Impact of Manual Addition of Vancomycin to Polymethylmethacrylate (PMMA) Cements
Abstract
1. Introduction
2. Results
3. Discussions
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kühn, K.D. PMMA Cements Are We Aware What We Are Using? Springer: Berlin/Heidelberg, Germany, 2014; ISBN 13 978-3-642-41535-7. [Google Scholar] [CrossRef]
- Chaiyakit, P.; Meknavin, S.; Honku, N.; Onklin, I. Debridement, antibiotics, and implant retention combined with direct intra-articular antibiotic infusion in patients with acute hematogenous periprosthetic joint infection of the knee. BMC Musculoskelet. Disord. 2021, 18, 557. [Google Scholar] [CrossRef]
- Choe, H.; Maruo, A.; Hieda, Y.; Abe, K.; Kobayashi, N.; Ike, H.; Kumagai, K.; Takeyama, M.; Kawabata, Y.; Inaba, Y. Novel local antibiotic antifungal treatment for fungal periprosthetic joint infection with continous local antibiotic perfusion: A surgical technique. Arthroplast. Today 2023, 24, 101245. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Takagi, T. Treatment experience with continous local antibiotic perfusion for periprosthetic joint infection. J. Orthop. Sci. 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Frank, B.; Simon, S.; Aichmair, A.; Dominkus, M.; Hofstaetter, J. Clinical impact of microbiology results in two-stage revision arthroplasty with spacer exchange. Arch. Orthop. Trauma Surg. 2023, 143, 4741–4754. [Google Scholar] [CrossRef]
- Trampuz, A.; Zimmerli, W. Prosthetic joint infections: Update in diagnosis and treatment. Swiss. Med. Wkly. 2005, 17–18, 243–251. [Google Scholar]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-joint infections. N. Engl. J. Med. 2004, 16, 1645–1654. [Google Scholar] [CrossRef]
- George, J.; Newman, J.; Klika, A.; Miller, E.; Tan, T.; Parvizi, J.; Higuera, C. Changes in antibiotic susceptibility of staphylococcus aureus between the stages of 2-stage revision arthroplasty. J. Arthroplast. 2018, 33, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Renz, N.; Trebse, R.; Akgün, D.; Perka, C.; Trampuz, A. Enterococcal periprosthetic joint infection: Clinical and microbiological findings from 8-year retrospective cohort study. BMC Infect. Dis. 2019, 19, 1083. [Google Scholar] [CrossRef]
- Frommelt, L. Antibiotic choices in bone surgery-local therapy using antibiotic-loaded bone cement. In Local Antibiotics in Arthroplasty; Walenkamp, G., Ed.; Georg Thieme Verlag: Stuttgart, Germany, 2007; pp. 59–64. [Google Scholar]
- Parvizi, J.; Gehrke, T. International consensus on periprosthetic joint infection: Let cumulative wisdom be a guide. J. Bone Jt. Surg. Am. 2014, 6, 441. [Google Scholar] [CrossRef]
