Anti-Biofilm and Anti-Inflammatory Properties of the Truncated Analogs of the Scorpion Venom-Derived Peptide IsCT against Pseudomonas aeruginosa
Abstract
:1. Introduction
2. Results
2.1. Hemolytic Activity of the Truncated IsCT Analogs
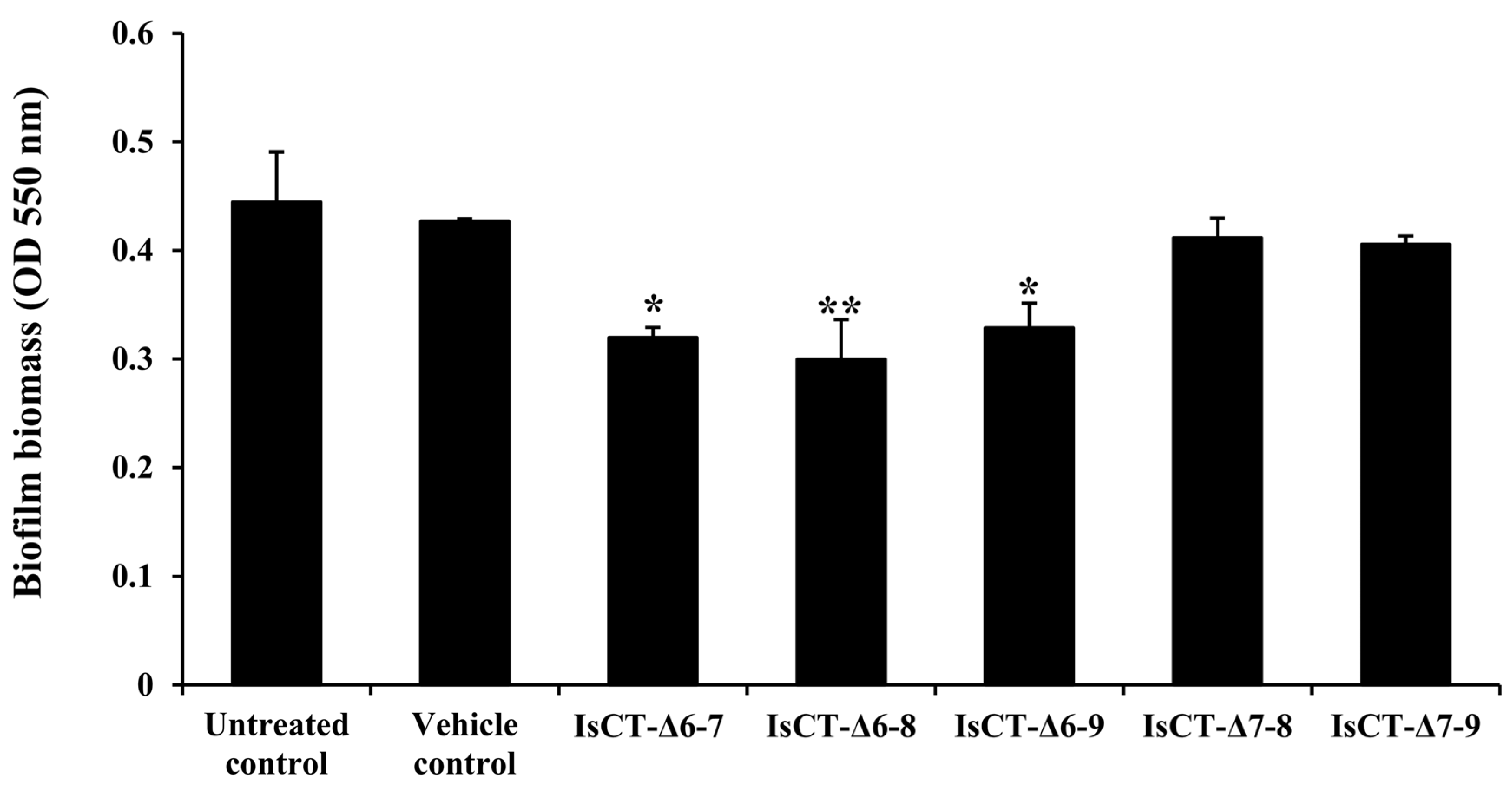
2.2. Anti-Biofilm Activities of the Truncated IsCT Analogs against P. aeruginosa
2.3. Effects of the IsCT-Δ6-8 Peptide on Growth of P. aeruginosa
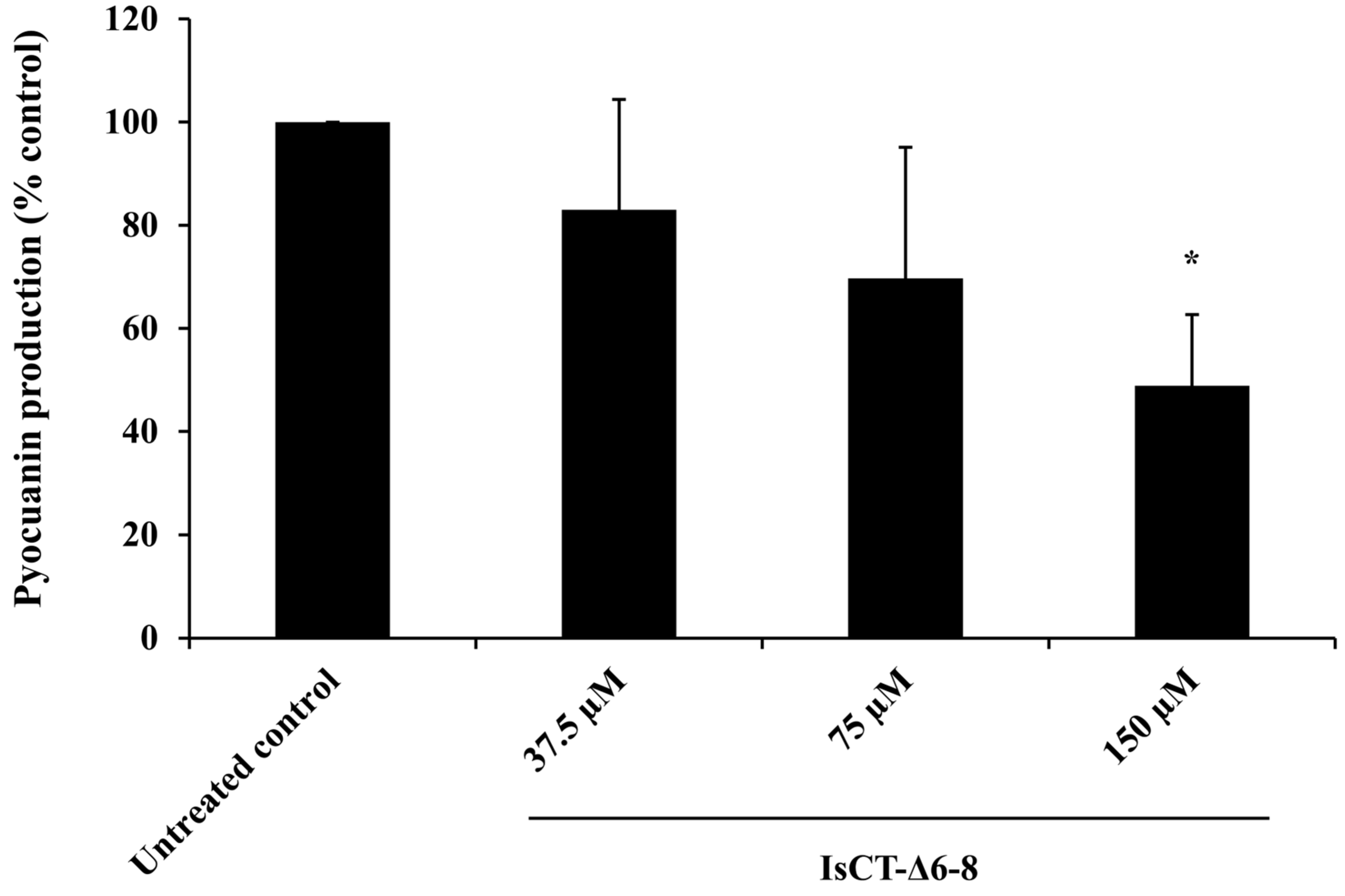
2.4. Effects of the IsCT-Δ6-8 Peptide on Pyocyanin Production of P. aeruginosa
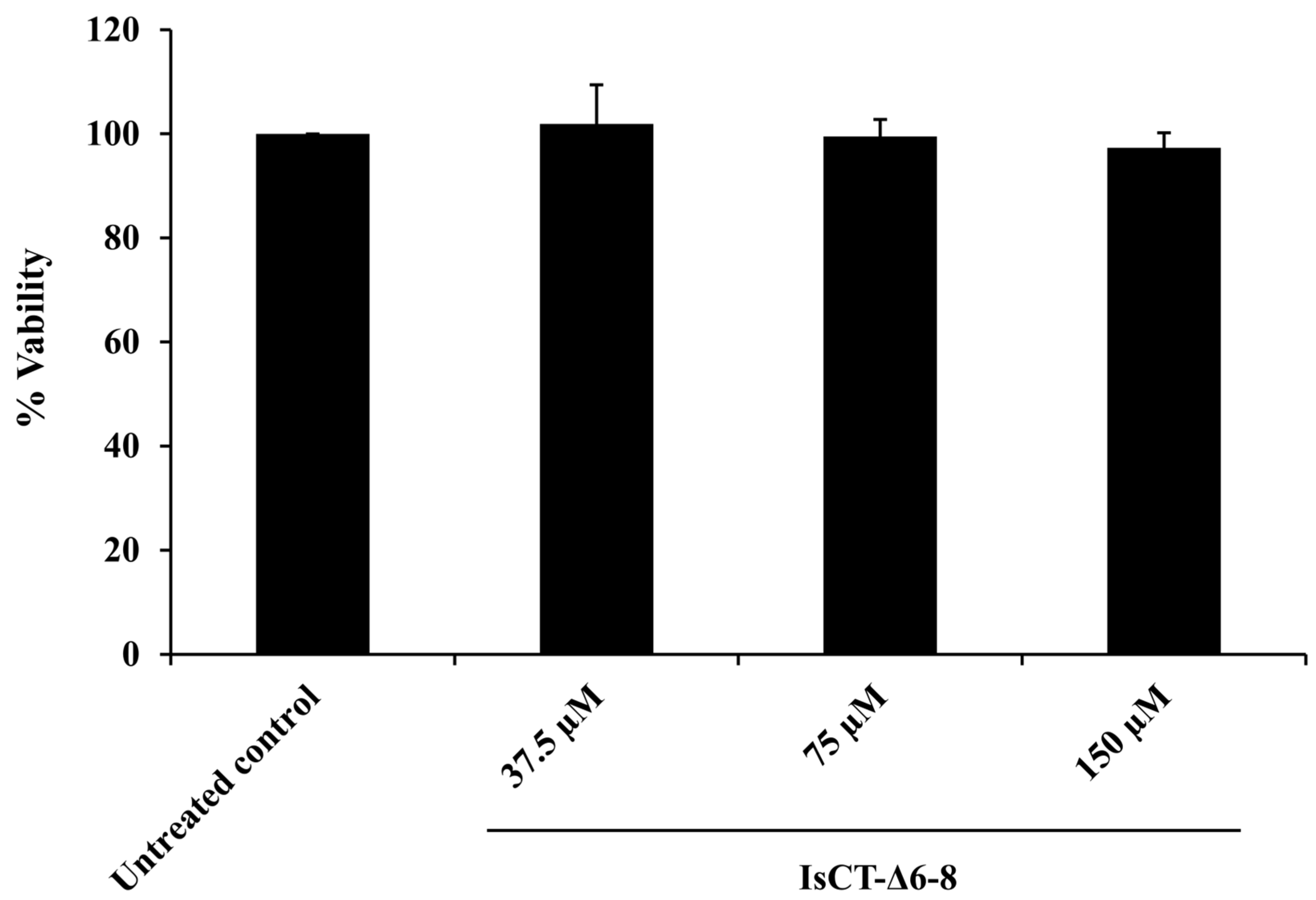
2.5. Effects of the IsCT-Δ6-8 Peptide on the Viability of RAW 264.7 Macrophages
