Clinacanthus nutans (Burm. f.) Lindau Extract Inhibits Dengue Virus Infection and Inflammation in the Huh7 Hepatoma Cell Line
Abstract
:1. Introduction
2. Results
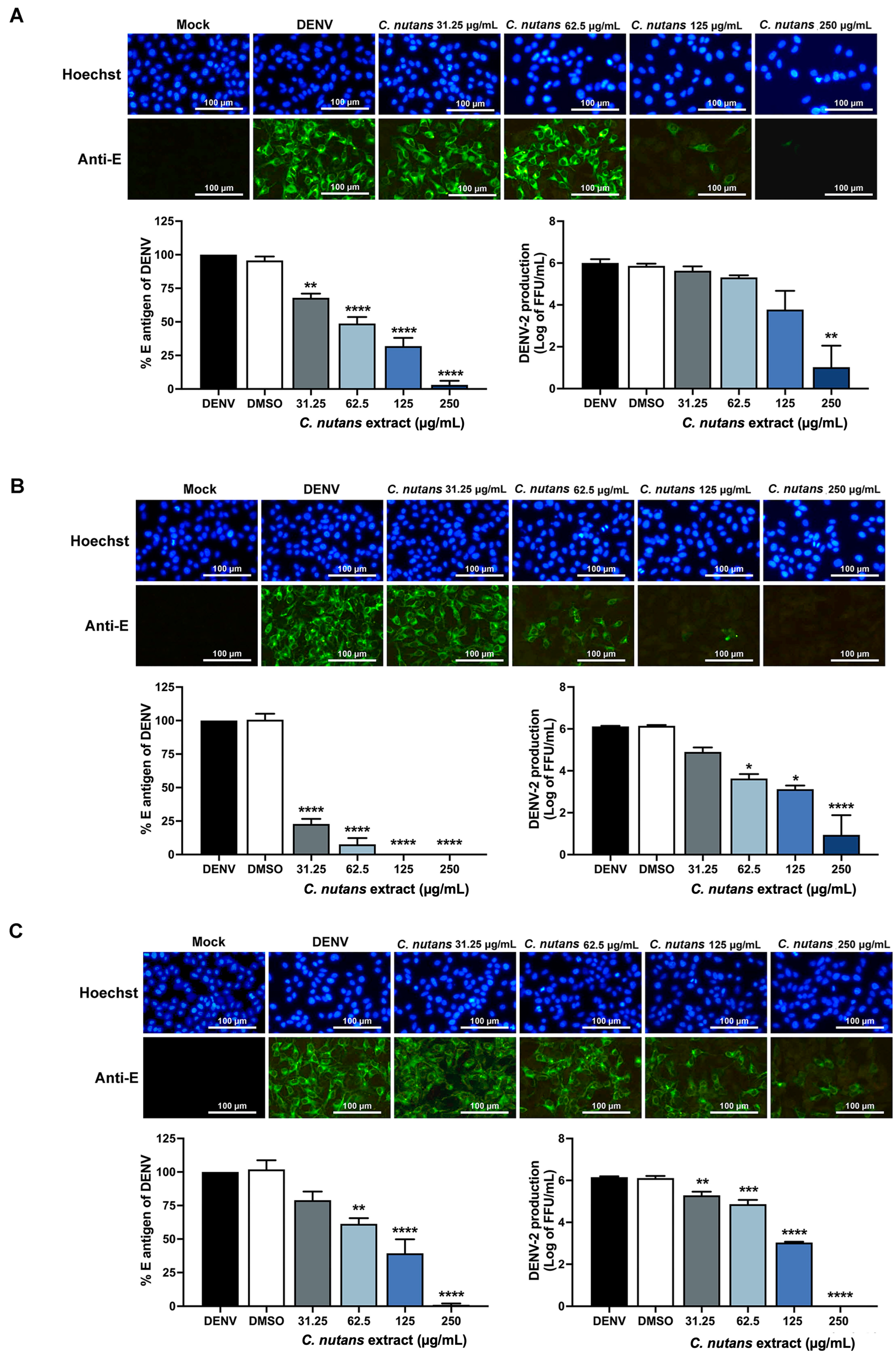
2.1. Inhibition of DENV-2-Infected Huh7 Hepatoma Cells Upon Treatment with Clinacanthus nutans Extract
2.2. Inhibition of DENV-2 Infection in the Early Steps of Infection by C. nutans Extracts
2.3. Treatment of C. nutans Extracts Inhibited the Virus Binding to Host Cells and Virus RNA Synthesis
2.4. Reduction in Inflammatory-Related Gene Expression in DENV-2 Infected Cells by C. nutans Extracts
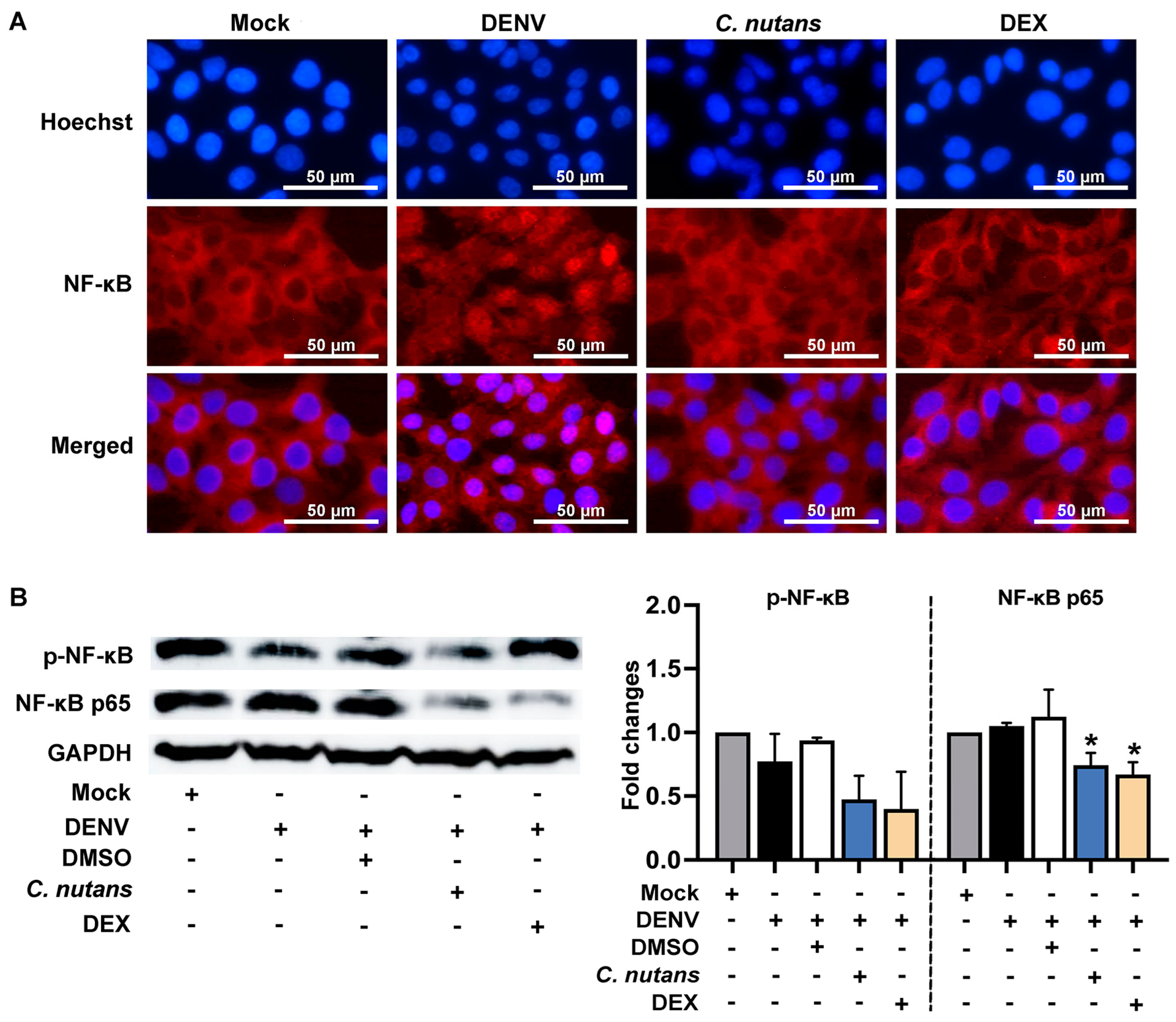
2.5. C. nutans Extract Suppressed the Inflammation of DENV-Infected Cells by Inhibiting the NF-κB Signaling Pathway
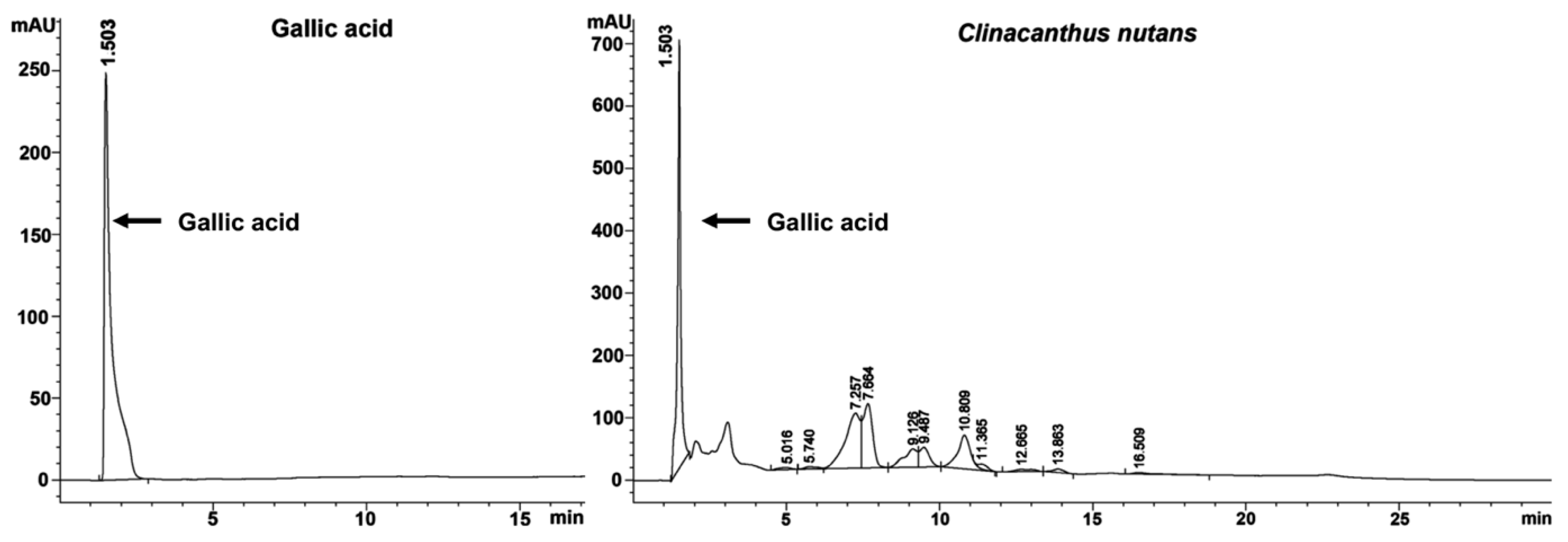
2.6. Gallic Acid Was a Bioactive Compound of C. nutans Extract
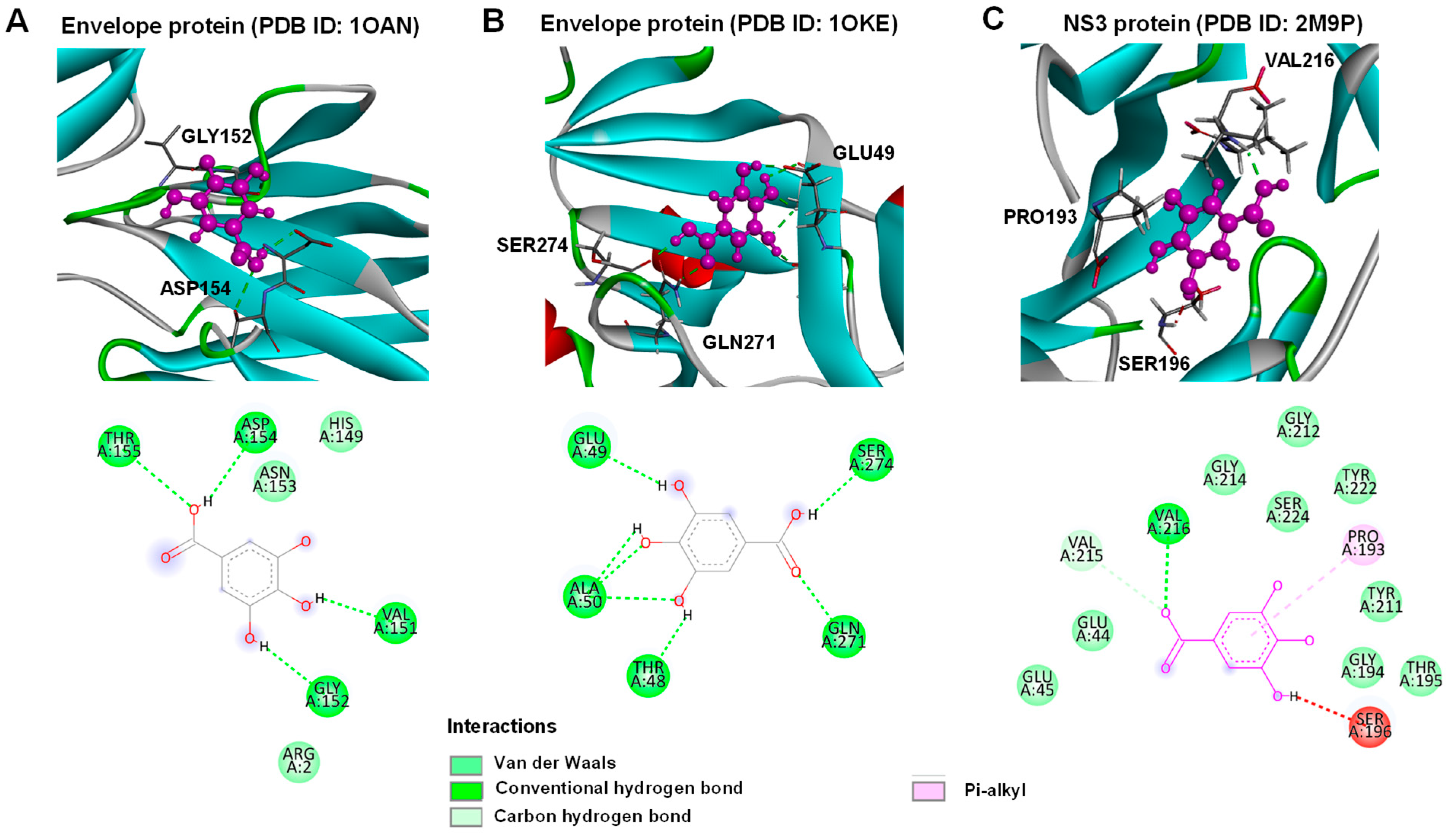
2.7. Gallic Acid Inhibited DENV Infection
3. Discussion
4. Materials and Methods
4.1. Plant and Extraction
4.2. Cell Cultures and DENV Propagation
4.3. Cell Viability Assay
4.4. DENV Infection and Time-of-Addition Assay
4.5. Focus Forming Unit (FFU) Assay
4.6. Cell-Based ELISA
4.7. Immunofluorescence Study (IFA)
4.8. Real-Time PCR
4.9. Immunoblotting Assay
4.10. Determination of Total Phenolic Content
4.11. Determination of the Phytochemical Composition of C. nutans Extract by HPLC