- Paz, E.; Sanz-Ruiz, P.; Abenojar, J.; Vaquero-Martin, J.; Forriol, F.; Del Real, J.C. Evaluation of elution and mechanical properties of high-dose antibiotic-loaded bone cement: Comparative “in vitro” study of the influence of vancomycin and cefazolin. J. Arthroplast. 2015, 8, 1423–1429. [Google Scholar] [CrossRef]
- Malhotra, A.; Lieb, E.; Berberich, C.; Kühn, K.D. PMMA cements in revision surgery. In Management of Periprosthetic Joint Infection. A Global Perspective on Diagnosis, Treatment Options, Prevention Strategies and Their Economic Impact; Kühn, K.D., Ed.; Springer: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Winkler, T.; Stuhlert, M.; Lieb, E.; Müller, M.; von Roth, P.; Preininger, B.; Trampuz, A.; Perka, C. Outcome of short versus long interval in two-stage exchange of periprosthetic joint infection: A prospective cohort study. Arch. Orthop. Trauma Surg. 2019, 139, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.; Carvalho, A.; Soares, D.; Abreu, M. Interval between two-stage exchanges: What is optimal and how do we know? Arthroplasty 2023, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Miola, M.; Bistolfi, A.; Fucale, G.; Crova, M.; Masse, A.; Verne, E. In vitro comparison between commercially and manually mixed antibiotic-loaded bone cements. J. Appl. Biomater. Biomech. 2010, 3, 166–174. [Google Scholar] [CrossRef]
- Miola, M.; Bistolfi, A.; Valsania, M.C.; Bianco, C.; Fucale, G.; Verne, E. Antibiotic-loaded acrylic bone cements: An in vitro study on the release mechanism and its efficacy. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 5, 3025–3032. [Google Scholar] [CrossRef] [PubMed]
- Neut, D.; van de Belt, H.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. The effect of mixing on gentamicin release from polymethylmethacrylate bone cements. Acta Orthop. Scand. 2003, 6, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Dunne, N.J.; Hill, J.; McAfee, P.; Kirkpatrick, R.; Patrick, S.; Tunney, M. Incorporation of large amounts of gentamicin sulphate into acrylic bone cement: Effect on handling and mechanical properties, antibiotic release, and biofilm formation. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2008, 3, 355–365. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Trotignon, J.P.; Loty, B.; Tcharkhtchi, A.; Verdu, J. Effect of antibiotics on the properties of poly(methylmethacrylate)-based bone cement. J. Biomed. Mater. Res. 2002, 6, 800–806. [Google Scholar] [CrossRef]
- Lautenschlager, E.P.; Jacobs, J.J.; Marshall, G.W.; Meyer, P.R., Jr. Mechanical properties of bone cements containing large doses of antibiotic powders. J. Biomed. Mater. Res. 1976, 6, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Lautenschlager, E.P.; Marshall, G.W.; Marks, K.E.; Schwartz, J.; Nelson, C.L. Mechanical strength of acrylic bone cements impregnated with antibiotics. J. Biomed. Mater. Res. 1976, 6, 837–845. [Google Scholar] [CrossRef]