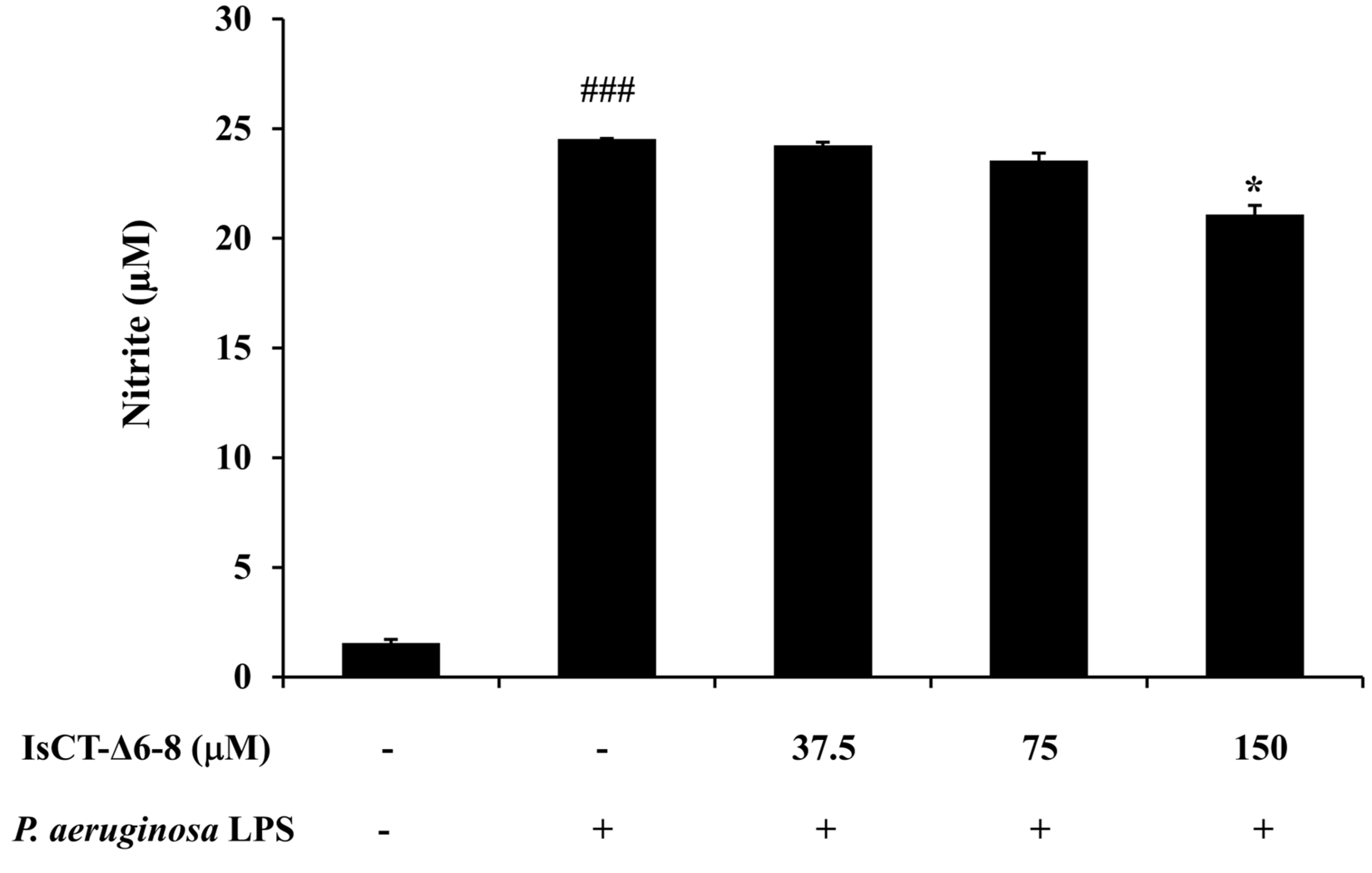
2.6. Effects of the IsCT-Δ6-8 Peptide on Nitric Oxide (NO) Production in P. aeruginosa LPS-Stimulated RAW 264.7 Macrophages
2.7. Effects of the IsCT-Δ6-8 Peptide on IL-6 Production in P. aeruginosa LPS-Stimulated RAW 264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Truncated IsCT Analogs and Their Physicochemical Properties
4.2. Hemolytic Assay
4.3. Bacterial Strain and Culture Condition
4.4. Biofilm Susceptibility Assay
4.5. Growth Curve Assay
4.6. Pyocyanin Assay
4.7. Cell Cultures
4.8. Cytotoxicity Assay
4.9. Measurements of Nitric Oxide (NO) and Interleukin (IL)-6