4.12. Molecular Docking of Dengue 2 Virus Envelope Proteins and NS3 Protease with Gallic Acid
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Dengue and Severe Dengue. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 5 December 2022).
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Li, Q.; Kang, C. Structures and dynamics of dengue virus nonstructural membrane proteins. Membranes 2022, 12, 231. [Google Scholar] [CrossRef]
- Zerfu, B.; Kassa, T.; Legesse, M. Epidemiology, biology, pathogenesis, clinical manifestations, and diagnosis of dengue virus infection, and its trend in Ethiopia: A comprehensive literature review. Trop. Med. Health 2023, 51, 11. [Google Scholar] [CrossRef]
- Ahmed, I.; Ahamed, R.; Nahar, S.; Bari, L.F.; Dewan, S.M. Immunization against dengue virus infection is coercive: A timely call. Health Sci. Rep. 2024, 7, e2170. [Google Scholar] [CrossRef]
- Acosta, E.G.; Castilla, V.; Damonte, E.B. Alternative infectious entry pathways for dengue virus serotypes into mammalian cells. Cell Microbiol. 2009, 11, 1533–1549. [Google Scholar] [CrossRef]
- Hou, J.; Baker, L.A.; Zhou, L.; Klein, R.S. Viral interactions with the blood-brain barrier: Old dog, new tricks. Tissue Barriers 2016, 4, e1142492. [Google Scholar] [CrossRef]
- Miller, J.L.; de Wet, B.J.; Martinez-Pomares, L.; Radcliffe, C.M.; Dwek, R.A.; Rudd, P.M.; Gordon, S. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 2008, 4, e17. [Google Scholar] [CrossRef]