- Lilikakis, A.; Sutcliffe, M.P. The effect of vancomycin addition to the compression strength of antibiotic-loaded bone cements. Int. Orthop. 2009, 3, 815–819. [Google Scholar] [CrossRef]
- Armstrong, M.S.; Spencer, R.F.; Cunningham, J.L.; Gheduzzi, S.; Miles, A.W.; Learmonth, I.D. Mechanical characteristics of antibiotic-laden bone cement. Acta Orthop. Scand. 2002, 6, 688–690. [Google Scholar] [CrossRef]
- Gallo, J.; Bogdanova, K.; Siller, M.; Svabova, M.; Lostak, J.; Kolar, M. Microbial and pharmacological characteristics of VancogenX. Acta Chir. Orthop. Traumatol. Cech. 2013, 1, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Schmolders, J.; Hischebeth, G.T.; Friedrich, M.J.; Randau, T.M.; Wimmer, M.D.; Kohlhof, H.; Molitor, E.; Gravius, S. Evidence of MRSE on a gentamicin and vancomycin impregnated polymethyl-methacrylate (PMMA) bone cement spacer after two-stage exchange arthroplasty due to periprosthetic joint infection of the knee. BMC Infect. Dis. 2014, 14, 144. [Google Scholar] [CrossRef] [PubMed]
- Watanakunakorn, C. Treatment of infections due to methicillin-resistant staphylococcus aureus. Ann. Intern. Med. 1982, 3, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Amerstorfer, F.; Fischerauer, S.; Sadoghi, P.; Schwantzer, G.; Kühn, K.D.; Leithner, A.; Glehr, M. Superficial vancomycin coating of bone cement in orthopedic revision surgery: A safe technique to enhance local antibiotic concentrations. J. Arthroplast. 2017, 5, 1618–1624. [Google Scholar] [CrossRef]
- Anagnostakos, K.; Furst, O.; Kelm, J. Antibiotic-impregnated PMMA hip spacers: Current status. Acta Orthop. 2006, 4, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, K.; Kelm, J.; Regitz, T.; Schmitt, E.; Jung, W. In vitro evaluation of antibiotic release from and bacteria growth inhibition by antibiotic-loaded acrylic bone cement spacers. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 2, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Deresinski, S. Vancomycin in combination with other antibiotics for the treatment of serious methicillin-resistant staphylococcus aureus infections. Clin. Infect. Dis. 2009, 7, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.H.; Chang, Y.H.; Chen, S.H.; Ueng, S.W.; Shih, C.H. High concentration and bioactivity of vancomycin and aztreonam eluted from simplex cement spacers in two-stage revision of infected hip implants: A study of 46 patients at an average follow-up of 107 days. J. Orthop. Res. 2006, 8, 1615–1621. [Google Scholar] [CrossRef]
- Kuechle, D.K.; Landon, G.C.; Musher, D.M.; Noble, P.C. Elution of vancomycin, daptomycin, and amikacin from acrylic bone cement. Clin. Orthop. Relat. Res. 1991, 264, 302–308. [Google Scholar] [CrossRef]