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raoofi, S.; Pashazadeh Kan, F.; Rafiei, S.; Hosseinipalangi, Z.; Noorani Mejareh, Z.; Khani, S.; Abdollahi, B.; Seyghalani Talab, F.; Sanaei, M.; Zarabi, F.; et al. Global prevalence of nosocomial infection: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0274248. [Google Scholar] [CrossRef]
- Hernández-Jiménez, P.; López-Medrano, F.; Fernández-Ruiz, M.; Silva, J.T.; Corbella, L.; San-Juan, R.; Lizasoain, M.; Díaz-Regañón, J.; Viedma, E.; Aguado, J.M. Risk factors and outcomes for multidrug resistant Pseudomonas aeruginosa infection in immunocompromised patients. Antibiotics 2022, 11, 1459. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Wood, S.J.; Kuzel, T.M.; Shafikhani, S.H. Pseudomonas aeruginosa: Infections, animal modeling, and therapeutics. Cells 2023, 12, 199. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef] [PubMed]
- Hadadi-Fishani, M.; Khaledi, A.; Fatemi-Nasab, Z.S. Correlation between biofilm formation and antibiotic resistance in Pseudomonas aeruginosa: A meta-analysis. Infez. Med. 2020, 28, 47–54. [Google Scholar]
- Ronda, M.; Pérez-Recio, S.; González Laguna, M.; Tubau Quintano, M.F.; Llop Talaveron, J.; Soldevila-Boixader, L.; Carratalà, J.; Cuervo, G.; Padullés, A. Ceftolozane/tazobactam for difficult-to-treat Gram-negative infections: A real-world tertiary hospital experience. J. Clin. Pharm. Ther. 2022, 47, 932–939. [Google Scholar] [CrossRef]
- Garcia-Clemente, M.; de la Rosa, D.; Máiz, L.; Girón, R.; Blanco, M.; Olveira, C.; Canton, R.; Martinez-García, M.A. Impact of Pseudomonas aeruginosa infection on patients with chronic inflammatory airway diseases. J. Clin. Med. 2020, 9, 3800. [Google Scholar] [CrossRef]
- Fernández-Billón, M.; Llambías-Cabot, A.E.; Jordana-Lluch, E.; Oliver, A.; Macià, M.D. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa biofilms. Biofilm 2023, 5, 100129. [Google Scholar] [CrossRef]
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis. Available online: https://iris.who.int/handle/10665/311820 (accessed on 12 July 2024).