- Huerre, M.R.; Lan, N.T.; Marianneau, P.; Hue, N.B.; Khun, H.; Hung, N.T.; Khen, N.T.; Drouet, M.T.; Huong, V.T.; Ha, D.Q.; et al. Liver histopathology and biological correlates in five cases of fatal dengue fever in Vietnamese children. Virchows Arch. 2001, 438, 107–115. [Google Scholar] [CrossRef]
- Povoa, T.F.; Oliveira, E.R.A.; Basilio-de-Oliveira, C.A.; Nuovo, G.J.; Chagas, V.L.A.; Salomao, N.G.; Alves, A.M.B.; Mota, E.M.; Paes, M.V. Correction: Peripheral Organs of Dengue Fatal Cases Present Strong Pro-Inflammatory Response with Participation of IFN-Gamma-, TNF-Alpha- and RANTES-Producing Cells. PLoS ONE 2018, 13, e0195140. [Google Scholar] [CrossRef]
- Conceicao, T.M.; El-Bacha, T.; Villas-Boas, C.S.; Coello, G.; Ramirez, J.; Montero-Lomeli, M.; Da Poian, A.T. Gene expression analysis during dengue virus infection in HepG2 cells reveals virus control of innate immune response. J. Infect. 2010, 60, 65–75. [Google Scholar] [CrossRef]
- Ferreira, R.A.; de Oliveira, S.A.; Gandini, M.; Ferreira Lda, C.; Correa, G.; Abiraude, F.M.; Reid, M.M.; Cruz, O.G.; Kubelka, C.F. Circulating cytokines and chemokines associated with plasma leakage and hepatic dysfunction in Brazilian children with dengue fever. Acta Trop. 2015, 149, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Alcalá, A.C.; Maravillas, J.L.; Meza, D.; Ramirez, O.T.; Ludert, J.E.; Palomares, L.A. Dengue virus NS1 uses scavenger receptor B1 as a cell receptor in cultured cells. J. Virol. 