- Penner, M.J.; Duncan, C.P.; Masri, B.A. The in vitro elution characteristics of antibiotic-loaded CMW and palacos-R bone cements. J. Arthroplast. 1999, 2, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Brien, W.W.; Salvati, E.A.; Klein, R.; Brause, B.; Stern, S. Antibiotic impregnated bone cement in total hip arthroplasty: An in vivo comparison of the elution properties of tobramycin and vancomycin. Clin. Orthop. Relat. Res. 1993, 296, 242–248. [Google Scholar] [CrossRef]
- Klekamp, J.; Dawson, J.M.; Haas, D.W.; DeBoer, D.; Christie, M. The use of vancomycin and tobramycin in acrylic bone cement: Biomechanical effects and elution kinetics for use in joint arthroplasty. J. Arthroplast. 1999, 3, 339–346. [Google Scholar] [CrossRef]
- Stevens, C.M.; Tetsworth, K.D.; Calhoun, J.H.; Mader, J.T. An articulated antibiotic spacer used for infected total knee arthroplasty: A comparative in vitro elution study of simplex and palacos bone cements. J. Orthop. Res. 2005, 1, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Ruiz, P.; Matas-Diez, J.; Villanueva-Martinez, M.; Dos Santos-Vaquinha Blanco, A.; Vaquero, J. Is dual antibiotic-loaded bone cement more effective and cost-efficient than a single antibiotic-loaded bone cement to reduce the risk of periprosthetic joint infection in aseptic revision knee arthroplasty? J. Arthroplast. 2020, 35, 3724–3729. [Google Scholar] [CrossRef]
- Blersch, B.; Barthels, M.; Schuster, P.; Fink, B. A low rate of periprosthetic infections after aseptic knee prosthesis revision using dual-antibiotic-impregnated bone cement. Antibiotics 2023, 12, 1368. [Google Scholar] [CrossRef]
- Ho, J.L.; Klempner, M.S. In vitro evaluation of clindamycin in combination with oxacillin, rifampin, or vancomycin against staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 1986, 2, 133–138. [Google Scholar] [CrossRef]
- Smith, P.F.; Booker, B.M.; Ogundele, A.B.; Kelchin, P. Comparative in vitro activities of daptomycin, linezolid, and quinupristin/dalfopristin against gram-positive bacterial isolates from a large cancer center. Diagn. Microbiol. Infect. Dis. 2005, 3, 255–259. [Google Scholar] [CrossRef]
- Kühn, K.D.; Lieb, E.; Berberich, C. PMMA bone cement: What is the role of local antibiotics. In Matrise Orthopaedic, Proceeding of N°243, Commission Paritaire 1218T86410; Heraeus: Lyon, France, 2016; pp. 12–18. ISSN 1148-2362. [Google Scholar]
- Frommelt, L. Guidelines on antimicrobial therapy in situations of periprosthetic THR infection. Orthopade 2004, 7, 822–828. [Google Scholar]