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Oh, D.H.; Lee, B.H. Bioactive Peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of antimicrobial peptides: Antimicrobial, anti-Inflammatory and antibiofilm activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Chung, P.Y.; Khanum, R. Antimicrobial peptides as potential anti-biofilm agents against multidrug-resistant bacteria. J. Microbiol. Immunol. Infect. 2017, 50, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.T.Y.; Gellatly, S.L.; Hancock, R.E.W. Multifunctional cationic host defence peptides and their clinical applications. Cell. Mol. Life Sci. 2011, 68, 2161–2176. [Google Scholar] [CrossRef]
- Tan, R.; Wang, M.; Xu, H.; Qin, L.; Wang, J.; Cui, P.; Ru, S. IImproving the activity of antimicrobial peptides against aquatic pathogen bacteria by amino acid substitutions and changing the ratio of hydrophobic residues. Front. Microbiol. 2021, 12, 773076. [Google Scholar] [CrossRef]
- Matthyssen, T.; Li, W.; Holden, J.A.; Lenzo, J.C.; Hadjigol, S.; O’Brien-Simpson, N.M. The potential of modified and multimeric antimicrobial peptide materials as superbug killers. Front. Chem. 2022, 9, 795433. [Google Scholar] [CrossRef]
- Dai, L.; Yasuda, A.; Naoki, H.; Corzo, G.; Andriantsiferana, M.; Nakajima, T. IsCT, a novel cytotoxic linear peptide from scorpion Opisthacanthus madagascariensis. Biochem. Biophys. Res. Commun. 2001, 286, 820–825. [Google Scholar] [CrossRef]
- Dai, L.; Corzo, G.; Naoki, H.; Andriantsiferana, M.; Nakajima, T. Purification, structure-function analysis, and molecular characterization of novel linear peptides from scorpion Opisthacanthus madagascariensis. Biochem. Biophys. Res. Commun. 2002, 293, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Shin, S.Y.; Kim, K.; Lim, S.S.; Hahm, K.-S.; Kim, Y. Antibiotic activity and structural analysis of the scorpion-derived antimicrobial peptide IsCT and its analogs. Biochem. Biophys. Res. Commun. 2004, 323, 712–719. [Google Scholar] [CrossRef]
- Tripathi, J.K.; Kathuria, M.; Kumar, A.; Mitra, K.; Ghosh, J.K. An unprecedented alteration in mode of action of IsCT resulting its translocation into bacterial cytoplasm and inhibition of macromolecular syntheses. Sci. Rep. 2015, 5, 9127. [Google Scholar] [CrossRef]
- Acevedo, I.C.C.; Silva, P.I., Jr.; Silva, F.D.; Araújo, I.; Alves, F.L.; Oliveira, C.S.; Oliveira, V.X., Jr. IsCT-based analogs intending better biological activity. J. Pept. Sci. 2019, 25, e3219. [Google Scholar] [CrossRef]
- de la Salud Bea, R.; Petraglia, A.F.; Ascuitto, M.R.; Buck, Q.M. Antibacterial activity and toxicity of analogs of scorpion venom IsCT peptides. Antibiotics 2017, 6, 13. [Google Scholar] [CrossRef]
- Liu, L.; Cao, X.; Ma, W.; Chen, L.; Li, S.; Hu, B.; Xu, Y. In-situ and continuous monitoring of pyocyanin in the formation process of Pseudomonas aeruginosa biofilms by an electrochemical biosensor chip. Sens. Actuators B Chem. 2021, 327, 128945. [Google Scholar] [CrossRef]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef]
- Jiang, Z.; Vasil, A.I.; Hale, J.D.; Hancock, R.E.W.; Vasil, M.L.; Hodges, R.S. Effects of net charge and the number of positively charged residues on the biological activity of amphipathic α-helical cationic antimicrobial peptides. Pept. Res. 2008, 90, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Dathe, M.; Wieprecht, T.; Nikolenko, H.; Handel, L.; Maloy, W.L.; MacDonald, D.L.; Beyermann, M.; Bienert, M. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett. 1997, 403, 208–212. [Google Scholar] [CrossRef]