2022, 96, e01664-21. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, N.M.; Wei, L.; Ho, N.N.; Neal, M.L.; Seferos, D.; Tongogara, T.; Mast, F.D.; Aitchison, J.D.; Kaushansky, A. Multiple receptor tyrosine kinases regulate dengue infection of hepatocytes. Front. Cell. Infect. Microbiol. 2024, 14, 1264525. [Google Scholar] [CrossRef] [PubMed]
- National Drug Information. 2023. Available online: http://ndi.fda.moph.go.th/drug_national (accessed on 15 January 2023).
- Chia, T.Y.; Gan, C.Y.; Murugaiyah, V.; Hashmi, S.F.; Fatima, T.; Ibrahim, L.; Abdulla, M.H.; Alswailmi, F.K.; Johns, E.J.; Ahmad, A. A Narrative Review on the Phytochemistry, Pharmacology and Therapeutic Potentials of Clinacanthus nutans (Burm. f.) Lindau Leaves as an Alternative Source of Future Medicine. Molecules 2021, 27, 139. [Google Scholar] [CrossRef] [PubMed]
- Zulkipli, I.N.; Rajabalaya, R.; Idris, A.; Sulaiman, N.A.; David, S.R. Clinacanthus nutans: A review on ethnomedicinal uses, chemical constituents and pharmacological properties. Pharm. Biol. 2017, 55, 1093–1113. [Google Scholar] [CrossRef] [PubMed]