- Cherednichenko, K.; Sayfutdinova, A.; Rimashevskiy, D.; Malik, B.; Panchenko, A.; Kopitsyna, M.; Ragnaev, S.; Vinokurov, V.; Voronin, D.; Kopitsyn, D. Composite bone cements with enhanced drug elution. Polymers 2023, 15, 3757. [Google Scholar] [CrossRef]
- Bertazzoni Minelli, E.; Benini, A.; Samaila, E.; Bondi, M.; Magnan, B. Antimicrobial activity of gentamicin and vancomycin combination in joint fluids after antibiotic-loaded cement spacer implantation in two-stage revision surgery. J. Chemother. 2014, 27, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Streuli, J.C.; Exner, G.U.; Reize, C.L.; Merkofer, C.; Scott, C.P.; Zbinden, R. In vitro inhibition of coagulase-negative staphylococci by vancomycin/aminoglycoside-loaded cement spacers. Infection 2006, 2, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Frommelt, L. Diagnosis and treatment of foreign-body-associated infection in orthopaedic surgery. Orthopade 2009, 9, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Metsemakers, W.J.; Morgenstern, M.; McNally, M.A.; Moriarty, T.F.; McFadyen, I.; Scarborough, M.; Athanasou, N.A.; Ochsner, P.E.; Kuehl, R.; Raschke, M.; et al. Fracture-related infection: A consensus on definition from an international expert group. Injury 2018, 3, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Fink, B.; Grossmann, A.; Fuerst, M.; Schafer, P.; Frommelt, L. Two-stage cementless revision of infected hip endoprostheses. Clin. Orthop. Relat. Res. 2009, 7, 1848–1858. [Google Scholar] [CrossRef] [PubMed]
- Qadir, R.; Sidhu, S.; Ochsner, J.L.; Meyer, M.S.; Chimento, G.F. Risk stratified usage of antibiotic-loaded bone cement for primary total knee arthroplasty: Short term infection outcomes with a standardized cement protocol. J. Arthroplast. 2014, 8, 1622–1624. [Google Scholar] [CrossRef] [PubMed]
- Mulazimoglu, L.; Drenning, S.D.; Muder, R.R. Vancomycin-gentamicin synergism revisited: Effect of gentamicin susceptibility of methicillin-resistant staphylococcus aureus. Antimicrob. Agents Chemother. 1996, 6, 1534–1535. [Google Scholar] [CrossRef] [PubMed]
- Watanakunakorn, C.; Bakie, C. Synergism of vancomycin-gentamicin and vancomycin-streptomycin against enterococci. Antimicrob. Agents Chemother. 1973, 2, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Watanakunakorn, C.; Tisone, J.C. Effects of a vancomycin-rifampin combination on enterococci. Antimicrob. Agents Chemother. 1982, 5, 915–916. [Google Scholar] [CrossRef]