- Amorim-Carmo, B.; Parente, A.M.S.; Souza, E.S.; Silva-Junior, A.A.; Araújo, R.M.; Fernandes-Pedrosa, M.F. Antimicrobial peptide analogs from scorpions: Modifications and structure-activity. Front. Mol. Biosci. 2022, 9, 887763. [Google Scholar] [CrossRef] [PubMed]
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Núñez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.M.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E.W. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, J.; Yin, Y.; Wang, G.; Yang, M.; Li, Y.; Zhang, Z.; Lai, R. The antimicrobial peptide ZY4 combats multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii infection. Proc. Natl. Acad. Sci. USA 2019, 116, 26516–26522. [Google Scholar] [CrossRef] [PubMed]
- Nagant, C.; Pitts, B.; Nazmi, K.; Vandenbranden, M.; Bolscher, J.G.; Stewart, P.S.; Dehaye, J.P. Identification of peptides derived from the human antimicrobial peptide LL-37 active against biofilms formed by Pseudomonas aeruginosa using a library of truncated fragments. Antimicrob. Agents Chemother. 2012, 56, 5698–5708. [Google Scholar] [CrossRef] [PubMed]
- Parducho, K.R.; Beadell, B.; Ybarra, T.K.; Bush, M.; Escalera, E.; Trejos, A.T.; Chieng, A.; Mendez, M.; Anderson, C.; Park, H.; et al. The antimicrobial peptide human beta-defensin 2 inhibits biofilm production of Pseudomonas aeruginosa without compromising metabolic activity. Front. Immunol. 2020, 11, 805. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Gonçalves, S.; Felício, M.R.; Maturana, P.; Santos, N.C.; Semorile, L.; Hollmann, A.; Maffía, P.C. Synergistic and antibiofilm activity of the antimicrobial peptide P5 against carbapenem-resistant Pseudomonas aeruginosa. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Teerapo, K.; Roytrakul, S.; Sistayanarain, A.; Kunthalert, D. A scorpion venom peptide derivative BmKn–22 with potent antibiofilm activity against Pseudomonas aeruginosa. PLoS ONE 2019, 14, e0218479. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Manefield, M. Pyocyanin promotes extracellular DNA release in Pseudomonas aeruginosa. PLoS ONE 2012, 7, e46718. [Google Scholar] [CrossRef]
- Chimi, L.Y.; Noubom, M.; Bisso, B.N.; Singor Njateng, G.S.; Dzoyem, J.P. Biofilm formation, pyocyanin production, and antibiotic resistance profile of Pseudomonas aeruginosa isolates from wounds. Int. J. Microbiol. 2024, 2024, 1207536. [Google Scholar] [CrossRef]
- Lau, G.W.; Hassett, D.J.; Ran, H.; Kong, F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 2004, 10, 599–606. [Google Scholar] [CrossRef]
- Sharma, G.; Rao, S.; Bansal, A.; Dang, S.; Gupta, S.; Gabrani, R. Pseudomonas aeruginosa biofilm: Potential therapeutic targets. Biologicals 2014, 42, 1–7. [Google Scholar] [CrossRef]
- Ciornei, C.D.; Novikov, A.; Beloin, C.; Fitting, C.; Caroff, M.; Ghigo, J.M.; Cavaillon, J.M.; Adib-Conquy, M. Biofilm-forming Pseudomonas aeruginosa bacteria undergo lipopolysaccharide structural modifications and induce enhanced inflammatory cytokine response in human monocytes. Innate Immun. 2010, 16, 288–301. [Google Scholar] [CrossRef]
- Gubernatorova, E.O.; Gorshkova, E.A.; Namakanova, O.A.; Zvartsev, R.V.; Hidalgo, J.; Drutskaya, M.S.; Tumanov, A.V.; Nedospasov, S.A. Non-redundant functions of IL-6 produced by macrophages and dendritic cells in allergic airway inflammation. Front. Immunol. 2018, 9, 2718. [Google Scholar] [CrossRef]