- Jiamton, S.; Chanyachailert, P.; Nanchaipruek, Y.; Jantanapornchai, N.; Patthamalai, P.; Limphoka, P.; Nokdhes, Y.-N.; Jantaravinid, J. Efficacy and Safety of Clinacanthus nutans Lindau Cream vs. Podophyllin for the Treatment of Adults with Condyloma Acuminata. Evid. Based Complement. Altern. Med. 2022, 2022, 1577716. [Google Scholar] [CrossRef]
- Chan, S.M.; Khoo, K.S.; Sekaran, S.D.; Sit, N.W. Mode-Dependent Antiviral Activity of Medicinal Plant Extracts against the Mosquito-Borne Chikungunya Virus. Plants 2021, 10, 1658. [Google Scholar] [CrossRef]
- Ismail, N.Z.; Adebayo, I.A.; Mohamad Zain, N.N.; Arsad, H. Molecular docking of compounds from Clinacanthus nutans extract detected by GC-MS analysis with the SARS-CoV-2 main protease and ACE2 protein. Nat. Prod. Res. 2022, 36, 2848–2852. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Chao, C.H.; Yeh, T.M. Roles of Macrophage Migration Inhibitory Factor in Dengue Pathogenesis: From Pathogenic Factor to Therapeutic Target. Microorganisms 2020, 8, 891. [Google Scholar] [CrossRef]
- Panya, A.; Jantakee, K.; Punwong, S.; Thongyim, S.; Kaewkod, T.; Yenchitsomanus, P.T.; Tragoolpua, Y.; Pandith, H. Triphala in Traditional Ayurvedic Medicine Inhibits Dengue Virus Infection in Huh7 Hepatoma Cells. Pharmaceuticals 2021, 14, 1236. [Google Scholar] [CrossRef] [PubMed]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. Crystal Structure of the Dengue 2 Virus Envelope Protein. Available online: https://www.wwpdb.org/pdb?id=pdb_00001oan (accessed on 7 July 2024).