- Hansen, E.; Kühn, K.D. (Eds.) Essentials of Cemented Knee Arthroplasty; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Bistolfi, A.; Massazza, G.; Verné, E.; Massè, A.; Deledda, D.; Ferraris, S.; Miola, M.; Galetto, F.; Crova, M. Antibiotic loaded cement in orthopaedic surgery: A review. Int. Sch. Res. Not. Orthop. 2011, 2011, 290851. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fink, B.; Vogt, S.; Reinsch, M.; Buchner, H. Sufficient release of antibiotic by a spacer 6 weeks after implantation in two-stage revision of infected hip prostheses. Clin. Orthop. Relat. Res. 2011, 11, 3141–3147. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, K.; Wilmes, P.; Schmitt, E.; Kelm, J. Elution of gentamicin and vancomycin from polymethylmethacrylate beads and hip spacers in vivo. Acta Orthop. 2009, 2, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, K. Therapeutic use of antibiotic-loaded bone cement in the treatment of hip and knee joint infections. J. Bone Jt. Infect. 2017, 1, 29–37. [Google Scholar] [CrossRef]
- Bertazzoni Minelli, E.; Della Bora, T.; Benini, A. Different microbial biofilm formation on polymethylmethacrylate (PMMA) bone cement loaded with gentamicin and vancomycin. Anaerobe 2011, 6, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Golge, U.H.; Oztemur, Z.; Parlak, M.; Tezeren, G.; Ozturk, H.; Bulut, O. Investigation of mechanical strength of teicoplanin and ciprofloxacin impregnated bone cement on day 1 and day 15. Acta Orthop. Traumatol. Turc. 2014, 3, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Meeker, D.; Cooper, K.; Renard, R.; Mears, S.; Smeltzer, M.; Barnes, C. Comparative study of antibiotic elution profiles from alternative formulations of polymethylmethacrylate bone cement. J. Arthroplast. 2019, 34, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Krampitz, B.; Steiner, J.; Trampuz, A.; Kühn, K.-D. Voriconazole Admixed with PMMA-Impact on Mechanical Properties and Efficacy. Antibiotics 2023, 12, 848. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G. Not all approved antibiotic-loaded PMMA bone cement brands are the same: Ranking using the utility materials selection concept. J. Mater. Sci. Mater. Med. 2015, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.; Janna, S. Estimation of the optimum loading of an antibiotic powder in an acrylic bone cement: Gentamicin sulfate in SmartSet HV. Acta Orthop. 2006, 4, 622–627. [Google Scholar] [CrossRef]
- Humez, M.; Domann, E.; Thormann, K.M.; Fölsch, C.; Strathausen, R.; Vogt, S.; Alt, V.; Kühn, K.-D. Daptomycin-Impregnated PMMA Cement against Vancomycin-Resistant Germs: Dosage, Handling, Elution, Mechanical Stability, and Effectiveness. Antibiotics 2023, 12, 1567. [Google Scholar] [CrossRef]
