Meta-Analysis. J. Leukoc. Biol. 2022, 111, 1069–1096. [Google Scholar] [CrossRef]
- de França, L.F.C.; da Silva, F.R.P.; di Lenardo, D.; Alves, E.H.P.; Nascimento, H.M.S.; da Silva, I.A.T.; Vasconcelos, A.C.C.G.; Vasconcelos, D.F.P. Comparative Analysis of Blood Parameters of the Erythrocyte Lineage between Patients with Chronic Periodontitis and Healthy Patients: Results Obtained from a Meta-Analysis. Arch. Oral Biol. 2019, 97, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L.; Darbar, U.; Rakmanee, T.; Donos, N. Anemia of Inflammation Associated with Periodontitis: Analysis of Two Clinical Studies. J. Periodontol. 2019, 90, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Angkananard, T.; Anothaisintawee, T.; McEvoy, M.; Attia, J.; Thakkinstian, A. Neutrophil Lymphocyte Ratio and Cardiovascular Disease Risk: A Systematic Review and Meta-Analysis. Biomed Res. Int. 2018, 2018, 2703518. [Google Scholar] [CrossRef]
- Do Lee, C. White Blood Cell Count and Incidence of Coronary Heart Disease and Ischemic Stroke and Mortality from Cardiovascular Disease in African-American and White Men and Women: Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 2001, 154, 758–764. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).