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. Crystal Structure of the Dengue 2 Virus Envelope Protein in Complex with n-Octyl-beta-D-glucoside. Available online: https://www.wwpdb.org/pdb?id=pdb_00001oke (accessed on 7 July 2024).
- Gibbs, A.; Tounge, B.; Steele, R. NMR Structure of an Inhibitor Bound Dengue NS3 Protease. 2014. Available online: https://www.wwpdb.org/pdb?id=pdb_00002m9p (accessed on 4 November 2023).
- Artpradit, C.; Robinson, L.N.; Gavrilov, B.K.; Rurak, T.T.; Ruchirawat, M.; Sasisekharan, R. Recognition of heparan sulfate by clinical strains of dengue virus serotype 1 using recombinant subviral particles. Virus Res. 2013, 176, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Wright, P.J.; Davidson, A.; Lobigs, M. Virulence attenuation of Dengue virus due to augmented glycosaminoglycan-binding affinity and restriction in extraneural dissemination. J. Gen. Virol. 2006, 87, 2791–2801. [Google Scholar] [CrossRef]
- Ang, F.; Wong, A.P.; Ng, M.M.; Chu, J.J. Small interference RNA profiling reveals the essential role of human membrane trafficking genes in mediating the infectious entry of dengue virus. Virol. J. 2010, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Che, P.; Tang, H.; Li, Q. The interaction between claudin-1 and dengue viral prM/M protein for its entry. Virology 2013, 446, 303–313. [Google Scholar] [CrossRef]
- Panya, A.; Bangphoomi, K.; Choowongkomon, K.; Yenchitsomanus, P.T. Peptide inhibitors against dengue virus infection. Chem. Biol. Drug Des. 2014, 84, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Panya, A.; Sawasdee, N.; Junking, M.; Srisawat, C.; Choowongkomon, K.; Yenchitsomanus, P.T. A peptide inhibitor derived from the conserved ectodomain region of DENV membrane (M) protein with activity against dengue virus infection. Chem. Biol. Drug Des. 2015, 86, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Panya, A.; Yongpitakwattana, P.; Budchart, P.; Sawasdee, N.; Krobthong, S.; Paemanee, A.; Roytrakul, S.; Rattanabunyong, S.; Choowongkomon, K.; Yenchitsomanus, P.T. Novel bioactive peptides demonstrating anti-dengue virus activity isolated from the Asian medicinal plant Acacia catechu. Chem. Biol. Drug Des. 2019, 93, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Saokaew, N.; Poungpair, O.; Panya, A.; Tarasuk, M.; Sawasdee, N.; Limjindaporn, T.; Chaicumpa, W.; Yenchitsomanus, P. Human monoclonal single-chain antibodies specific to dengue virus envelope protein. Lett. Appl. Microbiol. 2014, 58, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Pongmuangmul, S.; Phumiamorn, S.; Sanguansermsri, P.; Wongkattiya, N.; Fraser, I.H.; Sanguansermsri, D. Anti-herpes simplex virus activities of monogalactosyl diglyceride and digalactosyl diglyceride from Clinacanthus nutans, a traditional Thai herbal medicine. Asian Pac. J. Trop. Biomed. 2016, 6, 192–197. [Google Scholar] [CrossRef]
- Sookmai, W.E.T.; Pientong, C.; Sakdarat, S.; Kongyingyoes, B. The anti-papillomavirus infectivity of Clinacanthus nutans compounds. Srinagarind Med. J. 2011, 26, 260–263. [Google Scholar]
- Rodenhuis-Zybert, I.A.; Wilschut, J.; Smit, J.M. Dengue virus life cycle: Viral and host factors modulating infectivity. Cell Mol. Life Sci. 2010, 67, 2773–2786. [Google Scholar] [CrossRef] [PubMed]
- Khanam, A.; Gutierrez-Barbosa, H.; Lyke, K.E.; Chua, J.V. Immune-Mediated Pathogenesis in Dengue Virus Infection. Viruses 2022, 14, 2575. [Google Scholar] [CrossRef] [PubMed]