- Villanueva-Martinez, M.; Sanz, P.; Berberich, C. Spacer management. In Management of Periprosthetic Joint Infection. A Global Perspective on Diagnosis, Treatment Options, Prevention Strategies and Their Economic Impact; Kühn, K.D., Ed.; Springer: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Hsieh, P.H.; Huang, K.C.; Tai, C.L. Liquid gentamicin in bone cement spacers: In vivo antibiotic release and systemic safety in two-stage revision of infected hip arthroplasty. J. Trauma 2009, 3, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.H.; Tai, C.L.; Lee, P.C.; Chang, Y.H. Liquid gentamicin and vancomycin in bone cement: A potentially more cost-effective regimen. J. Arthroplast. 2009, 1, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Rosslenbroich, S.B.; Raschke, M.J.; Kreis, C.; Tholema-Hans, N.; Uekoetter, A.; Reichelt, R.; Fuchs, T.F. Daptomycin: Local application in implant-associated infection and complicated osteomyelitis. Sci. World J. 2012, 2012, 578251. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.N.; Peggins, J.O.; Nochetto, C.B.; Smith, M.L.; Chiesa, O.A.; Moulton, K. LC/MS/MS measurement of gentamicin in bovine plasma, urine, milk, and biopsy samples taken from kidneys of standing animals. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 821, 22–30. [Google Scholar] [CrossRef]

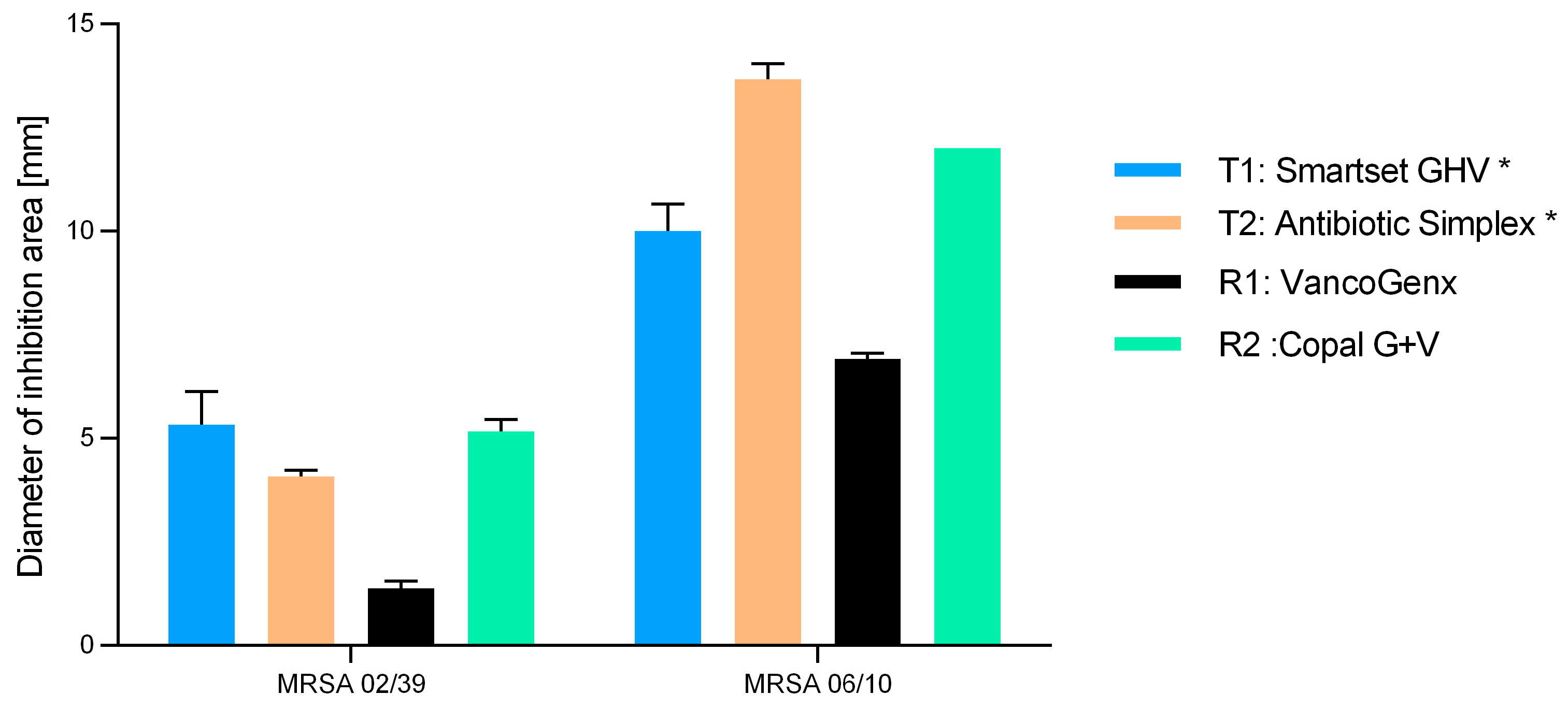
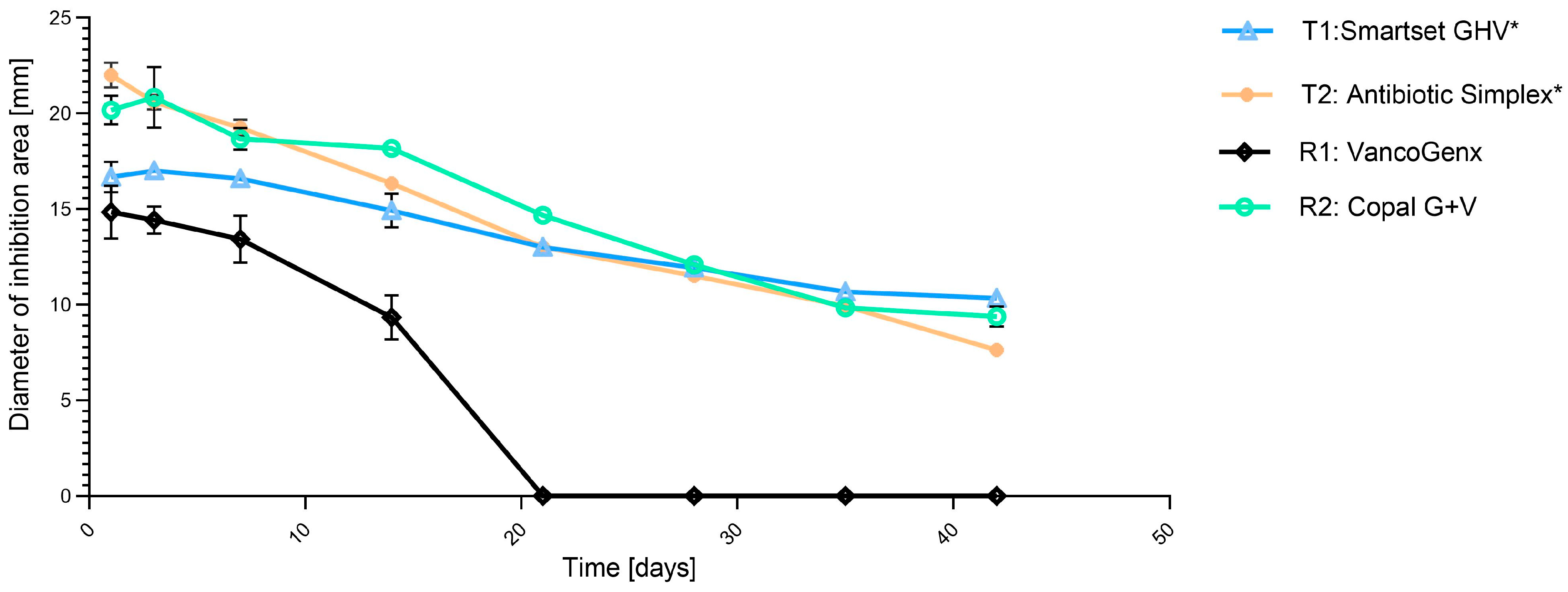


| Cement Tested | Industrial Added Antibiotics (Per 40 g PMMA) | Additionally (Manually) Added Vancomycin |
|---|---|---|
| Test group | ||
| T1: Smartset® GHV | 1.0 g Gentamicin | +2.0 g Vancomycin Hexal (CT1631) |
| T2: Antibiotic Simplex® T | 1.0 g Tobramycin | +2.0 g vancomycin Hexal (CT1631) |
| Reference group | ||
| R1: VancogenX® | 1.0 g Gentamicin plus 1.0 g Vancomycin | no |
| R2: Copal® G+V | 0.5 g Gentamicin plus 2.0 g Vancomycin | no |
| Antibiotic | Methicillin-Resistant S. aureus (MRSA 02/39) | Methicillin-Resistant S. Aureus (MRSA 06/10) | Methicillin-Susceptible S. aureus (DSM 799) |
|---|---|---|---|
| Gentamicin | >256 µg/mL (R) | 0.5 µg/mL (S) | 0.5 µg/mL (S) |
| Vancomycin | 0.75 µg/mL (S) | 1 µg/mL (S) | 0.5 µg/mL (S) |
| Tobramycin | >256 µg/mL (R) | 0.5 µg/mL (R) | 0.5 µg/mL (S) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kittinger, C.; Eder-Halbedl, M.; Kühn, K.D. Impact of Manual Addition of Vancomycin to Polymethylmethacrylate (PMMA) Cements. Antibiotics 2024, 13, 721. https://doi.org/10.3390/antibiotics13080721
Kittinger C, Eder-Halbedl M, Kühn KD. Impact of Manual Addition of Vancomycin to Polymethylmethacrylate (PMMA) Cements. Antibiotics. 2024; 13(8):721. https://doi.org/10.3390/antibiotics13080721
Chicago/Turabian StyleKittinger, Clemens, Michael Eder-Halbedl, and Klaus Dieter Kühn. 2024. "Impact of Manual Addition of Vancomycin to Polymethylmethacrylate (PMMA) Cements" Antibiotics 13, no. 8: 721. https://doi.org/10.3390/antibiotics13080721
APA StyleKittinger, C., Eder-Halbedl, M., & Kühn, K. D. (2024). Impact of Manual Addition of Vancomycin to Polymethylmethacrylate (PMMA) Cements. Antibiotics, 13(8), 721. https://doi.org/10.3390/antibiotics13080721