- Ramirez, T.; Shrestha, A.; Kishen, A. Inflammatory potential of monospecies biofilm matrix components. Int. Endod. J. 2019, 52, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xiang, D.; Tian, F.; Ni, M. Lipopolysaccharide from biofilm-forming Pseudomonas aeruginosa PAO1 induces macrophage hyperinflammatory responses. J. Med. Microbiol. 2021, 70, 001352. [Google Scholar] [CrossRef]
- Courtney, J.M.; Ennis, M.; Elborn, J.S. Cytokines and inflammatory mediators in cystic fibrosis. J. Cyst. Fibros. 2004, 3, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Burgner, D.; Rockett, K.; Kwiatkowski, D. Nitric oxide and infectious diseases. Arch. Dis. Child. 1999, 81, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2015, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Lear, S.; Cobb, S.L. Pep-Calc.com: A set of web utilities for the calculation of peptide and peptoid properties and automatic mass spectral peak assignment. J. Comput. Aided Mol. Des. 2016, 30, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A web server to screen sequences with specific α-helical properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Chaudhary, S.K.; Sharma, A.; Yadav, K.K.; Nema, N.K.; Sekhoacha, M.; Karmakar, S.; Braga, F.C.; Matsabisa, M.G.; Mukherjee, P.K.; et al. Anti-biofilm activity of Marula—A study with the standardized bark extract. J. Ethnopharmacol. 2014, 154, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, O.; Kiendrebeogo, M.; Rajaonson, S.; Diallo, B.; Mol, A.; Jaziri, M.; Baucher, M. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2010, 76, 243–253. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]


| Treatment | % Hemolysis a |
|---|---|
| Untreated control | 0.00 ± 0.00 |
| 1% Triton X-100 | 100.00 ± 0.00 |
| IsCT-Δ6-7, 150 μM | 0.83 ± 0.81 |
| IsCT-Δ6-8, 150 μM | 1.91 ± 0.81 |
| IsCT-Δ6-9, 150 μM | 0.47 ± 0.30 |
| IsCT-Δ7-8, 150 μM | 2.13 ± 1.00 |
| IsCT-Δ7-9, 150 μM | 0.44 ± 0.35 |
| Peptides | Amino Acid Sequence | No. of Amino Acid | Molecular Weight (Da) | Net Charge (z) | pI | Hydrophobicity (H) | Hydrophobic Moment (mH) |
|---|---|---|---|---|---|---|---|
| IsCT-Δ6-7 | ILGKIGIKSLF | 11 | 1188.51 | +2 | 10.73 | 0.779 | 0.171 |
| IsCT-Δ6-8 | ILGKIIKSLF | 10 | 1131.46 | +2 | 10.73 | 0.857 | 0.187 |
| IsCT-Δ6-9 | ILGKIKSLF | 9 | 1018.30 | +2 | 10.73 | 0.752 | 0.491 |
| IsCT-Δ7-8 | ILGKIWIKSLF | 11 | 1317.67 | +2 | 10.73 | 0.984 | 0.376 |
| IsCT-Δ7-9 | ILGKIWKSLF | 10 | 1204.51 | +2 | 10.73 | 0.902 | 0.214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jantaruk, P.; Teerapo, K.; Charoenwutthikun, S.; Roytrakul, S.; Kunthalert, D. Anti-Biofilm and Anti-Inflammatory Properties of the Truncated Analogs of the Scorpion Venom-Derived Peptide IsCT against Pseudomonas aeruginosa. Antibiotics 2024, 13, 775. https://doi.org/10.3390/antibiotics13080775
Jantaruk P, Teerapo K, Charoenwutthikun S, Roytrakul S, Kunthalert D. Anti-Biofilm and Anti-Inflammatory Properties of the Truncated Analogs of the Scorpion Venom-Derived Peptide IsCT against Pseudomonas aeruginosa. Antibiotics. 2024; 13(8):775. https://doi.org/10.3390/antibiotics13080775
Chicago/Turabian StyleJantaruk, Pornpimon, Kittitat Teerapo, Supattra Charoenwutthikun, Sittiruk Roytrakul, and Duangkamol Kunthalert. 2024. "Anti-Biofilm and Anti-Inflammatory Properties of the Truncated Analogs of the Scorpion Venom-Derived Peptide IsCT against Pseudomonas aeruginosa" Antibiotics 13, no. 8: 775. https://doi.org/10.3390/antibiotics13080775