- Imad, H.A.; Phumratanaprapin, W.; Phonrat, B.; Chotivanich, K.; Charunwatthana, P.; Muangnoicharoen, S.; Khusmith, S.; Tantawichien, T.; Phadungsombat, J.; Nakayama, E.; et al. Cytokine Expression in Dengue Fever and Dengue Hemorrhagic Fever Patients with Bleeding and Severe Hepatitis. Am. J. Trop. Med. Hyg. 2020, 102, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Leowattana, W.; Leowattana, T. Dengue hemorrhagic fever and the liver. World J. Hepatol. 2021, 13, 1968–1976. [Google Scholar] [CrossRef] [PubMed]
- Tarasuk, M.; Songprakhon, P.; Chieochansin, T.; Choomee, K.; Na-Bangchang, K.; Yenchitsomanus, P.T. Alpha-mangostin inhibits viral replication and suppresses nuclear factor kappa B (NF-kappaB)-mediated inflammation in dengue virus infection. Sci. Rep. 2022, 12, 16088. [Google Scholar] [CrossRef] [PubMed]
- Chelyn, J.L.; Omar, M.H.; Mohd Yousof, N.S.; Ranggasamy, R.; Wasiman, M.I.; Ismail, Z. Analysis of flavone C-glycosides in the leaves of Clinacanthus nutans (Burm. f.) Lindau by HPTLC and HPLC-UV/DAD. Sci. World J. 2014, 2014, 724267. [Google Scholar] [CrossRef] [PubMed]
- De Melo, G.O.; Muzitano, M.F.; Legora-Machado, A.; Almeida, T.A.; De Oliveira, D.B.; Kaiser, C.R.; Koatz, V.L.; Costa, S.S. C-glycosylflavones from the aerial parts of Eleusine indica inhibit LPS-induced mouse lung inflammation. Planta Med. 2005, 71, 362–363. [Google Scholar] [CrossRef]
- Ong, W.Y.; Herr, D.R.; Sun, G.Y.; Lin, T.N. Anti-Inflammatory Effects of Phytochemical Components of Clinacanthus nutans. Molecules 2022, 27, 3607. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, X.; Zhang, B.; Ren, M. The protective effect of vitexinin septic encephalopathy by reducing leukocyte-endothelial adhesion and inflammatory response. Ann. Palliat. Med. 2020, 9, 2079–2089. [Google Scholar] [CrossRef]
- Duan, S.; Du, X.; Chen, S.; Liang, J.; Huang, S.; Hou, S.; Gao, J.; Ding, P. Effect of vitexin on alleviating liver inflammation in a dextran sulfate sodium (DSS)-induced colitis model. Biomed. Pharmacother. 2020, 121, 109683. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, H.; Gu, X.; Yin, X. The natural flavonoid glycoside vitexin displays preclinical antitumor activity by suppressing NF-kappaB signaling in nasopharyngeal carcinoma. Onco Targets Ther. 2019, 12, 4461–4468. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ma, Y.; He, Y.; Yang, H.; Chen, Y.; Wang, L.; Huang, D.; Qiu, S.; Tao, X.; Chen, W. A Network Pharmacology-Based Investigation to the Pharmacodynamic Material Basis and Mechanisms of the Anti-Inflammatory and Anti-Viral Effect of Isatis indigotica. Drug Des. Dev. Ther. 2021, 15, 3193–3206. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, K.; Vaiyapuri, M. Orientin, a C-glycosyl dietary flavone, suppresses colonic cell proliferation and mitigates NF-kappaB mediated inflammatory response in 1,2-dimethylhydrazine induced colorectal carcinogenesis. Biomed. Pharmacother. 2017, 96, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Khoo, L.W.; Mediani, A.; Zolkeflee, N.K.Z.; Leong, S.W.; Ismail, I.S.; Khatib, A. Phytochemical diversity of Clinacanthus nutans extracts and their bioactivity correlations elucidated by NMR based metabolomics. Phytochem. Lett. 2015, 14, 123–133. [Google Scholar] [CrossRef]
- Lin, C.-M.; Chen, H.-H.; Lung, C.-W.; Chen, H.-J. Antiviral and Immunomodulatory Activities of Clinacanthus nutans (Burm. f.) Lindau. Int. J. Mol. Sci. 2023, 24, 10789. [Google Scholar] [CrossRef] [PubMed]
- Khoo, L.W.; Kow, S.A.; Lee, M.T.; Tan, C.P.; Shaari, K.; Tham, C.L.; Abas, F. A Comprehensive Review on Phytochemistry and Pharmacological Activities of Clinacanthus nutans (Burm.f.) Lindau. Evid. Based Complement. Altern. Med. 2018, 2018, 1–39. [Google Scholar] [CrossRef]
- Lam, K.Y.; Ling, A.P.K.; Koh, R.Y.; Wong, Y.P.; Say, Y.H. A Review on Medicinal Properties of Orientin. Adv. Pharmacol. Sci. 2016, 2016, 4104595. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef]
- Nikfarjam, B.A.; Hajiali, F.; Adineh, M.; Nassiri-Asl, M. Anti-inflammatory Effects of Quercetin and Vitexin on Activated Human Peripheral Blood Neutrophils:-The effects of quercetin and vitexin on human neutro-phils. J. Pharmacopunct. 2017, 20, 127–131. [Google Scholar]
- Da Fonseca, J.M.; Reis, A.C.C.; Pereira, G.R.; de Moura, H.M.M.; Filho, J.D.S.; Silva, B.d.M.; Brandão, G.C. Chromatographic profile of xanthones and flavonoids in the anti-dengue extracts of Fridericia samydoides (Cham.) L.G. Lohmann (Bignoniaceae). Braz. J. Pharm. Sci. 2022, 58. [Google Scholar] [CrossRef]
- Anilkumar, K.; Reddy, G.V.; Azad, R.; Yarla, N.S.; Dharmapuri, G.; Srivastava, A.; Kamal, M.A.; Pallu, R. Evaluation of Anti-Inflammatory Properties of Isoorientin Isolated from Tubers of Pueraria tuberosa. Oxidative Med. Cell. Longev. 2017, 2017, 5498054. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Yu, Z.; Zheng, Y.; Wang, L.; Qin, X.; Cheng, G.; Ci, X. Isovitexin exerts anti-inflammatory and an-ti-oxidant activities on lipopolysaccharide-induced acute lung injury by inhibiting MAPK and NF-κB and activating HO-1/Nrf2 pathways. Int. J. Biol. Sci. 2016, 12, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Guo, W.; Gao, J.; Chen, J.; Olatunji, J.O. Clinacanthus nutans (Burm. f.) Lindau Ethanol Extract Inhibits Hepatoma in Mice through Upregulation of the Immune Response. Molecules 2015, 20, 17405–17428. [Google Scholar] [CrossRef]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jantakee, K.; Panwong, S.; Sattayawat, P.; Sumankan, R.; Saengmuang, S.; Choowongkomon, K.; Panya, A. Clinacanthus nutans (Burm. f.) Lindau Extract Inhibits Dengue Virus Infection and Inflammation in the Huh7 Hepatoma Cell Line. Antibiotics 2024, 13, 705. https://doi.org/10.3390/antibiotics13080705
Jantakee K, Panwong S, Sattayawat P, Sumankan R, Saengmuang S, Choowongkomon K, Panya A. Clinacanthus nutans (Burm. f.) Lindau Extract Inhibits Dengue Virus Infection and Inflammation in the Huh7 Hepatoma Cell Line. Antibiotics. 2024; 13(8):705. https://doi.org/10.3390/antibiotics13080705
Chicago/Turabian StyleJantakee, Kanyaluck, Suthida Panwong, Pachara Sattayawat, Ratchaneewan Sumankan, Sasithorn Saengmuang, Kiattawee Choowongkomon, and Aussara Panya. 2024. "Clinacanthus nutans (Burm. f.) Lindau Extract Inhibits Dengue Virus Infection and Inflammation in the Huh7 Hepatoma Cell Line" Antibiotics 13, no. 8: 705. https://doi.org/10.3390/antibiotics13080705
APA StyleJantakee, K., Panwong, S., Sattayawat, P., Sumankan, R., Saengmuang, S., Choowongkomon, K., & Panya, A. (2024). Clinacanthus nutans (Burm. f.) Lindau Extract Inhibits Dengue Virus Infection and Inflammation in the Huh7 Hepatoma Cell Line. Antibiotics, 13(8), 705. https://doi.org/10.3390/antibiotics13080705





